?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Strontium and magnesium doped ceria solid solutions (Ce0.99Sr0.01O1.995 and Ce0.99M0.01O1.995) were synthesized by a cost effective solid state reaction. The doped and un-doped CeO2 samples were sintered at 1200 °C, 1300 °C and 1400 °C to investigate the effect of sintering temperature and doping on density, structural and morphological properties. The density was measured by Archimedes’ method. It is observed that the density increases with increasing sintering temperature and with doping of strontium. The crystal structure and surface morphology have been characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). XRD and SEM reveals that the synthesized samples are single phase with a cubic fluorite structure, and the grains formed are of different sizes. The grain size depends on sintering temperature and type of doping. The lattice parameter increases with sintering temperature and substitution of Sr in ceria. The Grain size of Sr-doped ceria decreases, whereas that of Mg-doped ceria increases. EDS spectra show that the samples are free of contaminants. The Ce0.99Sr0.01O1.995 shows a more open structure than un-doped ceria.
Keywords:
1 Introduction
Among various types of fuel cells, solid oxide fuel cells (SOFC) have multi-fuel capability, a high conversion efficiency of approximately 60% and flexibility in their operation. The electrolyte of an SOFC must be dense, have high ionic conductivity and zero electronic conductivity [Citation1,Citation2]. Yttria-stabilized zirconia (YSZ) can be used as an SOFC electrolyte, but the high operating temperature of ∼1000 °C necessary for oxygen ion conductivity would decrease the efficiency and stability of the cell [Citation3,Citation4]. Cerium oxide has a peculiar function different from other rare-earth oxides, as it tends to consist of non-stoichiometric compounds with +4 and +3 oxidation states of cerium. The redox property of ceria leads to oxygen vacancies resulting in a very high oxygen ion conductivity when compared with YSZ, even at intermediate temperatures of 600–800 °C. Ceria doped with alkali earth metal oxides such as CaO and SrO has been studied extensively [Citation5–Citation7]. The conductivity of CaO-doped ceria is much greater than calcia-stabilized zirconia (CSZ) [Citation8,Citation9]. Ceria solid solutions with the formula Ce1−xMxO2−δ, where M is a rare earth metal or lanthanide, show more open structures, possess higher oxygen ion conductivities and are potential electrolytes for low temperature SOFCs (LT-SOFC) [Citation10–Citation12]. To achieve high ionic and zero electronic conductivity, an SOFC electrolyte requires high density and low porosity. The open structure of doped cerium oxides have high ionic conductivities and can be synthesized by various routes. The crystal structure and relative density of solid ceria electrolytes increases with increasing sintering temperature [Citation13]. Alkali earth oxides such as MgO, CaO and SrO are soluble in the ceria lattice, and the resultant materials are suitable candidates for the electrolyte in LT-SOFCs. Mechanically mixed powders of CeO2 and Sm2O3 have been studied extensively [Citation14]. Gadolinium doped ceria (GDC) and samarium doped ceria (SDC) synthesized by the solid state reaction method have been studied extensively for the effects of sintering temperature on density, porosity, structural and electrical properties [Citation15–Citation17]. Ceria co-doped with rare earth and alkali earth metals have also been synthesized by the solid state reaction method. Their densities increased while their porosities decreased with sintering temperature yielding higher oxygen ion conductivities [Citation18,Citation19]. To explore simpler and cheaper synthesis methods for SOFC electrolytes and to study the effect of temperature on un-doped and Mg- and Sr-doped ceria, representative samples were prepared and investigated.
2 Experimental
2.1 Sample preparation
Commercially available powders of CeO2, MgO and SrCO3 (AR grade, Sigma Aldrich, USA, 99.9% purity) were used as starting materials. The powders of CeO2 and SrCO3 were mixed in the appropriate stoichiometric proportions (1 mole%) to make Ce0.99Sr0.01O1.995. The mixture was ground in agate and mortar to obtain a homogenized powder. The powder was calcinated at 800 °C for 2 h to decompose the SrCO3 and reground. Two mole% of polyvinylpyridine was added to the powder as a binder and was mixed thoroughly. The powder sample was uni-axially pressed by a pressure of 10 tons/sq inch to obtain disc-shaped pellets. Ce0.99Mg0.01O1.995 and un-doped CeO2 pellets were prepared in a similar manner. The prepared pellets were sintered at 1200 °C, 1300 °C and 1400 °C for 2 h in air, at a ramp of 2 °C/min and cooled to room temperature by the rate. The prepared un-doped CeO2 pellets were identified as CE12, CE13 and CE14; Sr-doped pellets as SC12, SC13 and SC14; and Mg-doped pellets as MC12, MC13 and MC14, which were sintered at temperatures of 1200 °C, 1300 °C and 1400 °C, respectively. Additionally, bulk densities (dB) were determined for the aforementioned samples. Finally, a total of six dense pellets (CE12, CE13 and CE14, Sr-doped pellets SC13 and SC14 and Mg-doped MC14) were used for characterization.
2.2 Characterization
2.2.1 Density
Bulk densities (dB) of the sintered samples were measured by Archimedes’ method. The theoretical densities were measured by the formula(1)
(1) where MC, MO and ‘M’ are atomic wt. of ceria; oxygen and the dopants Sr and Mg; and A is the lattice parameter.
2.2.2 Structure and morphology
The phase and structural properties of sintered pellets were studied by powder X-ray diffraction (XRD) using Cu Kα radiation (λ = 1.54 Å) as the radiation source at 40 kV and 30 mA. The crystalline size ‘D’ was measured by Scherrer's formula(2)
(2) where β is FWHM of the peak and θ is Bragg angle. The lattice parameter was calculated by using the relation
(3)
(3)
Surface morphology was characterized by scanning electron microscopy (ZEISS Evo series SEM) at an operating voltage of 15 kV. Grain size was measured from higher magnification SEM micrographs.
3 Results and discussion
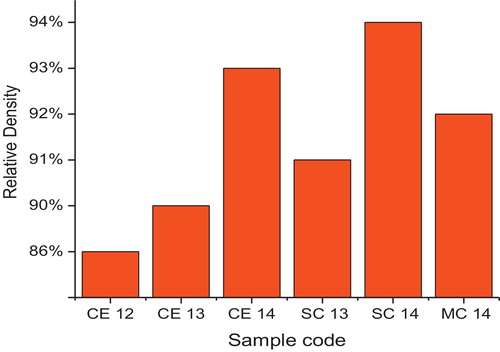
The measured bulk densities, dB, of samples are between 86% and 94% of their theoretical densities, dth. gives the relative densities of samples sintered at different temperatures. It is observed that the relative density increases with increasing sintering temperature, as shown in .
Table 1 Sample identification and densities.
Substitution of Sr in ceria leads to an increase in density of the ceria solid solution. The relative density is approximately 93% of the theoretical density at temperature 1400 °C for ceria and 94% for strontium doped ceria. The substitution and increase in the content of Sr in ceria [Citation10,Citation20] and Gd in ceria [Citation21] increases its density. However, in the case of Mg-doped ceria, no great change in density has been observed, probably because of its size mismatch and lesser atomic wt.
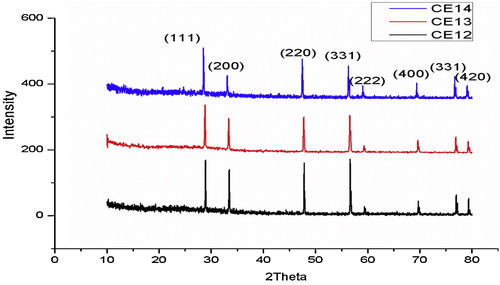
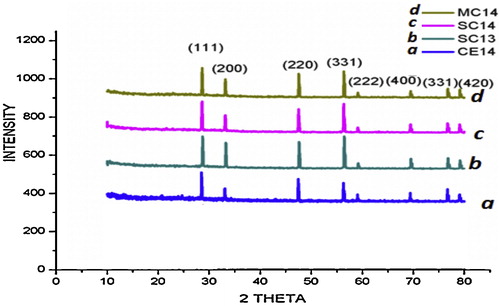
The X-ray diffraction patterns obtained for CE12, CE13 and CE14 are shown in , and diffraction patterns for CE14, SC13, SC14 and MC14 are shown in . All of the samples are single phase. The results are compared with JCPDS card no. 81-0792. The samples show the presence of (1 1 1), (2 0 0), (2 2 0), (3 1 1), (2 2 2), (4 0 0) and (3 3 1) diffraction peaks in the scanning range of 2θ = 20–90°, and exhibit cubic fluorite structure. The crystalline size and lattice parameters were calculated using Eqs. Equation(2)(2)
(2) and Equation(3)
(3)
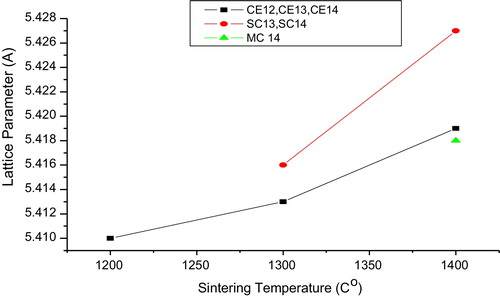
(3) . gives lattice parameters for the samples sintered at different temperatures. It is evident from that as the sintering temperature increases the (1 1 1) diffraction peak shifts towards a lower value of 2θ [Citation22]. Lattice parameters increase linearly with increasing temperature [Citation13,Citation23]. Deviation of the lattice parameter, Δa (Δa = |aCE − adoped|) from un-doped ceria CE14 was found at a maximum (0.008) for CS14 and at a minimum for MC14 as shown in . A range in crystal size from 19.3 to 29.08 nm is observed.
Table 2 Results of XRD and SEM.
From , it is clear that the peaks corresponding to doped samples shifted to lower 2θ values, which indicates an increase in the lattice parameter. As shown in , the lattice parameters of SC13 and SC14 are greater than that of CE13 and CE14. This could be attributed to the difference in size of the two metal atoms with the size of Ce+4 (0.97 Å) being less than that of Sr +2 (1.21 Å) [Citation15,Citation19,Citation24]. Whenever a material is doped with a dopant of higher atomic radii (rd > r) the diffraction peak maximum shifts towards a lower value of 2θ; conversely, it shifts to a higher 2θ value when rd < r [Citation20,Citation22,Citation25]. In the present study, the (1 1 1) peak of MC14 shifts towards the right when compared with that of CE14, as rMg (0.72 Å) < rCe (0.96 Å).
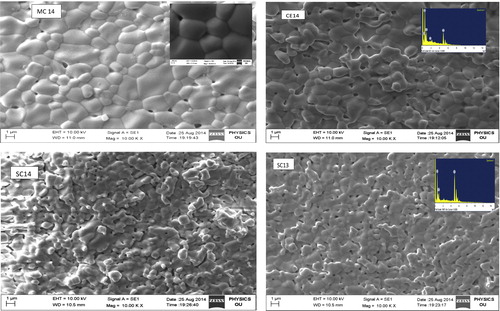
Micro structures of the sintered pellets are shown in . No pores are observed in high temperature sintered samples, which is consistent with their measured densities. The linear intercept method is applied and higher magnification micrographs are used to measure grain sizes. The grains observed are of varying size. The average grain size for CE14 is 5.5 μm, and that of SC14 is 3 μm. It is evident that the substitution of Sr into the Ceria lattice decreases the grain size, which may be due to the mismatch of size of atomic radii of Ce+4 and Sr+2. However, in the case of Mg-doped ceria the grain size (6.3 μm) increases, which could be due to rMg (0.72 Å) < rCe (0.96 Å). However, as sintering temperature increases, an increment in the grain size is observed.
4 Conclusion
The sintering and doping of Sr and Mg in ceria leads to a change in density, lattice parameter and grain size. The density and lattice parameters of un-doped ceria, Sr-doped and Mg-doped ceria increase with sintering temperature. The alkali earth metals can be used as a sintering aid in ceria. XRD analysis reveals that the lattice parameter increases with sintering temperature and doping. The grain size of cerium oxide decreases with the doping of Sr, giving a more open structure that could exhibit high ionic conductivity.
Notes
Peer review under responsibility of Taibah University.
References
- N.Q.MinhT.TakahashiScience and Technology of Ceramic Fuel Cells1995Elsevier978-0-444-89568-4
- R.BoveSolid oxide fuel cells: principles, design and state of the art in industriesS.BasuRecent Trends in Fuel Cell Science and Technology2007Springer and Anamaya PublishersNew Delhi, India0 387 35537 5 (Chapter 11)
- H.InabaH.TagawaCeria-based solid electrolytes: reviewSolid State Ionics831996116
- M.BiswasP.Kumar OjhaE.Moses JaysinghC.Durga PrasadSynthesis of nano crystalline yttria stabilized zirconia for SOFCNanomater. Nanotechnol.220115558
- O.Poh ShingMechanical synthesis and characterization of calcia doped ceria oxide ion conductorMater. Sci. Eng.172011
- K.YamashitaHydrothermal synthesis and low temperature conduction properties of substituted ceria ceramicSolid State Ionics8119955360
- S.BanerjeeP.S.DeviUnderstanding the effect of calcium on the properties of ceria prepared by a mixed fuel processSolid State Ionics1792008661669
- V.V.KhartonF.M.FigueirdoL.LavorroE.N.NaumovichCeria based materials for solid oxide fuel cellsJ. Mater. Sci.36200111051117
- H.AraiElectrical properties of calcia-doped ceria with oxygen ion conductionSolid State Ionics2041986241248
- N.JaiswalD.KumarS.UpadhyayCeria doped with calcium and strontium (Sr): a potential candidate for intermediate temperature fuel cellIonics2020144554
- C.VeranitisagulA.KaewvilaiW.WattanathanaN.KoonsaengA.LaobutheeElectrolyte materials for solid oxide fuel cells derived from metal complexes: gadolinia doped ceriaCeram. Int.38201224032409
- M.DudekCeramic electrolyte in Ce0.8−xGd2O3SrO system – preparation, properties and application for solid oxide fuel cellsInt. J. Electrochem. Sci.7201228742889
- M.G.ChourasiaJ.Y.PatilS.H.PawarL.D.JadhavStudies on structural, morphological and electrical properties of Ce1−xGdxO2−x/2Mater. Chem. Phys.10920083944
- H.YahiroY.EguchiK.EguchiH.AraiOxygen ionic conductivity of ceria-samarium oxide system with fluorite structureJ. Appl. Electrochem.181998527531
- L.D.JadhavS.H.PawarM.G.ChourashiyaEffect of sintering temperature on structural and electrical properties of gadolinium doped ceria (Ce0.9Gd0.1O1.95)Bull. Mater. Sci.30200797100
- M.Buchi SureshJ.RoyThe effect of strontium doping on densification and electrical properties of Ce0.8Gd0.2O2−δ electrolyte for IT-SOFC applicationsIonics182012291297
- T.-H.YehC.-C.ChouIonic conductivity investigation in samarium and strontium co-doped ceria systemPhys. Scr.T1292007
- Y.ZhengS.HeL.GeM.ZhouH.ChenL.GuoEffect of Sr on Sm-doped ceria electrolyteInt. J. Hydrogen Energy36201151285135
- S.RameshK.C.James RajuStructural and ionic conductivity studies of doped ceria electrolyteElectrochem. Solid-State Lett.152011B24B26
- N.JaiswalD.KumarS.UpadhyahO.PrakashEffect of Mg and Sr co-doping on the electrical properties of ceria-based electrolyte material for intermediate temperature solid oxide fuel cellsJ. Alloys Compd.5772013456462
- Y.MaCeria based Nanostructural Material for Low Temperature Solid Oxide Fuel Cells (Dissertation PhD thesis)2012Royal Institute of Technology, KTHStockholm
- E.Y.PikalovaCeO2 based materials doped with lanthanides for applications in intermediate temperature electrochemical cell devicesInt. J. Hydrogen Energy36201161386157
- S.RameshK.C.James RajuC.Vishnuvardhan ReddySynthesis and characterization of co-doped ceria ceramic by sol–gel methodTrans. Ind. Ceram. Sci.702011143147
- F.-Y.WangS.ChenQ.WangS.YuS.ChengStudy of Gd and Mg Co-doped ceria electrolyte for intermediate temperature solid oxide fuel cellCatal. Today972004189194
- H.ZouY.S.LinN.RaneT.HeSynthesis and characterization of nanosized ceria powders and high-concentration ceria solsInd. Eng. Chem. Res.43200430193025