Abstract
In this paper, polyaniline is deposited using a spraying technique onto a flexible current collector for pseudocapacitor applications. The polyaniline is characterized using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and optical absorption studies. The energy density of a pseudocapacitor is higher than that of double-layer supercapacitors due to Faradaic reactions. A high specific capacitance of 594.92 F/g is obtained at a scan rate of 5 mV/s with a scanning potential window of (−0.8 to 0.8 V). The results show an increase in the energy density of 82.63 W h/kg at a potential difference of 1 V using a 4 M potassium hydroxide aqueous electrolyte.
1 Introduction
Electronically conducting polymers (ECPs), such as polypyrrole (PPy), polyaniline (PANI), polythiophene (PTh) and poly[3,4-ethylenedioxythiophene] (PEDOT), can store and release charges through redox processes associated with π-conjugated polymer chains [Citation1–Citation5]. When oxidation occurs (also referred to as p-doping), ions from the electrolyte are transferred to the polymer backbone and, on reduction (“undoping”), they are released back into solution. Generally, p-dopable polymers are more stable than n-dopable polymers [Citation5]. The doping/undoping process occurs throughout the bulk of the electrodes, offering the opportunity to achieve high values of specific capacitance. Among the conducting polymers, polyaniline (PANI) has attracted much attention because of its low cost, environmental stability, controllable electrical conductivity, and easy processability [Citation5].
Electrochemical supercapacitors combine the advantages of dielectric capacitors, which can deliver high power within a very short time, and rechargeable batteries, which can store high amounts of energy. Supercapacitors have found an increasingly important role in power source applications, such as hybrid electric vehicles, short-term power sources for mobile electronic devices, among others. The materials studied for supercapacitors are mainly of three types: carbon, metal oxide, and conducting polymer. Due to the Faradaic reaction, the energy density of a supercapacitor consisting of electro-active materials with several oxidation states or structures (e.g., transition metal oxides and conducting polymers) is expected to be higher than that of double-layer capacitors. Conducting polymers offer the advantages ease of synthesis and low cost. In this paper, PANI is prepared chemically for use as a pseudocapacitor electrode [Citation6].
2 Experimental work
2.1 Materials
Aniline (C6H5NH2, 99%) was purchased from CDH. Ammonium persulphate, [(NH4)2S2O8, 98.5%] was purchased from WINLAB. Hydrochloric acid (HCl, 37%) and camphor sulphonic acid were purchased from Merck. These materials were used for the preparation of polyaniline. Potassium hydroxide (KOH) and sodium hydroxide (NaOH) were obtained from local agents.
2.2 Polyaniline preparation
Aniline (0.2 M) is added to 0.2 M HCl and then kept for 1 h at room temperature. Ammonium persulphate (APS) in 20 mL of distilled water is then slowly added to the suspension under stirring. The molar ratio of aniline, hydrochloric acid and APS is 1:1:1. The reaction is conducted by the in situ polymerization method in an ice bath for 1 h and then left at rest to polymerize for 24 h at room temperature. The prepared mixture is filtered and then rinsed with distilled water. PANI is simultaneously dedoped by 25 mL of 8 M sodium hydroxide at 90 °C for 5 h. The emeraldine salt is dried in air and then at 60 °C for 24 h. PANI is doped with camphor sulphonic acid (CSA) and then dissolved in chloroform [Citation7–Citation10].
2.3 Preparation of flexible PANI electrode
A flexible plastic sheet is coated with gold using a thermal vacuum evaporator. Camphor sulphonic acid doped PANI dissolved in chloroform is sprayed on the active area (1 cm × 1 cm) of the gold-coated plastic sheet using an air compressor and a spray gun. The samples are left in an oven at 70 °C to dry for 12 h prior to performing the electrochemical measurement. Electrochemical measurements are performed using two electrodes measurements in an electrochemical cell. The used electrolyte is aqueous potassium hydroxide (c = 4 M) [Citation11–Citation15].
2.4 Electrochemical measurements
The electrochemical performance is analyzed for supercapacitor electrodes in a two-electrode system via cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) using a Potentiostat/Galvanostat/ZRE Gamry G750 instrument. The galvanostatic charge-discharge is performed using a Gamry Reference 3000 instrument. The cyclic voltammetry (CV) response of the electrodes is measured at different scan rates varying from 1 mV/s to 300 mV/s, and the electrolyte is 4 M potassium hydroxide. Impedance spectroscopy measurements are performed at a dc bias of 0.1 mA, with a sinusoidal signal of 1 mA over a frequency range of 1 MHz to 0.05 Hz.
2.5 Characterization
The UV-Visible Thermo (Evolution 600) spectrophotometer that covers the range of 190 to 900 nm is used to characterize the optical properties of PANI. A Fourier Transform Infrared PerkinElmer (Spectrum BX) Spectrophotometer is used to cover the spectral range from 400 to 4000 cm−1. An XRD machine (X-Ray 7000 Shimadzu-Japan) equipped with a Cu-Kα radiation source (λ = 0.15418 nm) operating at 30 kV and 30 mA is operated at a scanning rate of 4° min−1 over 2θ values between 5° and 90° to characterize the structural properties. Scanning electron microscopy (SEM) is performed using a JEOL JSM 6360LA instrument operated at an acceleration voltage of 20 kV. A Raman Microscope Senterra Bruker, Germany, at a wavelength 532 nm is used to characterize the chemical bonding structure.
3 Results and discussion
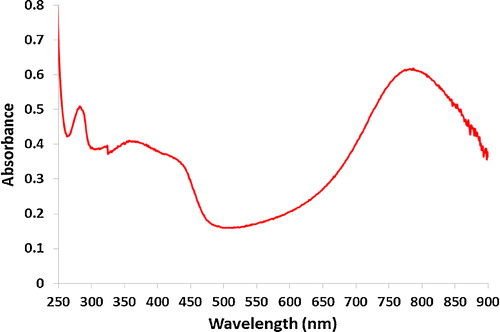
shows the UV–vis spectrum of PANI doped with CSA in chloroform. Two electronic transitions are observed at 357 nm and 787 nm. The characteristic band at 357 nm can be attributed to the π–π* transition on the polymer chain. The characteristic band at 787 nm is attributed to a polaron due to CSA doping and corresponds to localization of electrons [Citation14,Citation16].
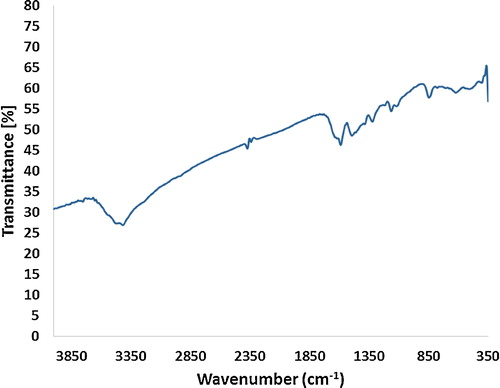
shows the FTIR spectrum of a chemically synthesized polyaniline in its undoped state. PANI shows vibration bands at 1588, 1495, 1322, 1164, 848 and 622 cm−1. The 1588 cm−1 vibration band is due to the C=C double bond of the quinoid rings, whereas the 1495 cm−1 vibration band arises due to the vibration of the C=C double bond associated with the benzenoid ring. The origin of the 1322 cm−1 is related to various stretching and bending vibrations associated with a C–N single bond. The vibration band at 1164 cm−1 is due to the presence of C–N double bonds. The vibration band at 848 cm−1 is attributed to C–H vibration [Citation10,Citation14,Citation16–Citation20].
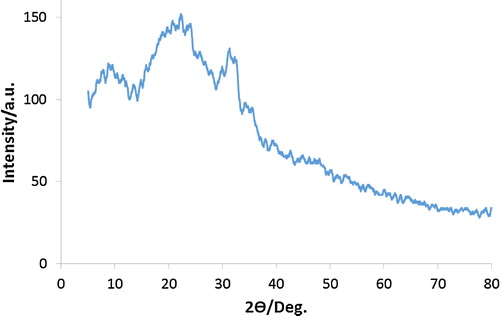
shows the XRD pattern of undoped polyaniline. For the PANi pattern, three characteristic peaks appear at 10°, 23° and 32° corresponding to (011), (020) and (200) amine salt crystals, respectively. The amorphous nature of the sample is confirmed by the XRD spectrum, which shows a broad band at 23° and low intensity peaks related to poorly crystallized PANI [Citation21,Citation22].
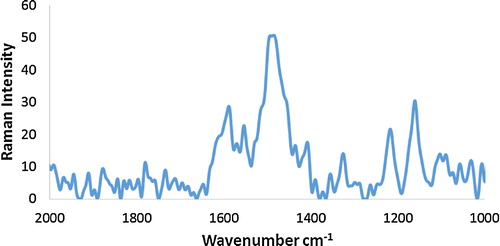
shows the Raman spectrum of PANI. The spectrum of neat PANI shows bands at 1160, 1217, 1485 and 1588 cm−1 corresponding to the C–H bending of the quinoid ring, C–N+• stretching of the bipolaron structure, N–H bending of the bipolaronic structure and C–C stretching of the benzenoid ring, respectively [Citation10,Citation16–Citation21].
shows the SEM images of the PANI doped with CSA sprayed onto a substrate of gold-coated flexible plastic substrate at different magnifications. The SEM images of CSA doped polyaniline consist of smooth fibre shapes. From the images, the low magnification (1500×) image confirms the interconnected fibrous network of the polyaniline electrode. These fibres are relatively smooth and are randomly distributed on the substrate with many pores with a diameter and length ranging from 1 to 10 μm, as observed from the high magnification (10,000×) image. It can be inferred that the fibre network of polyaniline is porous and has a large surface area.
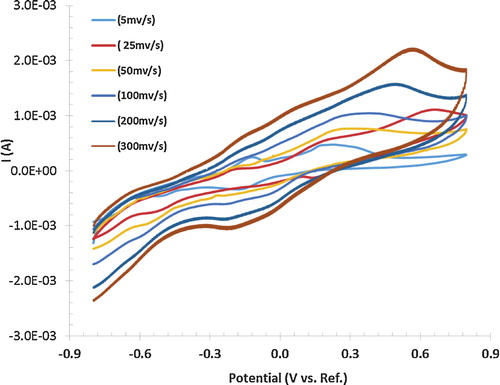
shows cyclic voltammetry (using two probes connection) of polyaniline electrodes deposited onto a gold coated flexible current collector at a scan rate of 5–300 mV/s with a scanning potential window of (−0.8 to 0.8 V). The CV curve area is gradually enlarged with the increase of the voltage scan rate. This result indicates pseudo capacitive behaviour. The oxidation and reduction peaks appeared at 0.6 V and −0.2 V. The calculated specific capacitance is 594.92 F/g at scan rate of 5 mV/s, and the weight of active material is 8 mg. The calculated specific capacitance provides an increased energy density of 82.63 W h/kg at potential difference of 1 V [Citation10–Citation12].
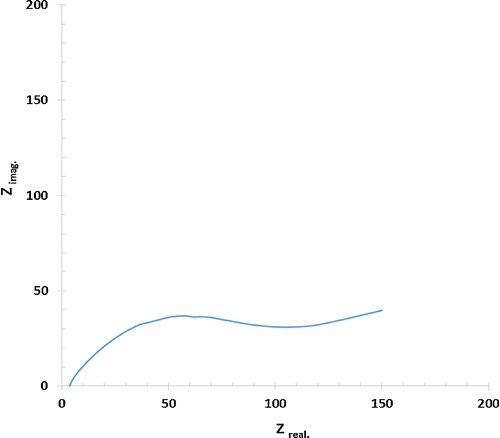
shows the Nyquist plot, which is a plot of the imaginary component (Z″) of the impedance against the real component (Z′), and the frequency response of the PANI electrode/electrolyte system. The pseudocapacitor Nyquist plot shows a straight line in the low-frequency region and a semicircle in the high-frequency region. The semicircle corresponds to charge transfer resistance Rp. The value of charge transfer resistance is found to be 120.2 Ω. The intersection of the curve at the X-axis represents the internal or equivalent series resistance (ESR). ESR is the sum of the electrolyte resistance, the intrinsic resistance of the active electrode material, and the contact resistance at the interface between active material and the current collector. The value of equivalent series resistance is found to be 3.7 Ω. This resistance determines the rate of the charge and discharge and, consequently, the power capability of the supercapacitor. The slope of the 45° portion of the curve is called the Warburg resistance and is a result of the frequency dependence of ion diffusion/transport in the electrolyte [Citation10–Citation13,Citation23].
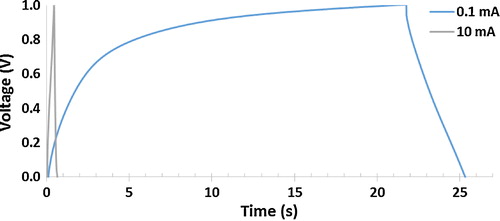
shows the galvanostatic charge/discharge curves of PANI at constant currents of 0.1 mA and 10 mA. The curve for 10 mA is highly linear and almost typically triangular. In addition, the discharge curve is nearly symmetric with its corresponding charge counterparts, indicating that the electrode has good capacitive characteristics. The curve for 0.1 mA charging occurs in nearly 21.7 s and discharges in 3.6 s.
4 Conclusions
A pseudocapacitor is fabricated using polyaniline that is sprayed onto a gold-coated plastic sheet as a flexible electrode. By using the spraying method, PANI is adhered and uniformly distributed along the surface of the current collector. The electrochemical properties of PANI reveal a superior capacitive performance. The fabricated pseudocapacitor provides an increased specific capacitance of 594.92 F/g and an increased energy density of 82.63 W h/kg. The power density of the pseudocapacitor is 82.63 kW/kg at a discharge time of 3.6 s.
Notes
Peer review under responsibility of Taibah University
References
- C.ArbizzaniM.MastragostinoPolymer based redox supercapacitorsElectrochim. Acta4119962126
- A.LaforgueaP.SimonaJ.F.FauvarqueaJ.F.SarraubP.LailleHybrid supercapacitors based on activated carbons and conducting polymersJ. Electrochem. Soc.148200111301134
- M.MastragostinoC.ArbizzaniF.SoaviPolymer based supercapacitorsJ. Power Sources972001812815
- C.ArbizzaniM.MastragostinoF.SoaviNew trends in electrochemical supercapacitorsJ. Power Sources1002001164170
- F.BéguinE.FrackowiakSupercapacitors Materials Systems and Applicationsfirst ed.2013Wiley-VCHGermany207233
- B.E.ConwayTransition from supercapacitor to battery behavior in electrochemical energy storageJ. Electrochem. Soc.138199115391548
- C.BianA.YuDedoped polyaniline nanofibres with micropores for high rate aqueous electrochemical capacitorSynth. Metals160201015791583
- G.A.SnookaP.KaoA.S.BestConducting polymer based supercapacitor devices and electrodesJ. Power Sources1962011112
- J.StejskalPolyaniline preparation of a conducting polymerPure Appl. Chem.742002857867
- H.WangQ.HaoX.YangL.LuX.WangA nanostructured graphene/polyaniline hybrid material for supercapacitorsNanoscale2201021642170
- H.GómezM.K.RamF.AlviP.VillalbaE.StefanakosA.KumarGraphene-conducting polymer nanocomposite as novel electrode for supercapacitorsJ. Power Sources196201141024108
- B.C.KimJ.S.KwonJ.M.KoJ.H.ParkC.O.TooG.G.WallacePreparation and enhanced stability of flexible supercapacitor prepared from Nafion/polyaniline nanofiberSynth. Metals16020109498
- Y.ZhangH.FengX.WuL.WangA.ZhangH.DongT.XiaX.LiL.ZhangProgress of electrochemical capacitor electrode materialsInt. J. Hydrogen Energy4200948894899
- D.S.DhawaleD.P.DubalV.S.JamadadeR.R.SalunkheC.D.LokhandeFuzzy nanofibrous network of polyaniline electrode for supercapacitor applicationSynth. Metals1602010519522
- K.S.RyuK.M.KimY.J.ParkN.G.ParkM.G.KangS.H.ChangRedox supercapacitor using polyaniline doped with Li salt as electrodeSolid State Ionics1522002861866
- Z.LuoL.ZhuaY.HuangH.TangEffects of graphene reduction degree on capacitive performances of graphene/PANI compositesSynth. Metals17520138896
- B.RajagopalanS.H.HurJ.S.ChungSurfactant-treated graphene covered polyaniline nanowires for supercapacitor electrodeNanoscale Res. Lett.102015183192
- W.WuD.PanY.LiG.ZhaoL.JingS.ChenFacile fabrication of polyaniline nanotubes using the self-assembly behavior based on the hydrogen bonding: a mechanistic study and application in high-performance electrochemical supercapacitor electrodeElectrochim. Acta1522015126134
- L.ZuX.CuiY.JiangZ.HuH.LianY.LiuY.JinY.LiX.WangPreparation and electrochemical characterization of mesoporous polyaniline-silica nanocomposites as an electrode material for pseudocapacitorsMaterials8201513691383
- Z.GaoF.WangJ.ChangD.WuX.WangF.XuS.GaoK.JiangChemically grafted graphene-polyaniline composite for application in supercapacitorElectrochim. Acta1332014325334
- F.P.DuJ.J.WangC.Y.TangC.P.TsuiX.L.XieK.F.YungEnhanced electrochemical capacitance of polyaniline/graphene hybrid nanosheets with graphene as templatesComposites532013376381
- G.Y.ZhaoH.L.LiPreparation of polyaniline nanowire arrayed electrodes for electrochemical supercapacitorsMicroporous Mesoporous Mater.1102008590594
- R.BolagamR.BoddulaP.SrinivasanSynthesis of highly crystalline polyaniline with the use of (Cyclohexylamino)-1-propanesulfonic acid for supercapacitorJ. Appl. Electrochem.4520155156