?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Seaweeds are potential renewable resources in the marine environment. The antibacterial activity of Jania rubens, Corallina mediterranea and Pterocladia capillacea were analyzed against human pathogenic bacteria. The present study was performed to investigate the phytochemical constituents of seaweeds, such as alkaloids, flavonoids, steroids, terpenoids and phlobatannins. In this study, we estimated phenols, flavonoids, tannins, pigments and mineral contents and determined the hydrogen peroxide scavenging activity, reducing power and total antioxidant activity of various extracts of selected seaweeds. Phytochemicals were extracted from the three seaweeds using various solvents, such as methanol, ethanol, acetone and chloroform. Among the various extracts, the methanolic extract was found to have the highest reducing power and total antioxidant capacity. We evaluated the seaweeds against Vibrio fluvialis, and Pterocladia capillacea was the most effective at controlling its growth. The highest zone of inhibition was recorded in the methanol extract. The chemical constituents of the seaweeds were characterized by GC–MS, which showed that they contain organic compounds, such as 1,2-benzenedicarboxylic acid.
1 Introduction
Since ancient times, macroscopic marine algae has been closely associated with human life and has been exhaustively used in numerous ways as a source of food, feed, fertilizer and medicine, and chiefly used for economically important phycocolloids [Citation1,Citation2]. Marine algae contain more than 60 trace elements in a concentration much higher than in terrestrial plants. They also contain protein, iodine, bromine, vitamins and substances of stimulatory and antibiotic nature. The phytochemicals from marine algae are extensively used in various industries such as food, confectionary, textile, pharmaceutical, dairy and paper, mostly as gelling, stabilizing and thickening agents. Seaweeds or marine macro algae are renewable living resources that are also used as food, feed and fertilizer in many parts of the world.
In addition to vitamins and minerals, seaweeds are also potentially good sources of proteins, polysaccharides and fibres [Citation3,Citation4]. Recently, Hebsibah and Dhana Rajan [Citation5] studied variations in the chemical constituents of the marine red alga Hypnea valentiae from the Tuticorin and Mandapam Coasts. Dinesh et al. [Citation6] studied the nutritive properties of 20 species of seaweeds from the Gulf of Mannar. Seenivasan et al. [Citation7] screened the antibacterial activity of extracts of marine algae representing Chlorophyta and Rhodophyta collected from the Vishakapatnaam Coast against two pathogens and also tested their ability to inactivate the enzyme penicillinase in vitro. Extracts of marine algae were reported to exhibit antibacterial activity [Citation8,Citation9]. Vanitha et al. [Citation10] reported the antibacterial action of nine seaweeds collected from the Kanyakumari Coast against human upper respiratory tract pathogens, which include both gram-positive and gram-negative bacteria.
Kandhasamy and Arunachalam [Citation11] determined the in vitro antibacterial properties of the seaweeds Caulerpa racemosa, Ulva lactuca, Gracilaria foliifera, Hypnea musciformis, Sargassum tenerrimum, S. myriocystem and Padina tetrastromatica collected from Koodankullam, and Tirunelveli against gram-negative and gram-positive pathogenic bacteria. Anitha et al. [Citation12] determined the antibacterial activity of methanol, diethyl ether, acetone and dichloromethane extracts of Padina Boergesenii collected against 10 human pathogenic bacteria. Marine resources are an unmatched reservoir of biologically active natural products, many of which exhibit structural features that have not been found in terrestrial organisms [Citation13]. There are numerous reports on compounds derived from macro algae with broad ranges of biological activities, such as the antimicrobial, antiviral, anti-tumour, anti-inflammatory, and neurotoxic [Citation14]. The present study was performed with three marine seaweeds: Jania rubens, Corallina mediterranea and Pterocladia capillacea red algae. The study was performed with the following objectives: (1) To investigate the preliminary phytochemical constituents present in the three seaweeds. (2) To estimate the biochemical composition and photosynthetic pigments of the selected seaweeds. (3) To analyze the mineral composition of the three seaweeds. (4) To evaluate the antibacterial activity of the three seaweeds. (5) To reveal the chemical constituents in the three seaweeds using GC–MS analysis.
2 Materials and methods
2.1 Collection and identification of seaweeds
The studied algal species were collected from the coastal area of Abu-Qir Alexandria – North Egypt. Algal samples were cleaned of epiphytes, and necrotic parts were removed. Then, cleaned samples were rinsed with sterile water to remove any associated debris. The cleaned fresh materials were shade air-dried and ground into fine powder, as described by Gonzalez del Val et al. [Citation15]. The samples were identified as, Jania rubens (Linnaeus), Corallina mediterranea (J. Agardh) and Pterocladia capillacea (Gmelin).
2.2 Preparation of seaweed extracts
Ten grams of powdered samples were extracted with 50 ml of solvents, such as methanol, ethanol, acetone and chloroform. The samples were kept in the dark for 72 h with intermittent shaking. After incubation, the solution was filtered through filter paper, and the filtrate was collected (crude extracts) and stored in the refrigerator until further use.
2.3 Gas chromatography and mass spectrometry analysis
Gas chromatography–mass spectrometry (GC–MS) analysis was performed using an Agilent GC-MC-5975C with a Triple–Axis Detector equipped with an auto sampler. The GC column used was fused with silica capillary column (length 30 m × diameter 0.25 mm × film thickness 0.25 μm) with helium at 1.51 ml for 1 min as a carrier gas. The mass spectrometer was operated in the electron impact (El) mode at 70 eV in the scan range of 40–700 m/z. The split ratio was adjusted to 1:10, and the injected volume was 1 μl. The injector temperature was 250 °C, and the oven temperature was kept at 70 °C for 3 min, rose to 250 °C at 14 °C min−1 (total run time 41 min). Peak identification of crude seaweed extracts were performed by comparison with retention times of standards, and the mass spectra obtained were compared with those available in NIST libraries (NIST 11 – Mass Spectral Library, 2011 version) with an acceptance criterion of a match above a critical factor of 80% according to Musharraf et al. [Citation16].
2.4 Estimation of flavonoid content
Total flavonoid content was determined according to the method of Chang et al. [Citation17]. A one-ml aliquot of each extract was mixed with 0.1 ml of 10% aluminium chloride and 0.1 ml of 1 M potassium acetate. Methanol (2.8 ml) was added and kept at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm. The flavonoid content was expressed in mg/g, and Quercetin was used as a standard compound.
2.5 Estimation of tannin content
Total tannin content was determined according to the method of Julkunen-Titto [Citation18]. Briefly, 50 μl of seaweed extract was mixed with 1.5 ml of 40% vanillin (prepared with methanol), and then 750 μl of HCl was added. The solution was shaken vigorously and left to stand at room temperature for 20 min in darkness. Absorbance against a blank was read at 500 nm. Catechin was used as standard.
2.6 Estimation of phenol content
The total phenol content was measured using the Folin–Ciocalteu method of Taga et al. [Citation19]. Extract (100 μl) was mixed with 2 ml of 2% Na2CO3 and allowed to stand for 2 min at room temperature. Then, 100 μl of 50% Folin–Ciocalteu phenol reagent was added. After incubation for 30 min at room temperature in darkness, the absorbance was read at 720 nm. The total phenol content of samples was expressed as mg gallic acid per gram.
2.7 Estimation of chlorophyll content
The amount of chlorophyll present in the algae was estimated by the method of Arnon [Citation20]. Fresh algal tissue (500 mg) was kept in a pestle and mortar with 10 ml of acetone and was ground well. The homogenate was then centrifuged at 3000 rpm for 15 min. Absorbance was measured at 645 nm and 663 nm. The chlorophyll content was determined using the following formulas:where A = absorbance at respective wavelength, V = volume of extract (ml), and W = fresh weight of sample.
2.8 Estimation of carotenoid content
The amount of carotenoid was estimated by the method of Kirk and Allen [Citation21]. The same chlorophyll extract was measured at 480 nm using a spectrophotometer to estimate the carotenoid content.
Carotenoid (μg/g fr. wt.) = A480 + (0.114 × A663) − (0.638 × A645)
2.9 Hydrogen peroxide scavenging assay
The free radical scavenging activity of the extracts were determined using a hydrogen peroxide assay as described by Gulcin et al. [Citation22]. A 10 mM hydrogen peroxide solution was prepared in phosphate buffer (0.1 M, pH 7.4). One ml of the extract was rapidly mixed with 2 ml of H2O2. The absorbance was measured at 230 nm after 10 min of incubation at 37 °C against a blank without H2O2. The percentage of scavenging of H2O2 was calculated using the formula:
% of scavenging = ((A0 − Ai) ÷ A0) × 100
2.10 Determination of total antioxidant capacity (TAC)
The total antioxidant activity of seaweed extracts was determined according to the method of Mitsuda et al. [Citation23]. Sulphuric acid (7.45 ml, 0.6 M), 0.99 g of sodium sulfate (28 mM) and 1.23 ml of ammonium molybdate (4 mM) were mixed together in 250 ml with distilled water and labelled as total antioxidant capacity (TAC). Seaweed extract (0.1 ml) was dissolved in 1 ml of TAC, and the absorbance was read at 695 nm after 15 min. Ascorbic acid was used as a standard.
2.11 Estimation of reducing power
The reducing power of the extract was determined by following the method of Yamaguchi et al. [Citation24]. Extract (0.75 ml) was mixed with 0.7 5 ml phosphate buffer (pH 6.6), and 0.75 ml of 1% potassium ferricyanide was added. The mixture was incubated at 50 °C for 20 min. Trichloroacetic (0.75 ml, 10%) acid was added and centrifuged at 3000 g for 10 min. The supernatant (1.5 ml) was mixed with 1.5 ml of water and 0.1% ferric chloride. The absorbance was read at 700 nm after 10 min of incubation.
2.12 Preliminary phytochemical tests
Phytochemical analysis was performed to determine the presence of different phytochemicals as described by Sadasivam and Manickam [Citation25].
2.13 Estimation of minerals
Oven dried samples was accurately weighed in 0.2 g quantities in a dry conical flask, and 10 ml of diacid mixture (2:5 of nitric and perchloric acid) were added. The contents of the conical flask were allowed to stand for a few hours for cold digestion. The mixture was then kept on a hot plate, and the contents were digested by increasing the temperature. The digestion was continued until the content became colourless. The digestion material was filtered through Whatman NO. 40 filter paper, and the filtrate collected was diluted to a suitable volume and fed into an ICP – Perkin Elmer Mayer Optical Emission Spectrophotometer (AOAC [Citation26]).
2.14 Effect of algal plant extracts on Vibro fluvialis
2.14.1 Determination of anti-bacterial effect
The microorganism for the antimicrobial activity assay used was Vibrio fluvialis obtained from National Institute of Oceanography and fisheries, Alexandria.
These bacteria were cultured in an LB broth at 37 °C and maintained on LB agar Luria Bertani in g/l: Tryptone, 5; Yeast extract, 5; NaCl, 10; and sterilized by autoclaving for 25 min at 121 °C. The pH was adjusted to 7 with 1 N NaOH or 1 N HCl prior to sterilization. Next, sterilization media were supplemented with 175 mg/ml mycostatin to inhibit fungal growth. All cultures were supplemented with 15% glycerol (w/v) and stored frozen at 4° C. The assay was performed as previously described by Senthil Kumar and Kamaraj [Citation27]. Each pathogen was grown on its isolation medium and incubated at 30 °C until visible growth was observed. Bacterial suspensions of each indicator pathogen were then plated with LB media on the agar plates. A well was cut in the middle of the plate, and 0.05 ml of extract was added to it. The plate was incubated at 30 °C for 24 h. Inhibition zones were scored as antibacterial activity measured in cm.
3 Results
3.1 Phytochemical screening
Important phytochemicals, such as alkaloids, triterpenoids, steroids, tannin, saponin, coumarins, terpenoids, quinine, phytosteroids, phlobatannins and flavonoids were screened for their presence and presented in .
Table 1 Preliminary phytochemical screening of red seaweed extracts.
3.2 Biochemical analysis
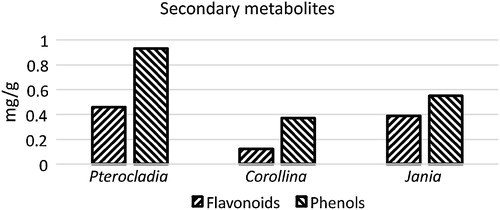
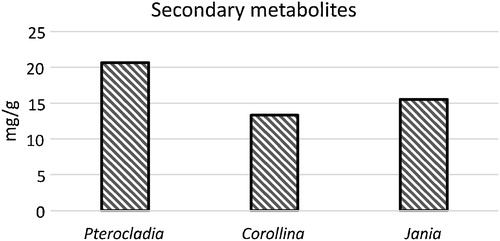
Total phenol, flavonoid and tannin content of selected species of seaweeds are presented in and . The highest total phenol, flavonoid and tannin content were 0.93, 0.46 and 20.68 mg/g, respectively, as recorded in P. capillacea; and the lowest contents were 0.37, 0.12 and 13.33 mg/g, respectively, as recorded in C. mediterranea.
3.3 Reducing power assay
Antioxidant activity in this assay has been reported to have a direct and positive correlation with reducing power, which was evaluated in the samples on the basis of their abilities to reduce Fe+3 complex to Fe+2. Greater absorbance values led to more superior reducing power of the extract. The highest amount of reducing power was observed in methanol extracts of three tested algae, followed by ethanol. The lowest amount of reducing power was recorded in chloroform extracts. In this assay, C. mediterranea > P. capillacea > J. rubens: 4.566 > 4.007 > 3.950, respectively at the same concentration, as shown in .
Table 2 Reducing power assay, hydrogen peroxide scavenging assay, and total antioxidant capacity of selected tested seaweeds.
3.4 Hydrogen peroxide scavenging assay
Antioxidant activity of the methanol extracts of J. rubens, C. mediterranea and P. capillacea were determined based on the percentage of free radical scavenging activity. In all extracts, higher scavenging activity was observed in methanol: P. capillacea > C. mediterranea > J. rubens: 39.634 > 38.632 > 38.382, respectively, followed by acetone Extract: P. capillacea > J. rubens > C. mediterranea: 37.102 > 37.010 > 36.221, respectively. The lowest activity was determined in ethanol extracts: P. capillacea > C. mediterranea > J. rubens: 15.939 > 15.136 > 10.538, respectively. Among the four different extracts, the methanol extract showed the greatest ability to decrease the pro-oxidant H2O2 as shown in .
3.5 Total antioxidant capacity (TAC)
The total antioxidant activity of acetone and chloroform extracts of the tested algae are nearly the same, while the total antioxidant activity of ethanol was recorded as the following: P. capillacea > J. rubens > C. mediterranea: 0.940 > 0.696 > 0.409, respectively, at the same concentrations. The minimal TAC was observed in J. rubens and is equal to 0.327 mg ascorbic acid equivalent in methanol extract, as shown in .
3.6 Photosynthetic pigments
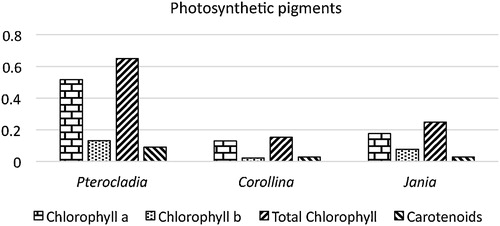
The photosynthetic pigments chlorophyll “a”, chlorophyll “b”, total chlorophyll and carotenoid content were estimated and presented in . The maximum chlorophyll “a” was 0.51, chlorophyll “b” was 0.132, total chlorophyll was 0.649 and carotenoid was 0.092 mg/g, and this was recorded in P. capillacea.
3.7 Mineral composition of seaweeds
The variation observed in the different seaweeds studied in the present study was presented in . Among the 13 minerals analyzed, the concentrations are Fe (0.475 mg/g), Mn (0.095 mg/g) were higher in J. rubens. Ca (450.752 mg/g), Mg (49.774 mg/g), Pb (0.0157 mg/g), Cu (0.0069 mg/g), Cd (0.0008 mg/g), Cr (0.0001 mg/g) were found to be highest in C. mediterranea. Na (29.495 mg/g), K (14.950 mg/g), Ni (0.0162 mg/g), Zn (0.0421 mg/g) and Co (0.0043 mg/g) were found to be highest in P. capillacea.
Table 3 Mineral composition of selected seaweeds (mg/g).
3.8 Antibacterial activity
The antibacterial activity of J. rubens, C. mediterranea and P. capillacea extracts on Vibrio fluvialis are presented in . The agar well diffusion method was used to evaluate the antibacterial activity by measuring the zone of inhibition against V. fluvialis. Among the three seaweeds screened for their antibacterial activity in the present investigation, P. capillacea was found to be superior over C. mediterranea and J rubens at controlling the growth of V. fluvialis. Among the three solvents tested, methanol, ethanol and acetone extracts of seaweeds exhibited the best activity.
Table 4 Antibacterial activity of three seaweed species.
3.9 GC–MS of collected marine algae
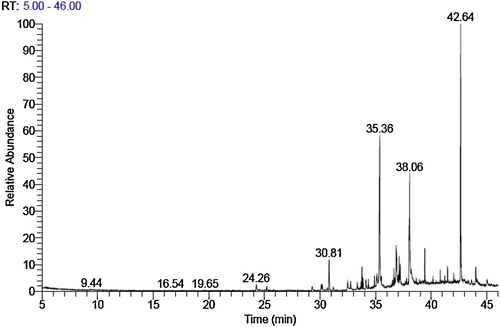
GC–MS analysis of crude ethyl acetate extract of tested algae showed a mixture of volatile compounds. A total of 55, 57 and 59 peaks were observed with retention times for J. rubens, C. mediterranea and P. capillacea, respectively, as shown in –. Chemical constituents were identified using spectral database NIST 11 software installed in the GC–MS.
3.10 GC–MS of J. rubens
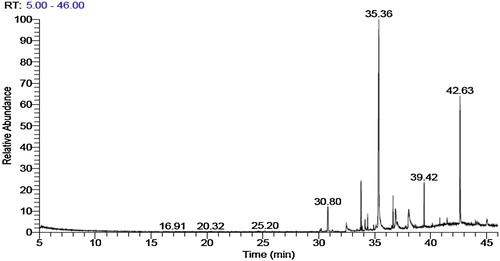
The GC–MS analysis of the crude extract revealed that the main chemical constituent was the organic compound 1-(+)-ascorbic acid 2,6-dihexadecanoate (retention time (RT) = 35.36 min, 35.48%) followed by icosapent (RT = 38.04 min, 6.91%), trans-13 octadecenoic acid (RT 36.85 min, 5.04%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (RT 33.77 min, 4.66%), heptadecane (RT = 30.80, 4.01%) and 1,2-benzenedicarboxylic acid (RT = 39.42 min) (3.10%) shown in .
Table 5 Chemical constituents of Jania rubens.
3.11 GC–MS of C. mediterranea
The data recorded in demonstrated that the most abundance six chemical constituents of C. mediterranea were cholesterol (RT = 42.64 min, 20.53%) followed by n-hexadecanoic acid (RT = 35.36 min, 17.65%), icosapent (RT = 38.05 min 13.56%), oleic acid (RT = 36.85 min, 6.69%), heptadecane (RT = 30.81 mn, 3.13%) and 1,2 benzenedicarboxylic acid (RT = 39.42 min, 2.01%).
Table 6 Chemical constituents of Corallina mediterranea.
3.12 GC–MS of P. capillacea
The main chemical constituents of P. capillacea are shown in : n-hexadecanoic acid is most abundant (RT = 35.41 min, 33.57%) followed by cholesterol (RT = 42.64 min, 11.03%), heneicosane (RT = 30.82 min, 8.50%), cis-5,8,11,14,17-eicosapentaenoic acid, methyl ester (RT = 38.05 min, 7.80), trans-13-octadecenoic acid (RT = 36.87 min, 6.73%), tetradecanoic acid (RT = 32.54 min, 3.79%) and 1,2-benzenedicarboxylic acid (RT = 39.42 min, 3.03%). It is possible that a bioactive compound primarily consisting of 1,2-benzenedicarboxylic acid may be involved in biological activity of other compounds.
Table 7 Chemical constituents of Pterochadia capillacea.
4 Discussion
Seaweeds are primitive non-flowering plants without roots, stems and leaves. They contain different vitamins, minerals, trace elements, proteins and bioactive substances [Citation9]. Many polysaccharides are recovered from seaweeds, with the most important of them being agar, alginic acid, laminarine, fucoidin, galactans, carrageenan, xylan and mannans [Citation13]. In the present study, phytochemical screening of the seaweeds showed the presence of alkaloids, flavonoids, triterpenoids, steroids, tannins, coumarins, terpenoids, quinine, phytosteroids and phlobatannins in all samples tested. Saponin was absent in the three samples.
Phenolic compounds are commonly found in plants, including seaweeds, and have been reported to show a wide range of biological activities including antioxidant properties [Citation28,Citation29]. Reports have revealed that phenolic compounds are one of the most effective antioxidants in red algae. Viswanathan et al. [Citation30], reported crude methanolic extracts of red seaweeds to yield results in the range of 1.5–4.1 mg GAE/g, which is higher in phenolic content than the red species studied in this work. The present study was promising, as algae polyphenolic compounds are effective antioxidants in delaying oil rancidity, and therefore, the seaweed extracts could have a potential effect in food application. Total flavonoids in the seaweeds ranged from 0.123 to 0.460 mg/g. Kahkonen et al. [Citation31] stated that flavonoids are probably the most important natural phenol due to their broad spectrum of chemical and biological activities, including antioxidant and free radical scavenging properties. Flavonoids have been reported as antioxidants of a wide range of reactive oxygen species and inhibitors of lipid peroxidation and as potential therapeutic agents against a wide variety of diseases. It has been reported that the presence of phytoconstituents such as flavonoids, tannins and polyphenols prevent a number of diseases through their free radical scavenging activity [Citation32], and these phenolic compounds, which include phenol, tannin and flavonoids, have been found in appreciable amounts in the three seaweeds.
The highest total amounts of chlorophyll and carotenoids were recorded in red alga P. capillacea and the lowest in red alga C. mediterranea.
Among the 13 minerals analyzed, high concentrations of Na, K, Ni, Zn and Co were found in P. capillacea, while Ca, Mg, Pb, Cu, Cd and Cr were found in C. mediterranea, and Fe and Mn were found in J. rubens. The mineral fractions of some seaweed even accounted for 40% of dry matter; however, in some cases, the mineral content of seaweeds is even higher than that of land plants and animal products [Citation33]. Seaweeds are known as excellent sources of minerals, especially sodium and iodine, due to their high polysaccharide content, which could also imply a high level of soluble and insoluble dietary fibre [Citation3,Citation34].
Antioxidant activity of marine algae may arise from pigments such as chlorophylls, carotenoids, vitamins and vitamin precursors, including cophenol, carotene, niacin, thiamine, ascorbic acid and phenolic compounds, such as polyphenols, hydroquinones and flavonoids. Phospholipids, particularly phosphatidylcholine, terpenoids, peptides, and other antioxidative substances, directly or indirectly contributed to the inhibition or suppression of oxidation processes [Citation35,Citation36]. The dried methanolic extracts of the three red algae species of seaweeds were found to have good antioxidant and antimicrobial activities. Similar results were previously obtained [Citation30]. Although a variety of solvents have been employed in screening algae for antioxidant and antimicrobial activity, it is still unclear what type of solvent is the most effective and suitable for extraction of seaweeds [Citation37,Citation38].
As discussed, methanol extracts of red algae had higher total phenolic content, H2O2 scavenging and total antioxidant capacity as compared to ethanol, acetone and chloroform extracts. The reducing power indicates that the antioxidant compounds are electron donors and can reduce the oxidized intermediates of the lipid peroxidation process, so they can act as primary and secondary antioxidants [Citation39,Citation40]. Overall, methanol was the most effective solvent for extraction of antioxidant properties from seaweeds, which may be due to methanol having a higher dielectric constant than ethanol, acetone and chloroform. We found that for the red seaweeds, methanol extracts of J. rubens, C. mediterranea and P. capillacea were more reactive than the other extracts.
Emergence of microbial disease in aquaculture industries implies serious losses. The use of commercial antibiotics for treatment of fish disease produces undesirable side effects. Marine organisms are a rich source of structurally novel biologically active metabolites [Citation41]. Therefore, cell extracts and active constituents of various algae may be potential bioactive compounds of interest in the pharmaceutical industry [Citation42].
Antimicrobial activity depends on both algal species and the solvents used for their extraction [Citation43]. The antimicrobial activity of algae extracts is generally assayed using various organic solvents, such as acetone, ether, chloroform, methanol [Citation44]. An organic solvent always provides a higher efficiency in extracting compounds for antimicrobial activity [Citation45,Citation46].
Previous studies of some marine algae belonging to selected species of marine benthic algae (Phaeophyceae and Rhodophyceae) collected from different coastal areas of Alexandria (Egypt) were investigated for their antibacterial and antifungal activities against fish pathogens. In vitro screening of organic solvent extracts from several marine macro algae, including Pterocladia capillacea (Gmelin), showed specific activity in inhibiting the growth of five virulent strains of bacteria pathogenic to fish species such as Vibrio anguillarum, V. tandara. Pseudomonas fluorescens and Aeromonas hydrophila [Citation47,Citation48].
Furthermore, extract of the red algae Pterocladia capillacea in saline and aqueous ethanol markedly inhibited the growth rate of V. vulnificus at very low concentrations [Citation49].
The methanol, dichloromethane, hexane, chloroform and volatile oil extracts of the red alga Jania rubens were tested in vitro for their antimicrobial activity against Gram-positive bacteria, Gram-negative bacteria and Candida albicans (ATCC 10239). The methanol and chloroform extracts showed more potent antimicrobial activity than the hexane, dichloromethane, and volatile oil extracts of J. rubens [Citation50].
The GC–MS analysis of crude extracts of J. rubens, C. mediterranea and P. capillacea revealed many components, with the main chemical constituents observed in high percentages being 1-(+)-ascorbic acid 2,6-dihexadecanoate, icosapent, trans-13-octadecenoic acid, 3,7,11,15-tetramethyl-2-hexadecen-1-ol heptadecane and 1,2-benzenedicarboxylic acid in J. rubens; cholesterol, n-hexadecanoic acid, icosapent, oleic acid, heptadecane and 1,2-benzenedicarboxylic acid in C. mediterranea; and n-hexadecanoic acid, cholesterol, heneicosane, tetradecanoic acid, cis-5,8,11,14,17-eicosapentaenoic acid methyl ester, trans-13-octadecenoic acid and 1,2-benzenedicarboxylic acid in P. capillacea. 1,2-Benzenedicarboxylic acid may be involved in antagonism against Vibrio fluvialis. Antibacterial activities of the algal extracts were reportedly due to the presence of lauric, palmitic (hexadecanoic acid), linolenic, linoleic, oleic, stearic (octadecanoic acid) and myristic acids (tetradecanoic acid) [Citation51]. Octadecanoic acid from neem extract was tested on three bacterial strains (S. aureus, E. coli and Salmonella sp.) and showed better inhibition activity against S. aureus than E. coli and Salmonella sp. [Citation52]. The present results were also in agreement with those in other previous reports [Citation53,Citation54]. Marine algae are an impending source of an extensive range of polyunsaturated fatty acids, carotenoids, phycobiliproteins, polysaccharides and phycotoxins [Citation55]. It was reported that lipids obstruct microbes by distracting the cellular membrane [Citation56] of bacteria, fungi and yeasts. These fatty acids may further distress the expression of bacterial virulence, which is significant for establishing infection. It has been demonstrated that fatty acids with a chain length greater than 10 carbon atoms induces lysis of bacterial protoplasts. Due to the harsh environments in which many macro algae exist, effective defence mechanisms have been established and consequently, a diverse source of bioactive compounds, including polysaccharides, polyphenols, fatty acids and peptides, with dissimilar structure and activities than those found in terrestrial plants have been discovered [Citation57]. Many species of macro algae possessed main constituents such as tetradecnoic acid, hexadecanoic acid, octadecanoic acid methyl ester [Citation58], which may reveal antagonism against bacteria [Citation59]. Lately, secondary metabolites and organic extracts obtained from U. fasciata have potential applications [Citation60], and the diverse derivatives of diterpenoids extracted from U. fasciata exhibited antagonism against Vibro parahaemolyticus and Vibro harveyi [Citation61]. Thus, in the present study, biological activity of J. rubens, C. mediterranea and P. capillacea against V. fluvialis was found to be due to the presence of various chemical constituents as described.
5 Conclusion
It can be concluded that marine macro algae are a rich source of structurally novel and biologically active metabolites. Secondary or primary metabolites produced by these macro algae may be potential bioactive compounds of interest in the pharmaceutical industry and medicinal compounds. The present investigation presents adequate data on the phytochemical constituents of biochemical composition, mineral composition, photosynthetic pigments and antibacterial potential of the three seaweed extracts for the synthesis of novel antibiotics. Bioactive compounds found in seaweeds await a major breakthrough for their potential application as natural antioxidants in different food and pharmaceutical products.
The present study suggests that these seaweed extracts possess antibacterial activity against bacterial pathogens, thus supporting their folkloric usage, promising a future scope for the use of these marine seaweeds against microbial populations. The characterization of the active compounds from seaweed extracts revealed the presence of octadecanoic acid, hexadecanoic acid, tetradecanoic acid and 1,2-benzenedicarboxylic acid.
Notes
Peer review under responsibility of Taibah University.
References
- T.LeveringH.A.HoppeO.J.SchimidMarine Algae. A Survey of Research and Utilization1969Granm be Gruyter Hamburg1420
- V.J.ChapmanSeaweeds and Their Usessecond ed.1970The Camelot Press Ltd.London and Southampton6385
- M.LahayeMarine algae as a source of dietary fibers: determination of soluble and insoluble dietary fiber contents in some sea vegetableJ. Sci. Food Agric.541991587594
- B.Darcy-VrillonNutritional aspects of the developing use of marine macroalgae for human food industryJ. Food Sci.441993523535
- B.Hebsibah ElsieM.S.Dhana RajanEvaluation of antibacterial activity and phytochemical screening of Gelidium acerosaJ. Pharm. Sci.22010704707
- G.DineshM.SekarR.KannanNutritive properties of seaweeds of Gulf of Mannar, Tami NaduSeaweed Res. Utiln.292007125132
- R.SeenivasanM.RekhaS.GeethaAntibacterial activity and phytochemical analysis of selected seaweeds from Mandapam coastJ. Appl. Pharm. Sci.22012159169
- I.MahasnehM.JamalM.ZibdehAntibiotic activity of marine algae against multiantibiotic resistant bacteriaMicrobios8319952326
- R.DharmeshK.StalinM.RamavatarA.K.SiddhantaAntioxidant activity and phytochemical analysis of a few Indian seaweed speciesIndian J. Geo-Mar. Sci.432014507518
- J.VanithaS.PrakashS.BhimbaB.V.LazarusAntibacterial action of seaweeds against human upper respiratory tract pathogensSeaweed Res. Utiln.252003181187
- M.KandhasamyK.D.ArunachalamEvaluation of in vitro antibacterial property of seaweeds of Southeast coast of IndiaAfr. J. Biotechnol.7200819581961
- A.AnithaR.BalamuruganN.S.SwarnakumarK.SivakumarEvaluation of seaweeds for biochemical composition and calorific contentSeaweed Res. Utiln.302008197202
- K.SarithaA.E.ManiM.PriyalaxmiJ.PattersonAntibacterial activity and biochemical constituents of seaweed U. lactucaGlob. J. Pharmacol.72013276282
- M.E.H.OsmanA.M.AboshadyM.E.ElshobaryProduction and characterization of antimicrobial active substance from some macroalgae collected from Abu-Qir (Alexandria) EgyptAfr. J. Biotechnol.12201368476858
- A.Gonzalez del ValG.PlatasA.BasilioScreening of antimicrobial activities in red, green and brown microalgae from Gran CanariaInt. Microbiol.420013540
- S.MusharrafM.A.AhmedN.ZehraM.ChoudharyA.U.RahmanBiodiesel production from microalgal isolates of Southern Pakistan and quantification of FAMEs by GC–MS analysisChem. Cent. J.62012110
- C.ChangM.YangH.WenJ.ChernEstimation of total flavonoid content in propolis by two complementary colorimetric methodsJ. Food Drug Anal.102002178182
- R.Julkunen-TittoPhenol constituents in the leaves of northern Willows methods for the analysis of certain phenolicsJ. Agric. Food Chem.331985213217
- M.TagaE.E.MillerD.E.PrattChia seeds as a source of natural lipids antioxidantsJ. Am. Oil Chem. Soc.611984928993
- D.I.ArnonCopper enzymes in isolated chloroplast, polyphenol oxidase in Beta vulgarisPlant Physiol.21949115
- Jto.KirkR.L.AllenDependence of chloroplast pigments synthesis on protein effects on actilioneBiochem. Biphys. Res. Commun.271965523530
- T.GulcinK.IrfanO.M.KufreviogluAntioxidant, antimicrobial, antiulcer and analgesic activities of nettleJ. Ethnopharmacol.902004205215
- H.MitsudaK.YuasumotoS.LuAntioxidation action of indole compounds during the autoxidation of linoleic acidEiyo to Shokuryo191996210214
- T.YamaguchiH.TakamuraT.Matobaet al.HPLC method for evaluation of free radical scavenging activity of foods by using 1,1-diphenyl-2-picryl hydrozlBiosci. Biotechnol.62199812011204
- S.SadasivamA.ManickamBiochemical Methods for Agricultural Sciences1996New Age International Ltd.New Delhi, India197
- AOACOfficial Methods of Analysis of AOAC International16th ed.1995OAC Int.Washington
- K.S.SenthilM.KamarajAntimicrobial activity of Cucumis anguria L. by agar well diffusion methodBot. Res. Int.420114142
- T.KudaT.KuniiH.GotoT.SuzukT.YanoVarieties of antioxidant and antibacterial properties of Ecklonia products harvested and processed in the Noto peninsula, JapanJ. Food Chem.1032007900905
- Y.AthukoralaN.KimY.J.JeonAntiproliferative and antioxidant properties of an enzymatic hydrolysate from brown algaeFood Chem. Toxicol.44200610651074
- S.ViswanathanC.EbcibaR.SanthiyaS.NallamuthuPhytochemical screening and In vitro antibacterial, antioxidant and anticancer activity of Amphiro FragilissimaInt. J. Innov. Res. Sci.: Eng. Technol.3201423198753
- M.KahkonenP.HopiaHj.VuorelaH.RauhaJ.P.KujalaAntioxidant activity of plant extract containing phenolic compoundsJ. Agric. Food Chem.471999349362
- X.J.DuanW.W.ZhangW.X.M.LiB.G.WangEvaluation of antioxidant property of extract and fraction obtained from red alga Polysiphonie urcelataFood Chem.95200610221037
- K.ItoK.HoriSeaweed. Chemical composition and potential food usesFood Rev. Int.51989101144
- R.A.S.HematFat and muscle dysfunctionRASHematAndropathy2007UrotextDublin, Ireland8385
- L.LuS.W.LiuS.B.JiangS.G.WuTannin inhibits HIV-1 entry by targetingActa Pharmacol. Sin.2004213218
- F.ShahidiNutraceuticals and functional foods. Whole versus processed foodsTrends Food Sci. Technol.542008587594
- T.ZhengY.ChenH.LuScreening for antibacterial and antifungal activities in some marine algae from the Fujian coast of China with three solventsChin. J. Oceanol. Limnol.192001327331
- N.ManchY.MelphaJ.Edwin JamesPhytochemical investigation of the three species of Ulva from Rasthacaud coast, IndiaJ. Chem. Pharm. Res.82014321329
- Gc.YenH.Y.ChenAntioxidant activity of various tea extracts in relation to their antimutagenicityJ. Agric. Food Chem.4319952732
- G.KokilamS.VasukiBiochemical and phytochemical analysis on Ulva fasciata and Caulerpa taxifoliaInt. J. Pharm. Pharm. Sci. Res.42014711
- M.A.BorowitzkaL.J.BorowitzkaVitamins and fine chemicals from microalgaeMicroalgal Biotechnology1992Cambridge University PressGreat Britain179
- E.RodriguesS.TilviC.G.NaikAntimicrobial activity of marine organisms collected off the coast of East IndiaJ. Exp. Biol. Ecol.3092004121127
- D.RadhikaC.VeerabahuR.PriyaAntibacterial activity of some selected seaweeds from the Gulf of Mannar Coast, South IndiaAsian J. Pharm. Clin. Res.520128990
- R.A.CordeiroV.M.GomesA.F.CarvalhoV.M.MeloEffect of proteins from the red seaweed Hypnea musciformis (Wulfen) lamouroux on the growth of human pathogen yeastsBraz. Arch. Biol. Technol.492006915921
- I.TuneyI.Cadirc iD.UnalA.SukatarAntimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey)Turk. J. Biol.302006171175
- M.PonnanikajamideenM.MaliniC.MalarkodiS.RajeshkumarBiochemical and phytochemical constituents of marine brown seaweed extracts from various organic solventsInt. J. Pharm. Ther.52014108112
- S.WefkyM.GhobrialStudies on the biodiversity of different solvent extracts of selected marine macroalgae against fish pathogensRes. J. Microbiol.32008673682
- K.KolanjinathanP.GaneshP.SaranrajPharmacological importance as seaweeds: a reviewWorld J. Fish Mar. Sci.62014115
- W.R.LiaoJ.Y.LInW.Y.ShiehW.L.JengR.HuangAntibiotic activity of lectins from marine algae against marine vibrioJ. Ind. Microbiol. Biotechnol.302003433439
- N.U.Karabay-YavasogluA.SukatarG.OzdemirZ.HorzumAntimicrobial activity of volatile components and various extracts of the red alga Jania rubensPhytother. Res.212007153156
- G.AgoramoorthyM.ChandrasekaranV.VenkatesaluM.J.HsuAntibacterial and antifungal activities of fatty acid methyl esters of the blind your eye mangrove from IndiaBraz. J. Microbiol.382007739742
- P.U.ZhongZ.Yu-qunY.Zhong-qiongX.JiaoJ.Ren-yongAntibacterial activity of 9-octadecanoic acid, hexadecanoic acid–tetrahydrofuran-3,4-diyl ester from Neem oilAgric. Sci. China9201012361240
- J.ZhouW.FangX.YangS.ZhouL.HuX.QiL.XieA non-luminescent and highly virulent V. harveryi strain is associated with “bacterial white tail disease” of Litopenaeus vannamei shrimpPLOS ONE2201229961
- S.K.SivakumarD.MasilamaniK.P.PrasannaEvaluation of marine macro alga, Ulva fasciata against bio-luminescent causing Vibrio harveyi during Penaeus monodon larvicultureAfr. J. Microbiol. Res.82014123132
- W.L.ChuBiotechnological applications of microalgaeIeJSME620122437
- G.BergassonH.HilmarssonH.ThormarH.ThormarAntibacterial, Antiviral and Antifungal Activities of Lipids2011John Wiley and Sons LimitedChichester, United Kingdom4780
- M.S.TierneyA.K.CroftM.HayesA review of antihypertensive and antioxidant activities in macroalgaeBot. Mar.532010387408
- M.BalamurugaG.G.SelvamT.ThinakaranK.SivakumarBiochemical study and GC–MS analysis of Hypnea musciformisAmerican-Euassian J. Sci. Res.82013117123
- S.S.Al-SaifN.Abdel-RaoufH.A.El-WaznaniI.A.ArefAntibacterial substances from marine algae isolated from Jeddah coast of Red Sea, Saudi ArabiaJ. Biol. Sci.18201323282357
- M.SilvaL.VieieraA.P.AlmeidaA.KijjoaThe marine macroalgae of the genus Ulva: chemistry, biological activities and potential applicationsOceanography12013101111
- K.ChakrabortyA.P.LiptonR.PaulrajK.VijayanAntibacterial labdane diterpenoids of Ulva fasciata from southwestern coast of IndianFood Chem.119201013991405