Abstract
Salvadora persica L., also known as the toothbrush tree (Miswak), has been used since ancient times as a chewing stick for oral hygiene. Miswak is a natural source of many unique phytochemicals, which are described by traditional medicine as a remedy for various disease symptoms with beneficial properties. The availability and richness of biologically active compounds and minerals, related to oral and dental health, in Miswak makes it a superior tool for oral hygiene and a barrier against general pathogens that enter the human body through the mouth. This study investigates the presence of antimicrobial agents in Miswak extracts based on their polarity in different solvents. The results show that Miswak contains more than one type of antimicrobial agent that inhibits the growth of both gram positive and negative bacteria. The zone of inhibition for three different extracts was measured in Escherichia coli, Staphylococcus aureus, Lactobacillus acidophilus, Streptococcus mutans and Pseudomonas aeruginosa; the results show a strong antimicrobial activity in the aqueous extract and less activity in alcoholic and nonpolar extracts.
1 Introduction
Oral hygiene is one of the most important daily routine practices and keeps the mouth and teeth clean and prevents many health problems [Citation1]. Modern dental care tools are designed to provide both a mechanical and chemical means of removing plaque and food residues from the surface and spaces between the teeth. Throughout history, people have been using different tools and chemicals to maintain their oral health, such as chewing sticks, tooth brushes, gum, mouth wash, toothpaste and floss, which are all believed to evolve from botanical origins [Citation2,Citation3]. Chewing sticks are considered the most popular among all of the dental care tools for their simplicity, availability, low cost and their traditional and/or religious value [Citation1,Citation3]. Chewing-sticks were used by the Babylonians more than 7000 years ago [Citation2]. Currently, in the Muslim world, the use of Miswak as a chewing stick is highly recommended as a Sunnah practiced by the prophet Mohammad (peace be upon him) and his companions to achieve daily dental care, and the prophet emphasised the importance of using Miswak for oral hygiene [Citation3].
Salvadora persica Linn. (Miswak) is a small evergreen tree that belongs to the Salvadoraceae family [Citation4]. The S. persica tree can reach up to 3 m tall and has thick succulent small leaves [Citation5]; new stem branches are green to greyish in colour while old branches are dark brown [Citation6]. The scientific name of S. persica was given to the tree after classifying the first sample in 1598 by the Spanish botanist, Dr. Laurent Garcin, who collected the specimen from the middle-east [Citation4]. The tree is globally known as the toothbrush tree or chewing-sticks; it has many local names in different geographical regions such as Miswak or Arak in the Arab world, Koyoji in Japan, Qesam in Hebrew, and Mastic in Latin [Citation7].
The suitability of Miswak chewing sticks as a dental care tool is achieved mechanically by the ability of its fibres to reach in between teeth and also chemically by the richness of its phyto-constituents, which are unique in their complexity and biological activity. Previous studies have demonstrated the richness of S. persica Miswak for the minerals and phytochemical components related to dental care, which is shown in . Many studies focus on extracted chemicals, such as β-sitosterol [Citation8], glycosides [Citation9] and organic compounds like pyrrolidine, pyrrole and piperidine derivatives [Citation10], which are biologically active and described as remedies for joint pain, spleen troubles, gonorrhoea, skin diseases and stomach ulcers [Citation6,Citation11]. Chlorides and fluorides in Miswak extracts were also shown to be important elements to remineralize teeth enamel along and showed antimicrobial activity [Citation12]. The Miswak sap is reported as a stimulant of saliva production with a pH buffering capacity [Citation13], the ability to remove the dental plaque layer [Citation14], remineralization of the enamel layer [Citation12] and shows a strong antimicrobial activity [Citation1].
Table 1 Phyto-chemicals found in S. persica and their benefits for oral and dental health.
Exploring the antimicrobial activity of S. persica is still in its early stages, where crude and alcoholic extracts have shown general effectiveness against some pathogenic bacteria and fungi [Citation2,Citation11,Citation15] such as Bacillus subtilis, Escherichia coli, Lactobacillus brevis, Proteus vulgaris, Staphylococcus aureus, Streptococcus mutans, Lactobacillus acidophilus, Pseudomonas aeruginosa, Aspergillus niger, and Candida albicans [Citation11,Citation15,Citation16]. The biological activity of a few of S. persica's fractionated phytochemicals were investigated and reported, such as benzyl isothiocyanate [Citation17] and β-sitosterol [Citation8], which inhibit the cariogenic and genotoxic compounds accumulated on the surface of the teeth. The toxicity of S. persica extracts was reported in high concentrations and exceeded 5 g/kg of the mammal's body weight [Citation18].
The polar nature of chemical compounds and the choice of solvents are major factors in determining the solutes and their activity in the performed extracts [Citation19]. Nevertheless, it has been reported that a high mineral content, dissolved inorganic compounds and anionic components in the extract, such as chlorides, fluorides, sulphur, cyanides, and heavy metals, possess a broad antimicrobial activity [Citation20,Citation21] by disrupting the bacterial cell wall, disrupting the transport system, inhibiting oxygen uptake, and leading to oxidative stress in bacteria or causing immediate toxicity and death [Citation21,Citation22]. The phytochemical constituents in S. persica were shown in an earlier study to be selective in their polarity, where alkaloids, flavonoids, quinones, and glycosides were more soluble in fresh samples with a higher water content [Citation16] than alcoholic extracts. However, nonpolar compounds in S. persica, such as essential and volatile oils, were reported to possess antimicrobial activity against human pathogenic bacteria and fungi [Citation23,Citation24], in addition to its role in buffering the pH of saliva and removing the plaque layer [Citation13].
In this study, the antimicrobial activity of Miswak extracts is investigated against known pathogenic bacteria and natural bacterial flora found in the saliva, compared to commercial toothpastes. The goal of this investigation is to find the difference between Miswak extracts performed by different polarity solvents; water (9), ethanol (5.2) and hexane (0) according to Snyder's polarity index along with the general effect of minerals found in S. persica and its antimicrobial activity.
2 Materials and methods
2.1 Miswak samples
Miswak samples were purchased from a local market at Al-Madinah Al-Munawara, KSA. The sticks were dried in a 55 °C oven for three days and then sliced into discs or ground into a fine powder using a coffee grinder. The Miswak extracts were prepared by adding 40 g of the Miswak powder to 200 ml of solvent (water, ethanol and hexane) in a closed container and soaked at room temperature for 48 h. The solvents were filtrated through a Whatman No. 1 filter paper and allowed to evaporate in a 40 °C oven for 72 h. The dried extracts were considered 100% pure and used to prepare different concentrations by using the same solvents (100, 250 and 500 μg/ml). Two commercial toothpaste brands, TP1 and TP2, were purchased from a local market and allowed to dry, ground and used as a control for all of the antimicrobial tests at the concentration of 100 μg/ml. The Miswak mouth wash was prepared by dissolving 250 mg of dried extract in 1 L of distilled water.
2.2 Saliva samples
Saliva samples were collected from 40 Taibah University students with their consent and in two stages; the first stage was immediate sampling of the saliva and the second stage was after 1 min of gargling for 45 s with Miswak mouth wash. All of the samples were collected from students who did not use Miswak and the collection time was between 10:00 am and 12:00 pm. The saliva samples were measured for their pH and used for the total bacteria count.
2.2.1 Bacterial count
The bacterial count was achieved by diluting the saliva samples 1–10 in distilled water and spreading a 100 μl of diluted saliva samples, before and after Miswak mouth wash, on LB agar media alone or supplemented with 100 μg/ml of water, ethanol and hexane extracts or 100 μg/ml of toothpastes, TP1 and TP2, for comparison. A total of three plates were cultured for each sample. The plates were incubated at 37 °C for 48 h and the colonies were counted using a colony counter. The experiment was repeated three times for statistical analysis.
2.3 Bacterial strains
Pathogenic bacterial strains; E. coli, Staph. aureus, Strep. mutans, L. acidophilus and P. aeruginosa were collected from a local hospital in Al-Madinah Al-Munawara and tested for their survival using extract discs.
2.4 Extract discs
The extract discs were prepared on 6 mm Whatman paper discs with a drop of 10 μl of water, ethanol and hexane extracts from the prepared concentrations; 100, 250 and 500 μg, respectively. Toothpaste controls, TP1 and TP2, were also blotted on 6 mm Whatman paper discs at the concentration of 100 μg. The discs were allowed to completely dry at room temperature before use.
2.4.1 Disc diffusion assay
A disc diffusion assay for antimicrobial activity was performed by growing the bacteria in 2 ml of liquid LB medium for 24 h at 37 °C in a shaking incubator. The actively growing bacteria were adjusted to a final density of 108 cfu/ml [Citation25] and used as a seed to inoculate 1 L of LB agar media before plating. After pouring the seeded LB agar, the extract discs were added on top of the solidified media along with a 10 μg ampicillin and 30 μg kanamycin commercial discs as controls. The plates with extract discs for each bacterial strain were kept at 4 °C for 2 h to allow extract diffusion from the discs and then the plates were transferred to a 37 °C incubator for 48 h. The zone of inhibition was measured around the discs.
2.5 Mineral content
The mineral content of S. persica dried powder was analysed for its chloride content according to the Mohr method [Citation26] and metals by digesting 0.5 g of dry Miswak powder in a HNO3/H2O2 solution, according to the method described by Pequerul et al. [Citation27] and measured the minerals by ICP-MS (7500cx, Agilent, JP) at the Chemistry Department of Taibah University.
2.6 Statistical analysis
The statistical analysis of samples was performed by calculating the average of the three replicates for each event and the three repeats of each experiment with the standard deviation. The “t” test for paired samples, simple regression and correlation factor “R” were used to evaluate the differences and the association between the samples, where P values < 0.05 were considered statistically significant.
3 Results
3.1 Mineral content
The mineral content profile of Miswak showed high levels of chloride in the water extract (71 mg/g) and lower levels in the ethanol extract (6.21 mg/g). Metals and trace metals were also found in high concentrations in the dried powder of Miswak as illustrated in .
Table 2 Metals (mg/g) and trace metals (μg/g) found in S. persica.
3.2 Saliva pH
The saliva pH of the collected samples was measured before and after gargling with the prepared Miswak mouth wash. The usage of Miswak mouth wash showed an increase of the saliva's pH from an average of 6.93–7.28 for all 40 samples with a P value < 0.001 and a correlation R factor of 0.3.
3.2.1 Total bacterial count
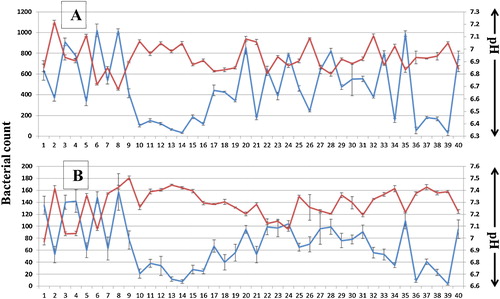
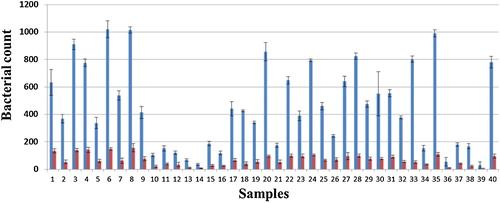
The total bacterial count from the collected saliva samples before and after using the Miswak mouth wash showed a significant decrease in the total number of surviving colonies for each sample with a correlation coefficient (R) of 0.89 and P < 0.001 as shown in .
Fig. 1 Total count of bacterial colonies grown on LB plates for 48 h (CFU/100 μl). Blue columns: saliva sample before gargling with Miswak mouth wash. Red columns: saliva samples after gargling with Miswak mouth wash. R = 0.89, P < 0.001.
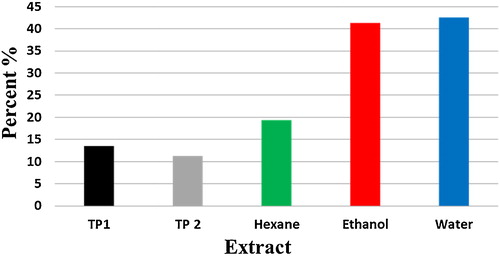
The addition of Miswak extracts, at the concentration of 100 μg/ml to the culture medium, negatively affected the growth of bacteria from the saliva samples. The plated saliva samples on LB agar supplemented with 100 μg/ml water extract showed a reduction of bacterial colony numbers by 42.5% and an R value of 0.732 (P < 0.05), the ethanol extract reduced the bacterial numbers by 41.3% with an R value of 0.758 (P < 0.05) and the hexane bacterial reduction was 19.4% with an R value of 0.716 (P < 0.05). However, commercial toothpastes, TP1 and TP2, also showed a reduction of 13.4% and 11.2% with R values of 0.45 and 0.83, respectively, as shown in . The effect of the saliva's pH on the total number of bacteria after using the Miswak mouth wash is illustrated in , where there was a reduction in the total number of bacteria and a reverse correlation with the increase of pH (P < 0.001).
3.3 Antibacterial activity
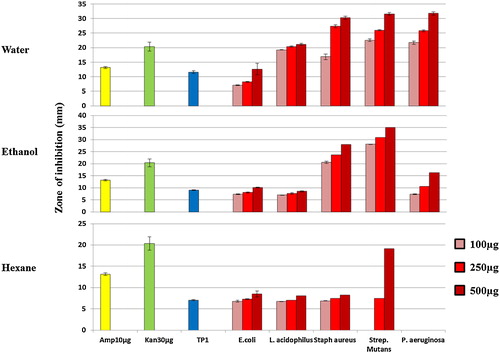
The antibacterial activity and the zone of inhibition measurements were conducted by the disc diffusion assay, where bacterial strains of E. coli, L. acidophilus, Staph. aureus, Strep. mutans and P. aeruginosa showed susceptibility to S. persica discs and water, ethanol and hexane extract paper discs as shown in .
4 Discussion
Miswak chewing sticks have been used for oral hygiene since ancient times [Citation2], providing clean teeth [Citation28], strong enamel [Citation5], and protecting from pathogens that enter the body through the mouth [Citation29]. The unique complexity of the Miswak phytochemicals and minerals, along with its long fibres, gives it an advantage as a tool for oral and dental health care through providing all of the necessary means of mechanical and chemical cleaning and maintaining healthy teeth and gums. The release of chemicals and minerals from Miswak at the time of usage stimulates saliva production and buffers its pH as shown in this study (), which confirms previous reports of similar findings [Citation13]. As a consequence, the antimicrobial activity of the released phytochemicals reduces the total number of bacteria [Citation23,Citation25,Citation29]. In this study, when the saliva samples were collected after 45 s of gargling with Miswak mouth wash, the total number of bacteria in the saliva samples decreased by ∼83% when compared to samples before gargling (). Statistically, the correlation R factor value (0.89) indicates a positive relationship between the change of pH and the total number of the saliva's bacteria with a P < 0.001. Although the total number of bacteria was reduced, the pH is not the only factor affecting the bacterial numbers; chlorides and minerals () are more abundant in the water extract of Miswak, which the mouth wash was prepared from, along with water soluble antimicrobial agents [Citation15,Citation21] that are all responsible for the reduction of bacterial numbers ().
The solubility of antimicrobial agents in different polarity solvents had different effects on the growth of the bacterial flora in the collected samples (). To investigate the effect of each extract on the selected pathogenic bacterial strains, a disc diffusion assay () was conducted to illustrate the difference. The results showed that water extract had the greatest effect on all of the tested bacteria in all three concentrations used, whereas the ethanol extract showed less inhibition, especially on L. acidophilus and P. aeruginosa. The hexane extract gave the least zone of inhibition among the three solvents in this study, Staph. aureus showed less susceptibility to hexane extract than the water or ethanol extracts and P. aeruginosa was not affected by the hexane extract at any of the concentrations used, as shown in . The high value of the measured inhibition zones in the alcohol extract confirms the results of previous reports showing the effect of alcohol extract on pathogenic bacteria [Citation16]. These results indicate that a combination of antimicrobial agents with different polarities exist in S. persica chewing sticks and act against gram positive and negative bacteria [Citation17]. For example, the chloride concentration in the water extract was 11.5 times more than the ethanol extract, which suggests that the solubility of such chlorides requires high polarity solvents and indicates that high polar solutes are responsible for inhibiting the growth of some bacterial strains, such as L. acidophilus (gram +ve) and P. aeruginosa (gram −ve), which were more susceptible to the water extract than the ethanol or hexane extracts. However, ethanol extract was more effective against Strep. mutans (gram +ve), which suggests that there are selective semi-polar antimicrobial agents in S. persica.
The water and ethanol solvents have a polarity index of 9 and 5.2, respectively, whereas hexane has a 0 polarity index according to Snyder's polarity index. Metals and ions are expected to dissolve best in water, followed by ethanol, but not in hexane. Nonpolar essential and volatile oils are hydrophobic; therefore, they are more likely to be dissolved in hexane but not in water. Hexane extract had the least inhibition effect on the tested bacterial strains; P. aeruginosa (gram −ve) did not show any inhibition with hexane extract (), whereas E. coli (gram –ve) was susceptible to hexane extract, which suggests that nonpolar solutes also have a selective antimicrobial activity.
5 Conclusions
The results of this study show that S. persica chewing sticks (Miswak) have a broad antimicrobial activity against gram positive and gram negative bacteria and contain more than one antimicrobial agent. Different antimicrobial agents have different polarity and solubility in selected solvents. More detailed investigations are required with fractionation studies using different polar and nonpolar solvents to identify the content of antimicrobial agents in S. persica.
Acknowledgements
The authors are thankful to Dr. Wael Al Sayed from the biology department for his help in obtaining the bacterial strains. We would also like to thank to Dr. Abdul-Aziz Amro from the Chemistry Department at Taibah University for his help in the chemical analysis.
Notes
Peer review under responsibility of Taibah University.
References
- H.S.HalawanyA review on Miswak (Salvadora persica) and its effect on various aspects of oral healthSaudi Dent. J.2420126369
- S.DuttaA.ShaikhThe active chemical constituent and biological activity of Salvadora persica (Miswak)Int. J. Curr. Pharmaceut. Rev. Res.312012 ISSN: 0976-822X
- E.RiggsC.van GemertM.GussyE.WatersN.KilpatrickReflections on cultural diversity of oral health promotion and preventionGlobal Health Promot.1912012606310.1177/1757975911429872
- H.AhmadK.RajagopalBiological activities of Salvadora persica L. (Meswak)Med. Aromat. Plants24201310.4172/21670412.1000129
- J.AkhtarK.SiddiqueS.BiM.MujeebA review on phytochemical and pharmacological investigations of Miswak (Salvadora persica Linn.)J. Pharm. BioAllied Sci.312011113117
- E.MirkamandarM.R.ShakibaieS.AdeliM.MehrabaniM.M.HayatbakhshS.EsmailianIn vitro antimicrobial activity of Salvadora persica extract on Helicobacter pylori strains isolated from duodenal ulcer biopsiesMicrobiol. Res.3120123841
- G.BosThe Miswak, an aspect of dental care in IslamMed. Hist.37119936879
- S.T.EzmirlyJ.C.ChengS.R.WilsonSaudi Arabian medicinal plants: Salvadora persicaPlanta Med.351979191192
- M.KamelK.OhtaniM.AssafLignin glycosides from stems of Salvadora persicaPhytochemistry31199224692471
- G.C.GallettiG.ChiavariPyrolysis/gas chromatography/ion-trap mass spectrometry of the “tooth brush” tree (Salvadora persica L.)Rapid. Commun. Mass. Spectr.71993651655
- H.SherM.NasserL.WijayaEthnobotanical and antibacterial potential of Salvadora persica L.: a well-known medicinal plant in Arab and Unani system of medicineJ. Med. Plants Res.5201112241229
- M.GaziT.DaviesN.Al-BagiehS.CoxThe immediate- and medium-term effects of Meswak on the composition of mixed salivaJ. Clin. Periodontol.1921992113117
- K.AlmasMiswak (chewing stick) and its role in oral healthPostgrad. Dent. Middle East31993214218
- F.A.Al-BayatiA.H.AI-KoubaisiA.Abdul WahidA.M.AmeenEffect of mouth wash extracted from Salvadora persica (Miswak) on dental plaque formation: a clinical trialJ. Med. Plants Res.4201014461454
- N.H.Al-BagiehA.IdowuN.O.SalakoEffect of aqueous extract of Miswak on the in vitro growth of Candida albicansMicrobios Lett.801994107113
- S.H.K.ParsadK.AnthonammaN.JyothirmayiK.D.SowjanyaV.R.L.SharlotteA.PriyankaS.J.MounikaIn vitro assay of herbaceous extracts of Salvadora persica L. against some pathogenic microbesRJPBCS242011860863
- A.SofrataE.M.SantangeloM.AzeemA.-K.Borg-KarlsonA.GustafssonBenzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against gram-negative bacteriaPLoS ONE68201110.1371/journal.pone.0023045
- M.AhmadH.ImranZ.YageenZ.RehmanA.RahmanN.FatimaT.SohailPharmacological profile of Salvadora persicaPak. J. Pharm. Sci.2432011323330
- I.DaroutA.ChristyN.SkaugIdentification and quantification of some potentially antimicrobial anionic components in Miswak extractIndian J. Pharmacol.3220001114
- S.T.EzmirlyM.M.ElnasrIsolation of glucotropaeolin from Salvadora persica L.J. Chem. Soc. Pakistan31981912
- J.A.LemireJ.J.HarrisonR.J.TurnerAntimicrobial activity of metals: mechanism, molecular targets and applicationsNat. Rev. Microbiol.112013371384
- J.M.YarbroughJ.B.RakeR.G.EagonBacterial inhibition of nitrate, inhibition of active transport, but not of group translocation and of intercellular enzymesAppl. Environ. Microbiol.391980831834
- F.AlaliM.HudaibT.AburjaiK.KhairallahN.Al-HadidiGC–MS analysis and antimicrobial activity of the essential oil from the stem of the Jordanian toothbrush tree Salvadora persicaPharmaceut. Biol.4282005577580
- U.NaomiK.ShiroY.SoichiroHelicobacter pylori infection and the development of gastric cancerN. Engl. J. Med.3452011784789
- F.A.Al-BayatiK.D.SulimanIn vitro antimicrobial activity of Salvadora persica L. extracts against some isolated oral pathogens in IraqTurk. J. Biol.3220085762
- D.A.SkoogD.M.WestF.J.HollerFundamentals of Analytical Chemistry7th ed.1996Thomson Learning, Inc.USA
- A.PequerulC.PerezP.MaderoJ.ValE.MongeA rapid wet digestion for plant analysisDev. Plant Soil Sci.53199336
- M.FarooqiJ.SrivastavaThe toothbrush tree (Salvadora persica)J. Crude Drug Res.8196812971299
- A.T.KhalilBenzylamides from Salvadora persicaArch. Pharm. Res.292006952956