?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Presently, there is increased attention and focus on heavy metals, which are becoming one of the most serious environmental problems due to their adverse health effects. These toxic heavy metals are not easily degraded and require removal from polluted water to protect people and the environment. The purpose of this work was to prepare two types of dithiocarbamate ligands, one aliphatic (diethyldithiocarbamate) and the other aromatic (diphenyldithiocarbamate), and to use them as chelators to remove Pb, Cd, Cu and Zn from polluted water. Dithiocarbamates were selected because they have good binding ability and can precipitate metal ions as complexes. The metal removal efficiency is compared between both ligands and also compared to the efficiency of activated carbon in an adsorption process to remove the same metals. The investigation results indicated that the diphenyldithiocarbamate ligand was more efficient in removing the studied metals than the diethyldithiocarbamate analogues. Additionally, the metal removal efficiency of the diphenyldithiocarbamate ligand was more effective than using the activated carbon method.
1 Introduction
Environmental pollution by toxic heavy metals has recently been of great concern and a serious problem due to the discharge and adverse effects of these heavy metals. These metals occur globally through anthropogenic activities [Citation1–Citation3] and are discharged into the environment directly or indirectly, reaching the air, water and food sources. These discharged metals are not biodegradable and may undergo transformations that have large environmental, public health, and economic impacts [Citation4–Citation6]. Additionally, most toxic heavy metals tend to accumulate in the vital organs of living organisms or plants [Citation7,Citation8]. The pollutants of concern in this investigation include lead, cadmium, copper and zinc. These metals have a number of applications in common consumer products and basic engineering works, paper and pulp industries, leather tanning, plastic stabilizers, photographic materials, fertilizers, pigments, batteries, etc. Moreover, cadmium and lead are known to have toxicological effects and to have a negative impact on the health of humans and living organisms [Citation9–Citation11]. Zinc and copper are relatively non-toxic to humans and animals at low doses. They are necessary for the proper functioning of living organisms and are involved in the metabolism of proteins and carbohydrates. However, exposure to high doses can be harmful and can cause many adverse health effects and damage to many biochemical processes [Citation12,Citation13].
Table 2 Metal removal efficiency of diethyldithiocarbamate.
Table 3 Metal removal efficiency of dibenzyldithiocarbamate.
Table 4 Metal removal efficiency using activated carbon.
These toxic heavy metals should be removed from polluted water to protect people and the environment. Different techniques have been used for the removal of dissolved and suspended heavy metals from polluted water. These include chemical precipitation–filtration, ion exchange, reverse osmosis, oxidation–reduction, solvent extraction, adsorption, cementation, plant leaf extraction, electrochemical treatment technologies and membrane separation [Citation4,Citation14–Citation19].
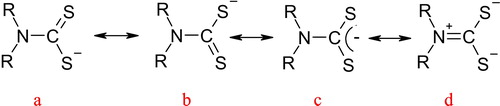
Finding new ways to remove heavy metals from water is one of the most important fields of the modern scientific research race. Dithiocarbamate ligands are one of the most fertile research materials in this field. Dithiocarbamate ligands are a type of dithiolate ligands, which comprise a number of mono- and di-negative charge ligand groups [Citation20]. demonstrates the dithiocarbamate (a, b, and c) and dithioureide (d) tautomeric forms. They have versatile binding abilities and form complexes with most transition metals [Citation21–Citation23].
Dithiocarbamates have received increasing attention in recent years because they have important applications in industry, agriculture and medical preparations. They are used in several pharmaceutical areas as fungicides, bactericides, insecticides, algaecides and NO-trapping agents [Citation24–Citation26]. They are used as effective antidotes for cadmium intoxication [Citation19]. Apart from their use in material science, they are also used as linkers for the improvement of electron transfer properties in molecular wire junctions [Citation27]. In addition, they have been used as corrosion inhibitors [Citation28], additives to improve the quality of lubricants [Citation24], and vulcanization accelerators in the rubber industry [Citation29].
Many studies have successfully used dithiocarbamate derivatives as ligands for the removal of some metal ions from aqueous solutions [Citation15,Citation29–Citation31]. Gaur et al. successfully used a copolymer containing a dithiocarbamate moiety to efficiently remove some metal ions from aqueous solutions [Citation32].
The present study addresses the use of dithiocarbamate derivative ligands for the removal of selected heavy metal ions (Cu, Cd, Pb and Zn) from polluted water. Dithiocarbamates have a strong chelating ability towards metal ions in various oxidation states due to the presence of nitrogen and sulfur atoms in various hybridized states, and they have an excellent ability to remove heavy metals due to their tendency to share electrons between nitrogen, sulfur atoms and metal ions [Citation31–Citation34]. Additionally, dithiocarbamates form insoluble stable coloured metal complexes in water [Citation29], which make them superior ligands for the removal of heavy metals from polluted water.
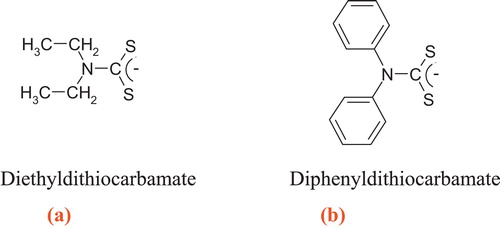
In this work, two types of dithiocarbamate ligands were prepared: the first is an aliphatic compound (diethyldithiocarbamate) (a), and the second is an aromatic compound (diphenyldithiocarbamate) (b). These ligands were used for the treatment of polluted water containing different concentrations of selected toxic heavy metals (Pb, Cd, Cu and Zn). The removal efficiency of these metals using this method and the activated carbon adsorption method to remove the same metals were then compared. The advantages and limitations of the application of these ligands were also evaluated.
2 Materials and methods
2.1 Preparation of reaction containers
In this study, 250-mL polyethylene containers were used. The containers were treated with 1 M HNO3 for 3 days and washed with distilled water.
2.2 Preparation of stock solutions
All solutions (5 ppm) of lead, copper and zinc used in the experiments were prepared by diluting 5–1000 mL from the reference solutions (1000 ppm) of lead, copper and zinc. The cadmium solution was prepared with a concentration of 4 ppm (due to the negative deviation of its solutions from Beer's law after the concentration of 5 ppm) by diluting 4–1000 mL from the reference cadmium solution (1000 ppm). The reference solutions were available with accurate concentrations from the supplier for the atomic absorption spectroscopy instrument.
2.3 Preparation of calibration curves
We have prepared standard solutions with 1, 2, 3, 4, 5, and 6 ppm concentrations for Pb, Cu and Zn by diluting 0.5, 1, 1.5, 2, 2.5, and 3 mL of the diluted 100 ppm reference solutions using 50-mL volumetric flasks. For cadmium, the 1, 2, 3, 4, and 5 ppm standard solutions were prepared by diluting 0.5, 1, 1.5, 2, and 2.5 mL of the diluted 100 ppm cadmium reference solution using 50-mL volumetric flasks. This is due to the negative deviation of cadmium solutions from Beer's law after the concentration of 5 ppm.
2.4 Instrumentation
The concentrations of Pb, Cu, Cd and Zn were determined using a UNICAM 929 Model Flame Atomic Absorption Spectrometer (AAS) equipped with an ATI unicam hollow cathode lamp. A mixture of acetylene as a fuel, air as an oxidizing agent and a Laminar flow burner were used. The ratio of the metal to the ligand was determined using a UV spectrophotometer (UNICAM UV2). For pH adjustment, we used a Metrohm pH Meter. The melting points of the prepared ligands were measured using an A9100 Electrothermal series digital melting point apparatus. IR spectra were recorded using a Thermo Scientific Nicolet 6700 FT-IR Spectrometer with KBr pellets.
2.5 Chemicals
The chemical substances used in this study were of high purity. Carbon disulfide (99.5%) was supplied by Riedel-deHaen, and diethylamine (99%) and diphenylamine (99%) were obtained from Fluka. Tetrahydrofuran (THF) (99.9%) and sodium hydride (60%) were obtained from ACROS, and sodium hydroxide (98%) was obtained from May & Baker.
2.6 Preparation of ligands
2.6.1 Preparation of sodium diethyldithiocarbamate salt
Sodium diethyldithiocarbamate salt was prepared according to the reported methods with some modification [Citation35,Citation36]. A 0.05 M NaOH solution was prepared by dissolving 2 g of NaOH in a minimum quantity of distilled water and mixed with 5.19 mL (0.05 mol) of diethylamine ((C2H5)2NH2) in a round-bottom flask. The mixture was stirred for several minutes using a magnetic stirrer, and 3.5 mL (0.05 mol) of a carbon disulfide (CS2) solution was added to the mixture dropwise until a pale yellow colour appeared. The mixture was then stirred for 2 h and heated to evaporate the as much water as possible. The mixture was left to cool at room temperature for 24 h, and yellowish-white crystals precipitated. The precipitate was washed with a 3:1 mixture of diethyl ether and ethanol and then air dried. The yield obtained was 85%, and the melting point was 97 °C. The infrared spectrum (IR) of the compound showed the characteristic absorptions of the C-NR2 and CS stretching modes in the range of 1500 and 980 cm−1, respectively. These observations are in line with those reported in the literature [Citation35].
2.6.2 Preparation of sodium diphenyldithiocarbamate salt
Sodium diphenyldithiocarbamate salt was prepared according to the reported method with slight modification [Citation36]. A solution of 12.7 g (0.075 mol) of diphenylamine (Ph2NH) and 1.80 g (0.075 mol) of NaH in 10 mL of anhydrous THF was prepared with constant stirring for 30 min using a magnetic stirrer. Then, a solution of 30 mL of CS2 was added dropwise to the above mixture within an hour, and the reaction mixture was left under stirring for 48 h; a pale yellow precipitate was formed. The solvent was then evaporated, and the precipitate was collected and washed many times with diethyl ether and left to dry for several days. The yield was 80% with a melting point of 119 °C. The IR spectrum of the compound showed the characteristic absorption of the C-NR2 and CS stretching modes in the range of 1500 and 980 cm−1, respectively. These results are similar to those reported in the literature [Citation36].
2.7 Determination of the ligand-to-metal ratio
In this study, we used ultraviolet spectroscopy for the determination of the ligand-to-metal ratio via the molar ratio method. The concentration of the metal was fixed at 5 ppm in a solution at a pH with the highest efficiency for removing each metal, as shown in , which was considered as a blank sample.
Table 1 pH values of solutions having highest metal removing efficiency.
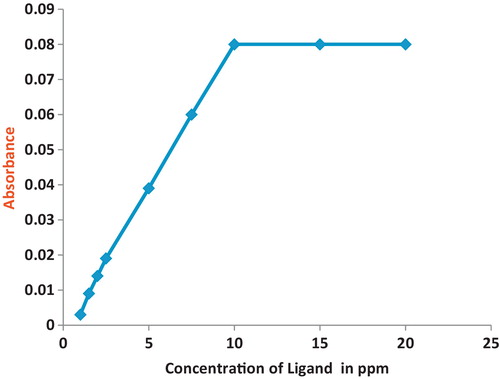
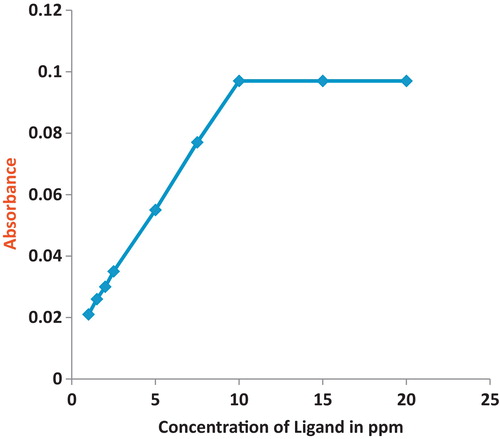
The sample needed to be analysed when containing the metal at the same previous conditions plus the ligand at ascending concentrations (1, 1.5, 2, 2.5, 5, 7.5, 10, 15, and 20 ppm). The absorption was measured for each sample, and a graph of the ligand concentration versus absorption was plotted. The plotted graph showed a direct correlation between the increase in concentration of the ligand and the absorption until the absorption became stable when the ligand concentration (10 ppm) was double the concentration of the metal. This showed that the ligand-to-metal ratio is 2:1, as shown in . Additionally, the melting points and IR spectral observations support the structure proposed for the metal complexes, which was similar to the reported one [Citation37]. This step was repeated for each metal and with every ligand. and are examples of the determination of the copper metal ratio in both ligands used.
2.8 Removal of heavy metals from aquatic solution
The metal solutions of appropriate concentrations were prepared by diluting the reference solution to 1000 ppm. The pH was determined using a pH meter and was adjusted using drops of 1 M perchloric acid (HClO4) or 1 M NaOH [Citation38]. A total of 20 mL of metal solution at the required concentration was placed in a polyethylene container, and the ligand was added in a 2:1 (ligand:metal) ratio. After precipitation, the precipitate was filtered and analysed using an atomic absorption spectrophotometer. Each sample analysis was repeated in triplicate, and the arithmetic mean was calculated to confirm the results.
A blank sample was prepared for each metal solution in the absence of ligand and at the same pH to confirm the initial concentration. Afterwards, the average removals of the selected metals from their solutions were studied by observing the decrease in concentration measurements using an atomic absorption spectrophotometer. The samples were agitated properly before the measurement process to ensure the homogeneity of the contents.
The same steps were repeated by adding activated carbon and taking into consideration the time factor in this case. Complexation formation occurred in the first case, while in the case of activated carbon, the adsorption process depended on the factor of time (in which efficiency is directly proportional to time). For this reason, we maintained equal time periods for both processes in this study. We also prepared a blank sample for each metal composed of the metal with the specific concentration and the aromatic dithiocarbamate ligand of a previously known percentage at an acidity value that would result in the highest removal efficiency for each metal. Then, we compared the quantity of the metal removed in this case with the quantity removed when using an aromatic dithiocarbamate ligand. It was found that the difference in both removal cases was 0.003 ppm, which indicated that the time period is very similar in both processes. This will yield a better score in the comparison of performance efficiency.
3 Results and discussion
The experimental observation details pertaining to the IR spectra are in agreement with the reported results in the literature [Citation36,Citation37,Citation39]. Additionally, the IR spectral observations support the proposed metal complex structure (), which was similar to that reported [Citation37]. The ligands were bidentate in all of the metal complexes, and the bidentate nature was observed in the 960–1010 cm−1 region [Citation36]. The main IR spectral peak positions of the dithiocarbamate complexes showed a thioureide band (C=N) in the range of 1460–1550 cm−1 and a C=S band in the range of 950–1040 cm−1, demonstrating the presence of a dithiocarbamate moiety [Citation36,Citation39].
3.1 Dependency of metal removal efficiency on ligand type
The process of removing each metal from its solution (i.e., 5 ppm) using the two dithiocarbamate ligands (aliphatic and aromatic) was studied using a ligand-to-metal ratio of 2:1 at five pH values of 3, 5.3, 7.3, 8.5, and 11. The results shown in Tables – indicated that both ligands, diethyldithiocarbamate and diphenyldithiocarbamate, have varying efficiencies in removing the selected metals. Additionally, the pH value of the medium plays an important role in the removing efficiency for each metal, as reported in similar studies [Citation40,Citation41]. The literature showed that dithiocarbamate compounds are unstable at pH values less than 4 [Citation42]. The results showed a removal efficiency change upon changing the pH for the same ligand with each metal. The final comparison between the results showed that the aromatic ligand (i.e., diphenyldithiocarbamate) has a higher removal efficiency compared to the aliphatic one. This is because it is more stable with a high bonding capacity for heavy metals [Citation43].
The results indicated that the lowest removal efficiency of the aliphatic ligand was 46% with Pb at a pH value of 5.30, while the removal efficiency of the aromatic ligand for the same metal at the same pH was 81%. Observations have indicated that the highest removal efficiency for both ligands and for all metals was at the same pH value. For lead and zinc, the pH value was 11, and for copper, the pH value was 8.50. However, for cadmium, there was an exception, as the removal efficiency of the aliphatic ligand was highest at a pH value of 5.30 and the removal efficiency of the aromatic ligand was highest at a pH value of 7.30. This finding supports the idea that the removal efficiency primarily depends on the type of the ligand used [Citation44] because the diphenyldithiocarbamate ligand contains an aromatic system that makes it more stable compared to the aliphatic ligand, which lacks this system. The aromatic system has a π electron system, which has empty antibonding orbitals that may accept electrons and reduce the electronic charge concentrated on the metal. This may have an effect on the stability of the complex formed as a whole. This phenomenon is known as metal–ligand charge transfer (MLCT) [Citation45].
3.2 Comparison of metal removal efficiency by dithiocarbamate ligands and activated carbon
Considering the observations obtained in this study, we found that time is an important factor in metal removal efficiency when using activated carbon. This is because this method depends on the principle of adsorption, which is directly proportional to time. Therefore, we found that the metal removal efficiency of the aromatic dithiocarbamate ligand is more effective at all experiment pH values for all studied metals (). This is because the complex formation process is a chemical reaction that occurs in a very short time, making this method that takes the factor of time into account the best.
Table 5 Comparison of metal removing efficiency by dithiocarbamate ligands and activated carbon at temp. 26 °C and for 5 min time period.
Despite its simplicity, the preparation of these ligands may be considered expensive from an economic point of view, as it requires the consumption of chemical materials. However, an advantage of this ligand that may reduce the preparation cost is the recovery possibility, where it can be used more than once in the purification process. This can be achieved by treating the complexes formed in the presence of an acid with a polar solvent such as water and an organic extract (toluene or xylene or benzene). The organic phase can then be evaporated to recover the ligand [Citation43].
There is another problem associated with using this type of ligand in purification, which is the possibility of water contamination. It was shown that ligands containing fewer than four carbon atoms exhibit phytotoxicity. This can be overcome by using ligands that have more than five carbon atoms.
In general, it was noted that the efficiency of metal removal by activated carbon is increased by increasing the pH value. Thus, for the purification of water using activated carbon with high efficiency, we have to sharply increase the pH, which may cause water toxicity. In contrast, using dithiocarbamate ligands has proven to be efficient in removing the studied metals in this experiment at pH ranges near that of ordinary water.
4 Conclusion
This study showed that the diphenyldithiocarbamate ligand was more effective in removing the studied heavy metal ions from aqueous solution compared to the aliphatic analogue at the same pH value. This is because the diphenyldithiocarbamate ligand is more stable with a high bonding capacity for heavy metals. Our observations showed that the heavy metal removal efficiency depends on the type of ligand used. Compared to the efficiency of activated carbon, diphenyldithiocarbamate was more efficient in removing the studied metals at all experimental pH values when the factor of time was taken into account. Therefore, diphenyldithiocarbamate was effective in removing the selected heavy metal ions from aqueous solutions, providing a potential approach for the treatment of polluted water containing multiple heavy metal ions.
Acknowledgements
The authors would like to acknowledge Khalid A.S. Alamodi for his assistance in the experimental work of this paper. We would like to thank Al al-Bayt University for the support. We are also grateful for a research grant from Deutsche DFG (to R. Abu-El-Halawa) and for the generous hospitality of Professor Thomas J. J. Müller University Düsseldorf and Professor G. Mass University of Ulm, Germany.
Notes
Peer review under responsibility of Taibah University.
References
- S.A.ZabinM.A.FoaadA.Y.Al-GhamdiNon-carcinogenic risk assessment of heavy metals and fluoride in some water wells in the Al-Baha region, Saudi ArabiaHum. Ecol. Risk Assess.146200813061317
- A.ZahraM.Z.HashmiR.N.MalikZ.AhmedEnrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah – feeding tributary of the Rawal Lake reservoir, PakistanSci. Total Environ.470–4712014925933
- S.A.ZabinS.M.HowladarAccumulation of Cu, Ni and Pb in selected native plants growing naturally in sediments of water reservoir dams, Albaha region, KSANat. Sci.13320151117
- F.FuQ.WangRemoval of heavy metal ions from wastewaters: a reviewJ. Environ. Manag.922011407418
- X.LiuQ.SongY.TangW.LiJ.XuJ.WuF.WangP.C.BrookesHuman health risk assessment of heavy metals in soil–vegetable system: a multi-medium analysisSci. Total Environ.463–4642013530540
- X.GaoF.ZhouC.A.ChenReview: pollution status of the Bohai Sea: an overview of the environmental quality assessment related trace metalsEnviron. Int.6220141230
- N.RascioF.Navari-IzzoReview: heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting?Plant Sci.1802011169181
- H.AliE.KhanM.A.SajadReview: phytoremediation of heavy metals – concepts and applicationsChemosphere912013869881
- ATSDR (Agency for Toxic Substances and Disease Registry)Toxicological Profile for Cadmium2012 Available online: http://www.atsdr.cdc.gov/toxprofiles/tp5.pdf (accessed on 15.03.15)
- ATSDR (Agency for Toxic Substances and Disease Registry)Toxicological Profile for Lead2007 Available online: http://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 15.03.15)
- G.ZhaoJ.LiX.RenC.ChenX.WangFew-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution managementEnviron. Sci. Technol.4520111045410462
- ATSDR (Agency for Toxic Substances and Disease Registry)Toxicological Profile for Zinc2005 Available online: http://www.atsdr.cdc.gov/toxprofiles/tp60.pdf (accessed on 20.03.15)
- ATSDR (Agency for Toxic Substances and Disease Registry)Toxicological Profile for Copper2004 Available online: http://www.atsdr.cdc.gov/toxprofiles/tp132.pdf (accessed on 15.03.15)
- R.SalimM.M.Al-SubuS.QashoaRemoval of lead from polluted water using decaying leavesJ. Environ. Sci. Health A: Tox. Hazard. Subst. Environ. Eng.2910199420872114
- R.SayE.BirlikA.DenizliA.ErsözRemoval of heavy metal ions by dithiocarbamate-anchored polymer/organosmectite compositesAppl. Clay Sci.312006298305
- M.A.BarakatNew trends in removing heavy metals from industrial wastewaterArab. J. Chem.42011361377
- R.SalimR.Abu El-HalawaEfficiency of dry plant leaves (mulch) for removal of lead, cadmium and copper from aqueous solutionsProcess Saf. Environ.80B52002270276
- O.KhazaliR.Abu-El-HalawaK.Al-Sou’odRemoval of copper(II) from aqueous solution by Jordanian pottery materialsJ. Hazard. Mater.139120076771
- B.LiuX.LvX.MengG.YuD.WangRemoval of Pb(II) from aqueous solution using dithiocarbamate modified chitosan beads with Pb(II) as imprinted ionsChem. Eng. J.2202013412419
- C.HogathMetal–dithiocarbamate complexes: chemistry and biological activityMini Rev. Med. Chem.1212201212021215
- F.JianF.BeiP.ZhaoX.WangH.FunK.ChinnakaliSynthesis, crystal structure and stability studies of dithiocarbamate complexes of some transition elements (M = Co, Ni, Pd)J. Coord. Chem.5542002429437
- H.ZhenQ.XuY.HuJ.ChengCharacteristics of heavy metals capturing agent dithiocarbamate (DTC) for treatment of ethylene diamine tetraacetic acid-Cu (EDTA-Cu) contaminated wastewaterChem. Eng. J.2092012547557
- S.A.BeryramabadiA.MorsaliS.H.VahidiDFT characterization of 1-acetylpiperazinyldithiocarbamate ligand and its transition metal complexesJ. Struct. Chem.5342012665675
- M.K.YadavG.RajputA.tN.GuptaV.KumarM.G.B.DrewN.SinghExploring the coordinative behaviour and molecular architecture of new PhHg(II)/Hg(II) dithiocarbamate complexesInorg. Chim. Acta4212014210217
- I.P.FerreiraG.M.de LimaE.B.PaniagoJ.A.TakahashiC.B.PinheiroSynthesis, characterization and antifungal activity of new dithiocarbamate-based complexes of Ni(II), Pd(II) and Pt(II)Inorg. Chim. Acta4232014443449
- P.J.RaniS.ThirumaranS.CiattiniSynthesis and characterization of Ni(II) and Zn(II) complexes of (furan-2-yl)methyl(2-(thiophen-2-yl)ethyl)dithiocarbamate (ftpedtc): X-ray structures of [Zn(ftpedtc)2(py)] and [Zn(ftpedtc)Cl(1,10-phen)]Spectrochim. Acta A: Mol. Biomol. Spectrosc.137201511641173
- J.D.E.T.Wilton-ElyD.SolankiE.R.KnightK.B.HoltA.L.ThompsonG.HogarthMultimetallic assemblies using piperazine-based dithiocarbamate building blocksInorg. Chem.4720200896429653
- M.M.SinghR.B.RastogiB.N.UpadhyayM.YadavCorrosion inhibition of copper in aqueous chloride solution by diphenyl amine and cupric diphenyldithiocarbamateIndian J. Chem. Technol.619999399
- S.KanchiP.SinghK.BisettyDithiocarbamates as hazardous remediation agent: a critical review on progress in environmental chemistry for inorganic species studies of 20th centuryArab. J. Chem.720141125
- F.FuH.ZengQ.CaiR.QiuJ.YuY.XiongEffective removal of coordinated copper from wastewater using a new dithiocarbamate-type supramolecular heavy metal precipitantChemosphere69200717831789
- L.BaiH.HuW.FuJ.WanX.ChengL.ZhugeL.XiongQ.ChenSynthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ionsJ. Hazard. Mater.195152011261275
- J.GaurS.JainR.BhatiaA.LalN.K.KaushikSynthesis and characterization of a novel copolymer of glyoxal dihydrazone and glyoxal dihydrazone bis(dithiocarbamate) and application in heavy metal ion removal from waterJ. Therm. Anal. Calorim.112201311371143
- H.FuX.LvY.YangX.XuRemoval of micro complex copper in aqueous solution with a dithiocarbamate compoundDesalination Water Treat.391–32012103111
- Z.LiSynthesis of a carbamide-based dithiocarbamate chelator for the removal of heavy metal ions from aqueous solutionsJ. Ind. Eng. Chem.202014586590
- C.SuN.TangM.TanX.GanL.CaiSynthesis and characterization of light lanthanide complexes with monosubstituted dithiocarbamatesSynth. React. Inorg. Met. Org. Chem.271997291300
- D.C.OnwudiweP.A.AjibadeSynthesis and characterization of Zn(II), Cd(II), and Hg(II) alkyl-aryl dithiocarbamate: X-ray crystal structure of [(C6H5N(et)CS2)Hg(C6H5N(butyl)CS2)]Synth. React. Inorg. Met.402010279284
- A.AroraL.AroraSynthesis of transition metal diethyldithiocarbamates and their effect on nodulation and other growth characters in Munghean, Vigna radiataAsian J. Chem.1512003144150
- O.SayrafiR.SalimS.A.SayrafiRemoval of cadmium from polluted water using decaying leaves effects of type of laves and of concentration of cadmiumJ. Environ. Sci. Health A: Tox. Hazard. Subst. Environ. Eng.A3110199625032513
- D.C.OnwudiweT.ArfinC.A.StrydomR.J.KriekA study of the thermal and AC impedance properties of N-phenyldithiocarbamate complexes of Zn(II)Electrochim. Acta1092013809817
- G.ShengH.DongY.LiCharacterization of diatomite and its application for the retention of radiocobalt: role of environmental parametersJ. Environ. Radioact.1132012108115
- S.YangJ.HuC.ChenD.ShaoX.WangMutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutionsEnviron. Sci. Technol.45201136213627
- L.BaiaH.HuaW.FuaJ.WanaX.ChengbL.ZhugebL.XiongbQ.ChenaSynthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ionsJ. Hazard. Mater.1952011261275
- V. Duijn, F.M. Durville, V. Rosmalen, Process for removing heavy metals from aqueous media, European Patent No. 116989 A1, European Search Report (EUR) (1984).
- A.NieuwpoortH.ClaessenJ.VanderlindenDiphenyl dithiocarbamato complexes of molybdenum and tungstenInorg. Nucl. Chem. Lett.11121975869871
- G.L.MiesslerP.J.FisherD.A.TarrInorganic Chemistry5th ed.2013Prentice Hall ISBN: 10-0321811054