Abstract
Polyethylene microspheres (microparticles) were prepared using a modified chemical route. The prepared powder samples were characterized using scanning electron microscopy, Fourier transform infrared spectroscopy and differential scanning calorimetry. The scanning electron microscopy images show that the concentration of polyglycolic acid decreased the agglomeration and increased the degree of sphericity of the polyethylene microspheres. The results show that the polyethylene microparticles may be good candidates as drug carriers for pulmonary drug delivery.
1 Introduction
Various methods are available for the administration of drugs, including intravenous, intradermal, subcutaneous, oral (e.g., inhalation), transdermal (topical), transmucosal, and rectal administrations. The oral and transdermal methods are the simplest routes of drug administration. However, for the oral method, there is a risk of drug decomposition by the digestive organs, whereas when transdermal methods are used, the adsorption efficiency of a drug is generally low. The injection method results in a high adsorption efficiency, which is an early effect of the pharmacological actions of the injected drug, and there is no risk of drug decomposition by the digestive organs. However, because the method can result in pain at the injection site, it is difficult to administer injection-based drugs to patients with belonephobia. In recent years, there has been a growing interest in the pulmonary route of drug administration because pulmonary administration is simple, shows the effects of the pharmacological action of the drug early on, and has no risk of drug decomposition [Citation1–Citation3]. Pulmonary administration is a method that works via the lungs. Among the several types of drug carriers, including liposomes [Citation4,Citation5], micelles [Citation6–Citation9], and gels [Citation10–Citation12], polymeric “particles” are suitable for pulmonary drug administration [Citation13]. In this method, the drug-incorporated particle is aspirated into the lung and decomposed via transcytosis, and then the drug is absorbed into the body. The drug-absorption efficiency is high because the lungs have relatively large surface areas, thin alveolar epithelial cells, and significant blood capillaries. Some noteworthy properties are required for a drug carrier to be used for pulmonary administration. First, it is important to use biocompatible or biodegradable materials, such as polyethylene glycol (PEG) or poly (lactide-co-glycolide) (PLGA). Second, it is important to control the diameter and density of the particles because large particles (over 10 μm) can be trapped in the pharynx or the bronchi [Citation14–Citation16]. In contrast, small particles (less than 1 μm) are emitted during breathing. An appropriate diameter of particles for pulmonary drug delivery is reported to be 1–5 μm [Citation17]. For the preparation of such particles, solvent evaporation-based methods are used in which the resulting particles have a high density. Specifically, when porous particles are prepared, particles having a low density are obtained using this method [Citation18]. In contrast, particles having a low density are generally obtained with spray dry-based preparation methods. Third, it is important to modify the surface of the particles. Particles that have functional molecules (e.g., functional polymers) on the surface can bind to the tissue surface and, consequently, tend to be absorbed into the tissue. Generally, chemical conjugation of polymers to particles is expensive and time consuming because the conjugation chemistry must be optimized for each polymer-particle combination. Additionally, commercially available biocompatible and biodegradable polymers, such as PLGA and polylactide (PLA), are known to be materials in which the surface modification of the resulting particles is difficult. This is because PLGA and PLA contain almost no reactive functional groups. Thus, there is a need for microparticle preparation methods that prepare suitable carriers for pulmonary drug delivery. Bunn [Citation19] reported a novel technique to prepare nanoparticles that are modified with hydrophilic polymers on the surface of the particles via a “block copolymer-assisted” emulsification/evaporation process using the solvent evaporation method. They concluded that the spray dry-based method is suitable for the preparation of microparticles for pulmonary drug delivery.
In the present study, we discuss the preparation of polyethylene microparticles using a modified chemical route for pulmonary drug delivery. The prepared powder samples were characterized using scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR) and differential scanning calorimetry (DSC).
2 Experimental
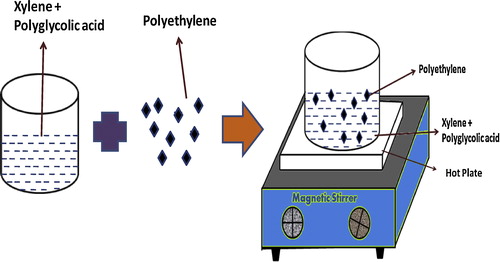
The constituents that were used for the preparation of the polyethylene microparticles are provided in . Small pieces of polyethylene were placed in a beaker containing 40 ml of xylene with a small amount of glycolic acid. Then, the solution was stirred using a magnetic stirrer at 100 °C until the powder form of PLGA was obtained. Sample powders with different concentrations of glycolic acid (0.3, 3, 5, 7 g) were prepared using this technique. After the synthesis, the powder samples were characterized at room temperature. shows the adopted synthesis method for the preparation of the polyethylene microparticles.
Table 1 Constituents used for the preparation of the polyethylene microparticles.
The morphology and spherical shape of the prepared particles were observed using scanning electron microscopy (SEM, JSM-6700F). The Fourier transform infrared spectra between 450 and 1500 cm−1 were obtained at room temperature with a Perkin Elmer Spectrum 400 FTIR spectrometer. Differential scanning calorimetry was performed using a DSC-Q20 V24.11 (heating rate: 50 °C/min; gas flow: air).
3 Results and discussion
3.1 Surface morphology of the polyethylene particles
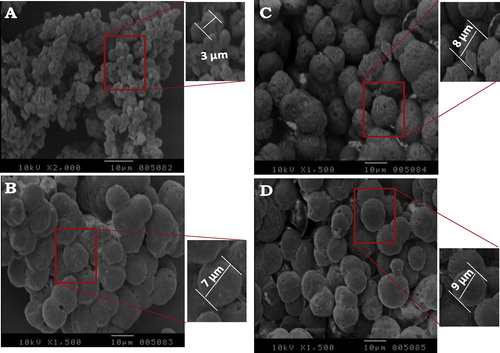
The SEM micrographs of the prepared materials at different concentrations of glycolic acid are shown in . The polyethylene microparticle sizes increased at a concentration of glycolic acid from 3 μm to 9 μm. The microparticles agglomerated when a lower concentration of acid was used. Additionally, when lower concentrations of glycolic acid were used, the polyethylene microparticles were not completely spherical. However, when 5 or 7 g were used, a high degree of sphericity and agglomeration of a few of the microparticles was observed.
3.2 FT-IR analysis
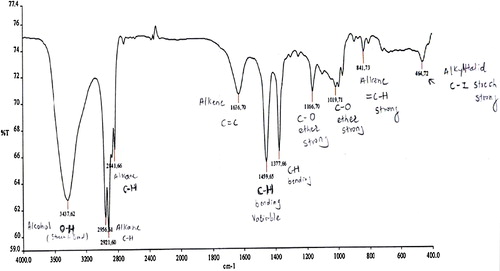
Although polyethylene may be considered at first approximation to be an infinite chain of CH2 groups, the chains are found in two different configurations. In the crystalline regions, the chain is found in a planar zig-zag configuration, as determined from X-ray diffraction studies [Citation20]. In the amorphous regions, which can constitute up to half of the specimen [Citation21], the chain configuration is essentially random and is restricted only by the conservation of the bond angles and distances [Citation22]. The random configuration of the amorphous regions becomes partially oriented when the sample is stretched [Citation21]. Thus, the usual spectrum of polyethylene represents a superposition of the spectra of two different types of chain configurations. displays the FT-IR spectra of the polyethylene (using 3 g of glycolic acid) microparticles in wavenumbers.
3.3 DSC analysis
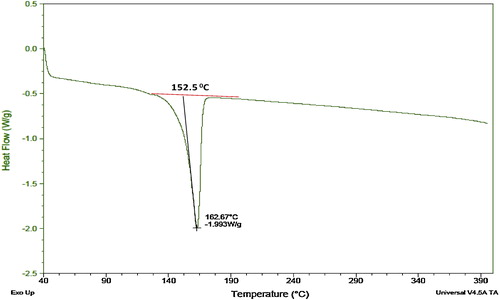
displays the DSC curve for the sample containing 3 g of glycolic acid. For this measurement, a sample weighing approximately 5 mg was heated at 10 °C/min. The figure shows the melting endotherm for one of the polyethylene samples during the initial “as received” heating. The results for the studied samples are summarized in , which shows that the onset melting temperature increased with the concentration of polyglycolic acid.
Table 2 “As received” DSC characterization of the polyethylene samples.
4 Conclusions
Polyethylene microspheres were successfully prepared using a modified chemical synthesis technique. The scanning electron microscopy images show that for a lower concentration of polyglycolic acid, the polyethylene microspheres agglomerate with a lower degree of sphericity. However, for a higher concentration, the agglomeration becomes lower, and the particle size and degree of sphericity of the microspheres increases. The results show that polyethylene microparticles may be good candidates as drug carriers for pulmonary drug delivery.
Notes
Peer review under responsibility of Taibah University.
References
- G.ScheuchM.J.KohlhaeuflP.BrandR.SiekmeierAdv. Drug Deliv. Rev.582006996
- D.I.DaniherJ.ZhuParticuology62008225
- S.AzarmiW.H.RoaR.LobenbergAdv. Drug Deliv. Rev.602008863
- M.G.BonicelliL.GiansantiM.IerinoG.ManciniJ. Colloid Interface Sci.35520111
- T.GuanY.MiaoL.XuS.YangJ.WangH.HeX.TangC.CaiH.XuInt. J. Pharm.4102011180
- K.KataokaA.HaradaY.NagasakiAdv. Drug. Deliv. Rev.472001113
- Y.UchidaY.MurakamiColloids Surf. B: Biointerfaces792010198
- Y.UchidaY.MurakamiColloids Surf. B: Biointerfaces842011346
- U.KedarP.PhutaneS.ShidhayeV.KadamNanomedicine62010714
- I.M.El-SherbinyH.D.C.SmythInt. J. Pharm.3952010132
- Y.MurakamiM.MaedaBiomacromolecules620052927
- Y.MurakamiM.MaedaMacromolecules3820051535
- F.ItoK.MakinoColloids Surf. B: Biointerfaces39200417
- C.KleinstreuerZ.ZhangZ.LiRespir. Physiol. Neurobiol.1632008128
- M.B.BroichsitterJ.GaussC.B.PackhaeuserK.LahnsteinT.SchmehlW.SeeterT.KisselT.GesslerInt. J. Pharm.3672009169
- S.S.ParkA.S.WexlerAerosol Sci.392008266
- W.YangJ.I.PetersR.O.WilliamsIIIInt. J. Pharm.3562008239
- J.LeeY.J.OhS.K.LeeK.Y.LeeJ. Control. Release146201061
- C.W.BunnTrans. Faraday Soc.351939482
- S.KrimmA.V.TobolskyJ. Polym. Sci.7195157
- A.CharlesbyJ. Polym. Sci.101953201
- S.KrimmJ. Chern. Phys.221954567