?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The corrosion inhibition effect of the synthesized compound 3-methyl-6-oxo-4-(thiophen-2-yl)-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile (TPP) on the corrosion of MS in 1 M HCl was investigated using weight loss, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) methods. The results obtained for TPP showed a good inhibition effect on the MS in the 1 M HCl solution. The inhibition efficiency was found to increase with a corresponding increase in the TPP concentration, and the best inhibition of 96.83% occurred at a TPP concentration of 200 ppm. The potentiodynamic polarization studies revealed that TPP behaved as mixed-type of inhibitor that predominantly inhibited the cathodic reaction. The impedance parameters were indicative of the high inhibitory efficiency and the adsorption of TPP on MS. Amongst the Frumkin, Temkin and Langmuir isotherms, the studied compound more closely followed the Langmuir adsorption isotherm. The scanning electron microscopy (SEM) examinations of the electrode surface confirmed the existence of such an adsorbed film.
1 Introduction
Mild steel is the most widely used metal in industrial applications due to its excellent mechanical properties and low cost [Citation1–Citation3]. Acids are used in industry during chemical cleaning, descaling, pickling, oil-well acidizing, petrochemical processes, erecting boilers, drums, heat exchangers, tanks, etc., and this leads to a corrosive attack [Citation4–Citation7]. Corrosion inhibitor solutions are widely used to prevent or minimize material loss for materials that are in contact with acid solutions. Heterocyclic compounds containing sulfur, oxygen, nitrogen and aromatic rings are very effective and efficient inhibitors for the metals in acidic medium [Citation8–Citation17].
This work was intended to state the inhibitory properties of the synthesized TPP compound on the corrosion of MS in 1 M HCl, and these properties were determined through the utilization of weight loss, potentiodynamic polarization, and electrochemical impedance spectroscopy (EIS) techniques. The mild steel surface morphologies in 1 M HCl with and without the presence of TPP were also studied. A survey of the literature revealed that TPP had not been investigated as a corrosion inhibitor until now.
2 Experimental
2.1 Inhibitor
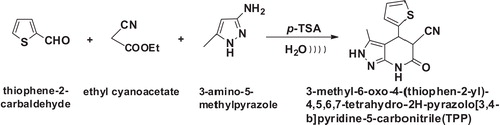
TPP was synthesized according a previously described method [Citation18]. The outline of the synthesis is given in . The melting points were recorded on a Toshniwal apparatus and are uncorrected. The purity of the compounds was confirmed on thin layers of silica gel in various non-aqueous solvent systems, e.g., benzene: ethyl acetate (9:1) and n-hexane: ethyl acetate (7:3). IR spectra (KBr) were recorded using a Shimadzu FT IR-8400s spectrophotometer, and 1H NMR and 13C NMR spectra were recorded using a Bruker DRX-300 instrument at 300 MHz and 75 MHz, respectively, in DMSO-d6 relative to tetramethylsilane as an internal reference. The mass spectra of the representative compounds were recorded using a Waters Xevo Q-Tof spectrometer at 70 eV, as shown in .
Table 1 Structure and analytical data for TPP.
2.2 Materials and solutions
The mild steel possessed the following composition (wt %) and was used for the weight loss studies: C 0.17%; Mn 0.46%; Si 0.026%; Cr 0.050%; P 0.012%; Cu 0.135%; Al 0.023%; Ni 0.05%; and balance Fe. The dimensions of the steel for the weight loss study and the electrochemical studies were 2.5 cm × 2 cm × 0.025 cm and 8 cm × 1 cm × 0.025 cm, respectively, and the samples were subsequently abraded with SiC abrasive papers of grade 600, 800, 1000 and 1200; washed with acetone; and finally dried at the ambient temperature. In the electrochemical study, a 1 cm2 area of the MS strip was exposed and was utilized as the working electrode; the rest of the MS was covered with epoxy resin. The test solution of 1 M HCl was prepared by diluting analytical grade 37% HCl with double-distilled water.
2.3 Weight loss measurements
Weight loss experiments were performed in the absence and presence of TPP at different concentrations according to standard methods [Citation19,Citation20]. The corrosion rate (CR), inhibition efficiency (η%) and surface coverage (θ) were determined using the following equations:(1)
(1)
(2)
(2)
(3)
(3) where W is the average weight loss of MS specimens, a is the total surface area of the MS specimen, t is the immersion time (3 h) and D is the density of MS in (g cm−3), and in Eq. Equation(2)
(2)
(2) , CR and
are the corrosion rates of the MS in the absence and presence of the inhibitors, respectively.
2.4 Electrochemical measurements
The electrochemical experiments were performed by using a three-electrode cell that was connected to a Potentiostat/Galvanostat G300-45050 (Gamry Instruments Inc., USA). The Echem Analyst 5.0 software package was used for the fitting of the data. MS was used as the working electrode, a platinum electrode was used as an auxiliary electrode, and a saturated calomel electrode (SCE) was used as the reference electrode. All potentials reported were measured vs. the SCE. Tafel curves were obtained by changing the electrode potential automatically from −0.25 V to +0.25 V vs. an open circuit potential at a scan rate of 1.0 mV s−1. EIS measurements were performed under potentiostatic conditions over a frequency range from 100 kHz to 0.01 Hz with an amplitude of 10 mV in the AC signal.
2.5 Surface analysis
The surfaces of the MS samples were studied by SEM (model FEI Quanta 200F microscope) at 500× magnification. The MS samples were observed after 3 h of immersion in the absence and presence of a 200 ppm TPP solution.
3 Results and discussion
3.1 Weight loss measurements
3.1.1 Effect of inhibitor concentration
The corrosion inhibition efficiencies (η%) of the inhibitor (TPP) at different concentrations were evaluated by the weight loss technique, and the results are listed in . It is clear from the results that the weight loss decreased and the η% increased with a corresponding increase in the concentration of the inhibitor [Citation21,Citation22]. The maximum inhibition efficiency was 94.2%, and it was obtained at 200 ppm.
Table 2 Parameters obtained from weight loss measurements for MS in 1 M HCl containing different concentrations of TPP.
3.1.2 Effect of temperature
The activation energy (Ea), enthalpy (ΔH*), and entropy (ΔS*) values shown in were evaluated by performing weight loss measurements in the temperature range of 308–338 K in the absence and presence of the optimum concentration of TPP. The activation energy (Ea), enthalpy (ΔH*), and entropy (ΔS*) were calculated using the following equations:(4)
(4)
(5)
(5) where Ea is the activation energy for corrosion of MS in 1 M HCl, R is the gas constant, A is the Arrhenius pre-exponential factor, T is the absolute temperature, h is Plank’s constant and N is Avogadro’s number.
Table 3 Thermodynamic parameters in absence and presence of optimum concentration of TPP.
A plot of the corrosion rate ln CR vs. 1000/T resulted in straight lines, as shown in . The values of Ea in 1 M HCl in the absence and presence of the inhibitor were determined from the slope. A plot of ln (CR/T) against 1000/T, as shown in , shows straight lines with a slope of (−ΔH*/R) and an intercept of [(ln (R/Nh)) + (ΔS*/R)], from which the values of ΔH* and ΔS* were calculated.
Fig. 1 Arrhenius plots of log CR vs. 1000/T for MS in 1 M HCl in the absence and the presence of TPP at optimum concentration.
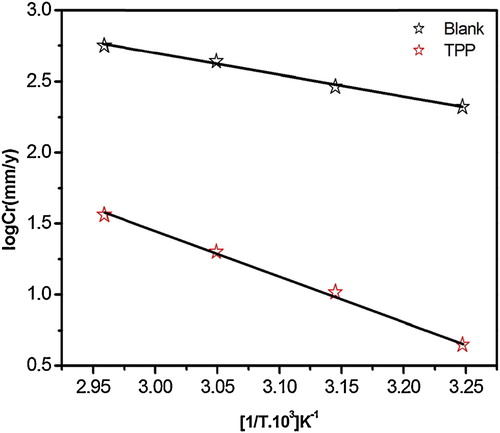
Fig. 2 Arrhenius plots of log CR/T vs. 1000/T for MS in 1 M HCl in the absence and presence of TPP at optimum concentration.
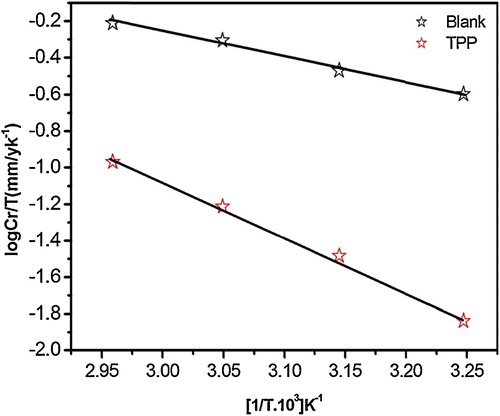
The apparent increase in Ea and ΔH* suggested the creation of an energy barrier to the corrosion reaction in the presence of the inhibitor. The higher value of ΔS* for the inhibited solution might be the result of the adsorption of the TPP molecules in the 1 M HCl solution (quasi substitution) [Citation23,Citation24].
3.1.3 Adsorption isotherm
The adsorption isotherm can be determined by assuming that the inhibition effect was mainly due to adsorption at the metal/solution interface. The adsorption process depends upon the electronic characteristics of the TPP, the nature of the metal surface, the temperature, steric effects and the varying degrees of the surface-site activity. The surface coverage values, θ, for different concentrations of TPP were used to determine the best fitting adsorption isotherm [Citation25].
By fitting the degree of surface covered (θ) to the adsorption isotherms, including the Frumkin, Temkin and Langmuir isotherms, the best fit was obtained for the Langmuir isotherm by plotting (C/θ) vs. C, which resulted in a straight line with a coefficient (R2) value close to unity, as shown in . The equation can be represented as:(6)
(6)
Fig. 3 (a) Frumkin, (b) Temkin and (c) Langmuir adsorption isotherms for of TPP on MS surface in 1 M HCl.
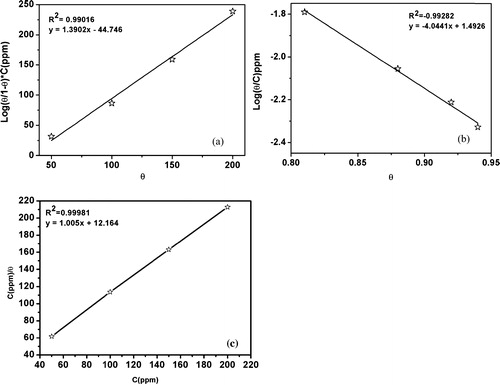
The equation can be rearranged to:(7)
(7) where Cinh is the concentration of the inhibitor and θ is the surface coverage.
Kads is related to the standard free energy of adsorption, ΔGads, through the following equation:(8)
(8) where R is the gas constant and T is the absolute temperature. The value of 55.5 is the concentration of water in the solution in mol L−1.
It has been reported in the literature that values of ΔGads around −20 kJ mol−1 indicate electrostatic interactions i.e., physisorption. Conversely, a ΔGads value near or greater than −40 kJ mol−1 indicates chemisorption [Citation7,Citation26]. The adsorption of the inhibitor molecules on the metal surface cannot be considered to be a purely physical or chemical phenomenon. In the present investigation, the calculated value for ΔGads at the optimum concentration and 308 K was −38 kJ mol−1, and this suggests both a physical and chemical mode of adsorption [Citation27,Citation28].
3.2 Electrochemical measurements
3.2.1 Potentiodynamic polarization measurements
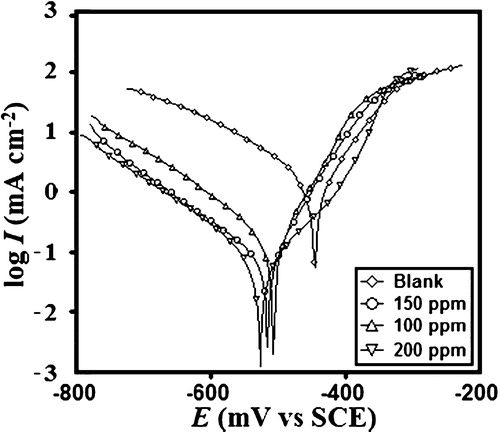
Tafel polarization curves were recorded for the MS in 1 M HCl solutions without and with the various concentrations of TPP; these curves are shown in . The polarization data, including the corrosion potential (Ecorr), corrosion current density (Icorr) and anodic (βa) and cathodic (βc) slopes, are listed in . The inhibition efficiency (η%) values were calculated from the given equation:(9)
(9) where Icorr and Icorr(i) are the corrosion current densities in the absence and presence of the inhibitor, respectively.
Table 4 Polarization parameters of MS in 1 M HCl solution at different concentration of TPP derivatives.
If the change in the Ecorr value was greater than 85 mV, a chemical compound could be recognized as an anodic- or a cathodic-type inhibitor. The maximum shift of Ecorr in the present case was 82 mV towards the cathodic direction, which indicated that TPP was a mixed type inhibitor and that it predominately inhibited the cathodic reaction. It was clear from the results that the values of Icorr decreased with the increasing concentration of TPP, which indicated an effective corrosion mitigation of the mild steel in 1 M HCl [Citation29]. The maximum decrease in Icorr was 59 μA cm−2 at 200 ppm. The anodic and cathodic Tafel slopes were found to change with changes in the inhibitor concentration, indicating that the anodic and cathodic corrosion were inhibited by TPP [Citation30,Citation31].
3.2.2 Electrochemical impedance spectroscopy (EIS)
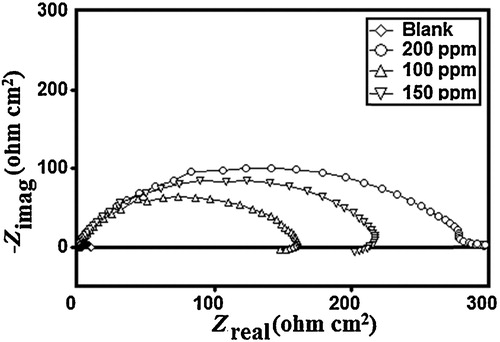
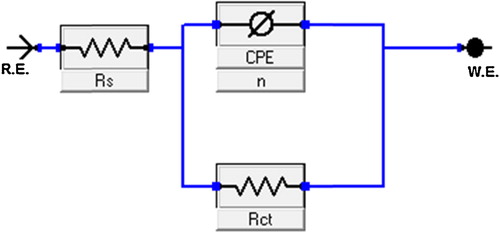
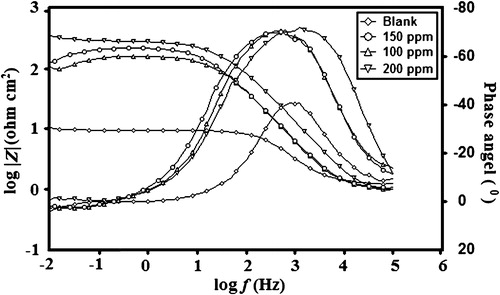
The Nyquist loops and related parameters in the absence and presence of TPP are shown in and . The Nyquist diagrams show a single semicircle shifted along the real impedance axis (Zreal), indicating that the corrosion of MS in 1 M HCl was controlled by a charge-transfer process. In the evaluation of Nyquist plots, the difference in the real impedance at lower and higher frequencies is commonly considered to be a charge transfer resistance [Citation32]. The equivalent circuit model used for the analysis is shown in , and it consists of the solution resistance (Rs), the charge-transfer resistance (Rct) and the constant phase angle element (CPE). The inhibition efficiency was calculated using the charge transfer resistance (Rct) as follows:(10)
(10) where Rct(inh) and Rct are the values of the charge transfer resistance in the presence and absence of inhibitors in 1 M HCl, respectively.
Table 5 Electrochemical impedance parameters and the corresponding inhibition efficiencies of mild steel in 1 M HCl in the absence and presence of different concentrations of TPP.
The results showed that the addition of different concentrations of TPP increased the Rct values and lowers the Cdl [Citation33,Citation34]. The increase in Rct was attributed to the increase in resistance. The decrease in the Cdl values was attributed to the adsorption of TPP on the metal surface. The values of the double layer capacitance, Cdl, can be represented as:(11)
(11) where Y0 is the CPE coefficient, n is the CPE exponent (phase shift), and ω is the angular frequency. The thickness of this protective layer (d) is correlated with Cdl by the following equation [Citation35]:
(12)
(12) where ɛ is the dielectric constant, ɛ0 is the permittivity of the free space and A is the surface area of the electrode. A Bode plot [log |Z| vs. log f] and a phase angle plot [α° vs. log f] are shown in . It is reported in literature that an ideal capacitor phase angle and slope value should be −90° and −1, respectively [Citation36]. The obtained results listed in , shows the deviations of the Bode and phase angle values from the blank acidic solution, which indicates the capacitive performance of the inhibited system.
Table 6 Slopes of the Bode plots (−S) and maximum phase angles (α°) for MS in 1 M HCl solution with increasing concentrations of TPP.
3.3 Surface analysis
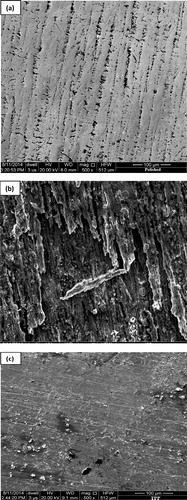
The surface morphologies of the MS samples in the presence and absence of TPP were observed through SEM analysis. The changes that occurred in the MS samples are shown in (a–c). (a) shows the clean polished surface of MS. (b) shows the MS surface in 1 M HCl. (c) shows the MS surface in 1 M HCl containing TPP. The smooth surface shown in (c) of the MS was attributed to the adsorption of the inhibitor molecules on the MS surface.
4 Mechanism of adsorption and inhibition
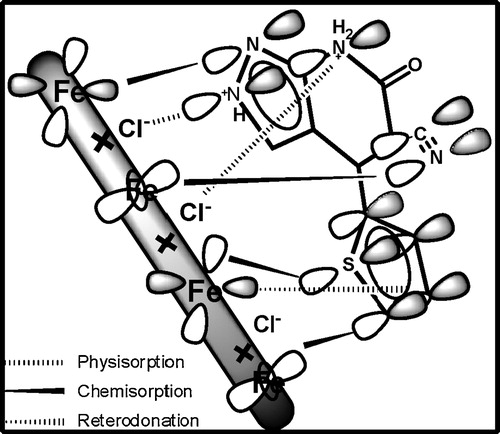
The mechanism of adsorption depends upon the interaction between the TPP molecules and the MS surface in 1 M HCl. The adsorption of TPP on the MS surface cannot solely be considered either physical or chemical. Because of this complex nature of adsorption, it is difficult to explain it through a single adsorption mechanism. Therefore, the following adsorption and inhibiting phenomena were proposed.
| 1. | TPP in 1 M HCl exists in protonated form, which will be in equilibrium with the corresponding neutral form by the following equation: The TPP molecules were adsorbed onto the mild steel surface via physical adsorption between the charged mild steel surface and the charged TPP molecules. | ||||
| 2. | The chemical adsorption of the TPP arises through the donation of free electron pairs of heteroatoms to vacant d-orbitals of the iron. | ||||
| 3. | The donor–acceptor interactions between the π-electrons of the aromatic ring and the d-orbitals of the metallic surface atoms (retrodonation) [Citation37–Citation39]. The skeletal representation of the adsorption of the TPP molecule onto the mild steel surface is presented in . | ||||
5 Conclusions
The results obtained for TPP showed that it acted as a good inhibitor with a 96.8% inhibition efficiency at a concentration of 200 ppm. The potentiodynamic polarization data indicated that the TPP was a mixed type inhibitor with a predominance in the cathodic direction. EIS data revealed a decrease in the Cdl values that resulted from either a decrease in the local dielectric constant or an increase in the thickness of the electrical double layer. The adsorption of the inhibitor molecules on the mild steel surface was found to obey the Langmuir adsorption isotherm. All of these data supported the theory of the good inhibition tendency of TPP.
Acknowledgement
Priyanka Singh would like to thank the Ministry of Human Resource Development (MHRD), New Delhi, India, for the financial assistance and the facilitation of our study.
Notes
Peer review under responsibility of Taibah University.
References
- M.A.MigahedA.M.Abdul-RaheimA.M.AttaW.BrostowSynthesis and evaluation of a new water soluble corrosion inhibitor from recycled poly(ethylene terphethalate)Mater. Chem. Phys.1212010208214
- D.AsefiM.AramiN.M.MahmoodiElectrochemical effect of cationic gemini surfactant and halide salts on corrosion inhibition of low carbon steel in acid mediumCorros. Sci.522010794800
- S.H.YooY.W.KimK.ChungS.Y.BaikJ.S.KimSynthesis and corrosion inhibition behavior of imidazoline derivatives based on vegetable oilCorros. Sci.5920124254
- R.SolmazInvestigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-dimethylaminobenzylidene)rhodanineCorros. Sci.792014169176
- Y.RenY.LuoK.ZhangG.ZhuX.TanLignin terpolymer for corrosion inhibition of mild steel in 10% hydrochloric acid mediumCorros. Sci.50200831473153
- S.A.UmorenO.OgbobeI.O.IgweE.E.EbensoInhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additivesCorros. Sci.50200819982006
- I.A.AdejoroF.K.OjoS.K.ObafemiCorrosion inhibition potentials of ampicillin for mild steel in hydrochloric acid solutionJ. Taibah Univ. Sci.201510.1016/j.jtusci.2014.10.002
- S.K.ShuklaM.A.QuraishiR.PrakashA self-doped conducting polymer polyanthranilic acid: an efficient corrosion inhibitor for mild steel in acidic solutionCorros. Sci.50200828672872
- G.QuartaroneL.RonchinA.VavasoriC.TortatoL.BonaldoInhibitive action of gramine towards corrosion of mild steel in deaerated 1.0 M hydrochloric acid solutionsCorros. Sci.6420128289
- S.VishwanathamN.HaldarFurfuryl alcohol as corrosion inhibitor for N80 steel in hydrochloric acidCorros. Sci.50200829993004
- X.LiS.DengH.FuThree pyrazine derivatives as corrosion inhibitors for steel in 1.0 M H2SO4 solutionCorros. Sci.53201132413247
- M.BouklahA.OuassiniB.HammoutiA.El IdrissiCorrosion inhibition of steel in 0.5 M H2SO4 by [(2-pyridin-4-ylethyl)thio]acetic acidAppl. Surf. Sci.25020055056
- A.OuchrifM.ZegmoutB.HammoutiS.El-KadiriA.Ramdani1,3-Bis(3-hyroxymethyl-5-methyl-1-pyrazole) propane as corrosion inhibitor for steel in 0.5 M H2SO4 solutionAppl. Surf. Sci.2522005339344
- G.K.GommaCorrosion of low-carbon steel in sulphuric acid solution in presence of pyrazole–halides mixtureMater. Chem. Phys.551998241246
- M.AbdallahM.M.El-NaggarCu+2 cation+3,5-dimethyl pyrazole mixture as a corrosion inhibitor for carbon steel in sulfuric acid solutionMater. Chem. Phys.712001291298
- A.S.FoudaA.S.EllithyInhibition effect of 4-phenylthiazole derivatives on corrosion of 304L stainless steel in HCl solutionCorros. Sci.512009868875
- A.DonerR.SolmazM.OzcanG.KardasExperimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solutionCorros. Sci.53201129022913
- A.RahmatiSynthesis of 4-aryl-3-methyl-6-oxo-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile via a one-pot, three-component reactionTetrahedron Lett.51201029672970
- P.SinghA.SinghM.A.QuraishiE.E.EbensoCetirizine: a new and effective corrosion inhibitor for mild steel in 1 M HCl solutionInt. J. Electrochem. Sci.7201270657079
- P.SinghA.SinghM.A.QuraishiE.E.EbensoExperimental and theoretical investigation for inhibition action and adsorption behavior of montelukast sodium in 1 M HCl solutionInt. J. Electrochem. Sci.7201286128626
- P.SinghM.A.QuraishiE.E.EbensoInvestigation of gliclazide drug as novel corrosion inhibitor for mild steel in 1 M HCl solutionInt. J. Electrochem. Sci.720121227012282
- P.SinghM.A.QuraishiE.E.EbensoMicrowave assisted green synthesis of bis-phenol polymer containing piperazine as a corrosion inhibitor for mild steel in1 M HClInt. J. Electrochem. Sci.820131089010902
- D.K.YadavB.MaitiM.A.QuraishiElectrochemical and quantum chemical studies of 3,4-dihydropyrimidin-2(1H)-ones as corrosion inhibitors for mild steel in hydrochloric acid solutionCorros. Sci.52201035863598
- D.K.YadavB.MaitiM.A.QuraishiInhibition effect of some benzylidenes on mild steel in 1 M HCl: an experimental and theoretical correlationCorros. Sci.552012254266
- I.AhamadM.A.QuraishiBis (benzimidazol-2-yl) disulphide: an efficient water soluble inhibitor for corrosion of mild steel in acid mediaCorros. Sci.51200920062013
- S.Ben AounHighly efficient corrosion inhibition of carbon steel in aggressive acidic media with a pyridazinium based ionic liquidInt. J. Electrochem. Sci.820131078810804
- A.K.SinghM.A.QuraishiThe effect of some bis-thiadiazole derivatives on the corrosion of mild steel in hydrochloric acidCorros. Sci.52201013731385
- SudheerM.A.QuraishiElectrochemical and theoretical investigation of triazole derivatives on corrosion inhibition behavior of copper in hydrochloric acid mediumCorros. Sci.702013161169
- N.SoltaniM.BehpourS.M.GhoreishiH.NaeimiCorrosion inhibition of mild steel in hydrochloric acid solution by some double Schiff basesCorros. Sci.52201013511361
- D.K.YadavM.A.QuraishiApplication of some condensed uracils as corrosion inhibitors for mild steel: gravimetric, electrochemical, surface morphological, UV–visible, and theoretical investigationsInd. Eng. Chem. Res.5120121496614979
- D.K.YadavM.A.QuraishiElectrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solutionInd. Eng. Chem. Res.51201281948210
- D.K.YadavD.S.ChauhanI.AhamdM.A.QuraishiElectrochemical behavior of steel/acid interface: adsorption and inhibition effect of oligomeric anilineRSC Adv.201310.1039/c2ra21697c
- A.K.SatapathyG.GunasekaranS.C.SahooK.AmitP.V.RodriguesCorrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solutionCorros. Sci.51200928482856
- M.A.AminS.S.Abd El RehimH.T.M.Abdel-FatahElectrochemical frequency modulation and inductively coupled plasma atomic emission spectroscopy methods for monitoring corrosion rates and inhibition of low alloy steel corrosion in HCl solutions and a test for validity of the Tafel extrapolation methodCorros. Sci.512009882894
- K.F.KhaledM.A.AminCorrosion monitoring of mild steel in sulphuric acid solutions in presence of some thiazole derivatives – molecular dynamics, chemical and electrochemical studiesCorros. Sci.51200919641975
- I.AhamadR.PrasadM.A.QuraishiInhibition of mild steel corrosion in acid solution by Pheniramine drug: experimental and theoretical studyCorros. Sci.52201030333041
- A.DandiaS.L.GuptaP.SinghM.A.QuraishiUltrasound-assisted synthesis of pyrazolo[3,4-b]pyridines as potential corrosion inhibitors for mild steel in 1.0 M HClACS Sustain. Chem. Eng.1201313031310
- K.R.AnsariM.A.QuraishiA.SinghSchiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solutionCorros. Sci.792012515
- M.A.QuraishiK.R.AnsariD.K.YadavE.E.EbensoCorrosion inhibition and adsorption studies of some barbiturates on mild steel/acid interfaceInt. J. Electrochem. Sci.720121230112315