?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pure and silver-doped cadmium zinc sulphide phosphors have been synthesized by high-temperature solid-state reaction methods under a nitrogen atmosphere. The influence of silver concentration on the crystal structure and the luminescence of solid-state synthesized (Cd0.95Zn0.5)S crystals were investigated by X-ray diffraction (XRD), energy dispersive analysis of X-rays (EDX) and photoluminescence (PL) emission spectroscopy. The powder X-ray diffraction (PXRD) pattern of silver-doped (Cd0.95Zn0.5)S revealed a hexagonal crystalline phase. The morphology of the samples was studied by scanning electron microscopy (SEM), which confirmed the microcrystalline behaviour and good connectivity with grain. The photoluminescence spectra were obtained by irradiating the samples with 345-nm UV light. The effects of silver concentration on the photoluminescence spectra of the prepared phosphors were investigated in detail.
1 Introduction
Group II–VI semiconductor materials have been the focus of extensive theoretical and experimental studies for several decades. Moreover, these semiconductor materials are of great interest for both fundamental research and industrial development. Because of their nonlinear optical properties, electrical properties, tremendous luminescence quality, quantum cutting and other excellent physical and chemical properties, group II–VI microcrystalline semiconductors have potential applications as sensors, conductors, diodes and optical devices [Citation1–Citation4]. For numerous applications in the field of optics, the optical properties of materials are required to be tunable and thus controllable. The optical property tunability of these materials is imperative and would be beneficial to their applicability [Citation2,Citation5–Citation7]. (CdZn)S is a high band gap material used for various optoelectronic applications in the visible range of the electromagnetic spectrum. Because of its relatively high photoconductivity, high luminosity, tunability and morphological properties, it yields excellent results for various applications [Citation2,Citation8–Citation13].
Among the various methods for the preparation of Ag-doped (CdZn)S materials, solid-state reaction techniques are effective. This technique can provide homogenous internal and volumetric heating. Moreover, the solid-state reaction technique is capable of producing uniform particle size distribution and high purity with fast processing at low cost [Citation14]. The literature reveals that many authors have already studied different properties of (CdZn)S. Sao et al. synthesized Ag+-doped (Cd0.95Zn0.5)S phosphors in an air atmosphere with KCl as a flux, and they also studied the ML and TL properties. Mechanoluminescence properties of (Cd0.95Zn0.5)S mixed nanoparticles doped with silver is already reported by Sao et al. (2012) and Ratnesh et al. (2014) [Citation9,Citation13]. The present work intends to study the effect of silver ion concentration on the structural and photoluminescence behaviour of (Cd0.95Zn0.5)S phosphors synthesized by solid-state reaction methodology under an N2 atmosphere.
2 Experimental details
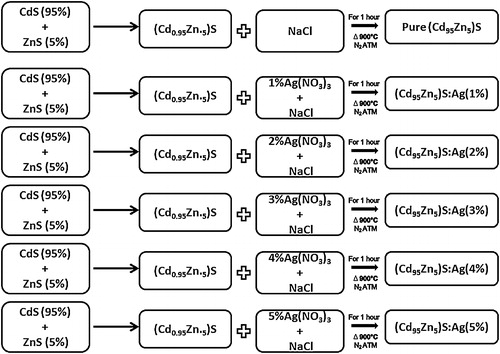
Pure and silver-doped (Cd0.95Zn0.5)S phosphors were prepared using solid-state reaction methods. Luminescence grade CdS and ZnS (Fluka, Switzerland) and silver nitrate (Ag(NO3)3; Merck) were acquired, as well as sodium chloride (NaCl; Merck) for use as a flux. The fixed CdS and ZnS contents (0.95% and 0.5%, respectively) were mixed with different Ag+ ion concentrations (1–5 mol%) for preparation of the phosphors. The mixture was placed in an alumina crucible, and then heated in a silica tubular furnace maintained at 900 °C under an inert atmosphere of flowing nitrogen gas. After the completion of heating, the mixture was allowed to cool to room temperature in the same furnace. Following cooling, the sample was immediately crushed to convert it into a fine powder with uniform crystal size. () [Citation2].
3 Results and discussion
3.1 X-ray diffraction (XRD) results
The XRD pattern of pure and Ag(1–5%)-doped (Cd0.95Zn0.5)S phosphor samples is shown in a The peak width shows the behaviour of the sample. The peak width decreases as the size of the crystal increases, thus the width increases as crystal size decreases. The size of the crystal was calculated using the full width at half maximum (FWHM) of all peaks obtained by the Debye Scherrer formula and then finding the average of these values [Citation18]. The formula used for this calculation was:where D is crystal size in nm, β is the full width at half maximum (FWHM), λ is the wavelength of X-ray source (1.54 Å), and θ is the angle of diffraction.
Fig. 1 (a) Powder X-ray diffraction pattern of pure and Ag (1–5%)-doped (Cd0.95Zn0.5)S phosphors. (b) Silver ion concentration vs. crystal size.
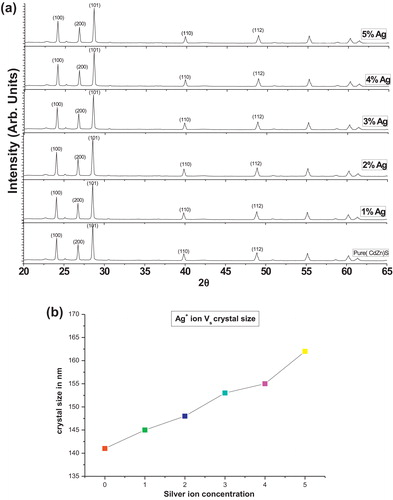
The crystal size of the sample varies from 100 to 160 nm for 0–5% Ag. The XRD analysis indicates that crystal size increases with increasing Ag+ concentration ( and b).
Table 1 Sample characteristics and their compositions (mol.%).
3.2 Scanning electron microscopy (SEM) results
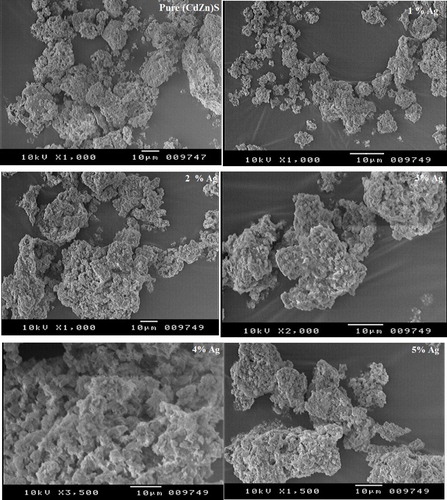
The morphologies are noticeably dependent on the preparation procedure and crystal composition. The surface morphology of the crystals was also observed by SEM, which provided images of the surface morphology of pure and Ag (1–5%)-doped (Cd0.95Zn0.5)S phosphors (). Based on the SEM images, the prepared phosphor shows microcrystalline behaviour and good connectivity with grain, which indicates that powder size and morphology are well controlled. No significant difference was observed in the XRD patterns and SEM micrographs; therefore, all samples are well crystallized into hexagonal (Cd0.95Zn0.5)S:Ag structures. These indicate that the enhancement in luminescence efficiency is not caused by grain morphology.
3.3 Energy dispersive analysis of X-rays (EDX) results
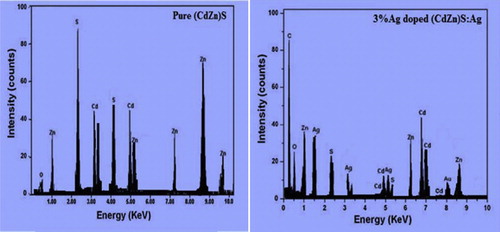
Energy dispersive X-ray analysis gives both qualitative and quantitative information about the elemental composition of the materials. From the EDX spectra, we can conclude that there are other materials, such as impurities or adducts, present in the samples. These impurities occur either accidently with the reagent molecules or are added for modification of the basic materials. The intensity of the spectra correlates with the amount of elements present in it. The EDX spectra of pure and silver-doped (Cd0.95Zn0.5)S phosphors are shown in . The results justified the synthesis of pure and doped (Cd0.95Zn0.5)S phosphors.
3.4 Photoluminescence results
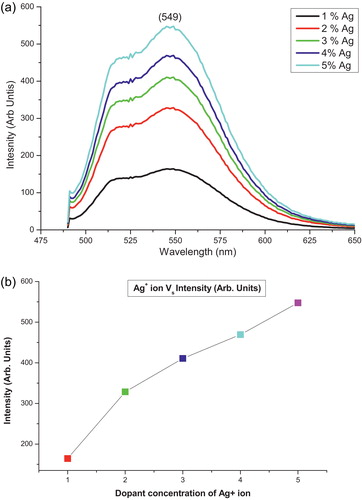
Photoluminescence (PL) is a very sensitive method that investigates defects and impurities. Thus far, numerous PL studies on Ag-doped (Cd0.95Zn0.5)S phosphors have been performed; however, little is known about the effect of higher concentrations of silver. Room temperature PL spectra of the (Cd0.95Zn0.5)S phosphors with different Ag ion concentrations (1–5 mol.%) are shown in a. The measurements were performed at an excitation wavelength of 345 nm. The PL spectra show broad peaks, which imply the superposition of multiple emission bands [Citation2]. These transitions belong to the defects produced by the silver ions. A small emission peak at 525 nm can be attributed to the transition from the shallow trap level, while the peak at 549 nm can be assigned to the radiative transition from a deep trap state due to the vacancy in the synthesized lattice. This was confirmed by Sethi et al. The peak intensities corresponding to 525 and 549 nm increased with increasing silver concentration [Citation2,Citation19] (b). Thus, the PL peak suggests that the sulphur vacancies are occupied by the Ag+ ion in the lattice host material of the (Cd0.95Zn0.5)S phosphors, which reduces the participation of the sulphur content in excitation and chemical reactions [Citation20].
4 Conclusion
The pure and silver-doped (Cd0.95Zn0.5)S phosphors were prepared using solid-state reaction methods. Formation of the phosphors was confirmed by EDX analysis. Other structural behaviour was defined by XRD and FESEM analysis. The structural and optical properties were investigated as a function of Ag+ ion concentration. Thus, crystal size increases with increasing Ag+ ion concentration. The average crystal size was in the 141–162 nm range. In the PL emission spectra, the emission peak was located in the green region at 549 nm. PL intensity also increases with increasing Ag+ ion concentration.
Notes
Peer review under responsibility of Taibah University.
References
- T.Prem KumarK.SankaranarayananTunability of structural, surface texture, compositional and optical properties of cdzns thin films by photo assistedchemical bath deposition techniqueChalcogenide Lett.6112009617622
- R.SethiL.KumarK.S.PrashantA.C.PandeyTunable visible emission of Ag-doped CdZnS alloyQuantum Nanoscale Res. Lett.5201096102
- R.K.TamrakarD.P.BisenOptical and kinetic studies of CdS:Cu nanoparticlesRes. Chem. Intermed.39201330433048
- R.K.TamrakarStudies on Absorption Spectra of Mn Doped CdS Nanoparticles2012LAP Lambert Academic PublishingVerlag ISBN 978-3-659-26222-7
- J.ZhangX.-W.DiZ.-I.LiuG.XuS.-M.XuX.ZhouMulticolored luminescent CdS nanocrystalsTrans. Nonferrous Met. Soc. Chin.1720071367
- K.SenthilD.MangalarjS.K.NarayandassStructural and optical properties of CdS thin filmAppl. Surf. Sci.169–1702001476479
- J.KaurN.S.SuryanarayanaV.DubeyEffect of temperature on the ML of Au doped (Zn, Cd) S mixed phosphorsChin. Chem. Lett.222011709712
- S.K.KulkarniaU.WinklerN.DeshmukhP.H.BorseR.FinkE.UmbachInvestigations on chemically capped CdS, ZnS and ZnCdS nanoparticlesAppl. Surf. Sci.4382001169170
- R.TiwariR.K.TamrakarN.K.SwamyV.DubeyMechanoluminescence properties of (Cd0.95Zn0.5)S mixed nanoparticles doped with silverUltra Sci.261B201416
- K.K.NandaS.N.SarangiS.MohantyS.N.SahuOptical properties of CdS nanocrystalline films prepared by a precipitation techniqueThin Solid Films32219982127
- R.TiwariP.B.TaunkR.K.TamrakarN.K.SwamdV.DubeySynthesis, characterization and thermoluminescence behavior of (Cd, Zn)S mixed phosphor doped with silverChalcogenide Lett.1132014P141P158
- R.S.ManeM.-Y.YoonH.ChungS.-H.HanCo-deposition of TiO2/CdS films electrode for photo-electrochemical cellsSol. Energy812007290
- S.K.SaoN.BrahmeD.P.BisenR.SharmaG.TiwariS.TiggaP.ChandrakarR.TamrakarThermoluminescence and mechanoluminescence studies of (Cd0.95Zn0.05)S:Ag doped phosphorRecent Res. Sci. Technol.482012117118 ISSN: 2076-5061
- R.K.TamrakarD.P.BisenC.S.RobinsonI.P.SahuN.BrahmeYtterbium doped gadolinium oxide (Gd2O3:Yb3+) phosphor: topology morphology, and luminescence behaviourInd. J. Mater. Sci.72014 (Article ID 396147)
- P.KlugL.E.AlexanderX-ray Diffraction Procedure1954WileyNew York
- W.WangF.HuangY.XiaA.WangPhotophysical and photoluminescence properties of co-activated ZnS:Cu:Mn phosphorsJ. Lumin.1282008610
- A.MurugadossA.ChattopadhayTuning photoluminescence of ZnS nanoparticles by silverBull. Mater. Sci.312008533