Abstract
A simple, rapid, and robust stability-indicating RP-HPLC method has been developed and validated to measure cefixime and dicloxacillin at a single wavelength (225 nm) in order to assay. The samples were isocratically eluted using a Capcell Pak C18 DDS5 column (4.6 mm × 250 mm with a particle size of 5 μm) with a mobile phase consisting of 5 mM phosphate buffer (with a pH adjusted to 5.4 using orthophosphoric acid): acetonitrile:methanol (42:55:03, v/v/v) delivered at a flow rate 1.0 mL min−1. A good linear response was obtained in the range from 0.5 to 25 μg mL−1. The LODs for CFX and CLX were found to be 0.020 and 0.018 μg mL−1, respectively, and the LOQs for CFX and CLX were 0.315 and 0.205 μg mL−1, respectively. The method was quantitatively evaluated in terms of linearity, precision, accuracy (recovery), selectivity and robustness in accordance with standard guidelines. The method is simple, convenient and suitable for analysing cefixime and dicloxacillin in bulk and in pharmaceutical formulations.
1 Introduction
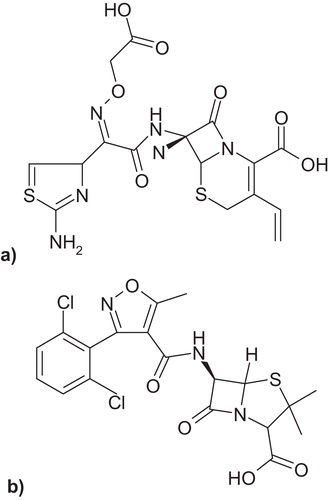
Chemically, cefixime (CFX) is (6R,7R)-7-[[2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethoxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, as shown in a. It provides broad and potent activity against various pathogens, especially gram-negative organisms, when given orally to treat susceptible infections such as gonorrhoea, otitis media, pharyngitis, lower respiratory tract infections and urinary tract infections [Citation1,Citation2].
Dicloxacillin (DLX), (2S,5R,6R)-6-[[3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, is shown in b. It is used to treat infections caused by susceptible gram-positive bacteria [Citation3]. Combination therapy is one choice for treating various bacterial infections.
A survey of the literature revealed that several analytical methods for analysing CFX and DLX as single components or in combination with other drugs (as formulations and in biological samples) have been described. However, very few methods for the simultaneous estimation of CFX and DLX in combination with other antibiotics in biological samples as single drug candidates in biological fluids have been described. The literature survey uncovered reports on analytical methods such as UV–VIS, HPLC, LC–MS, LC–MS–MS and HPTLC for the determination of CFX [Citation3–Citation16] and DLX [Citation3,Citation4,Citation16–Citation29] alone or in combination with other antibiotics (as formulations and in biological samples). In addition, the methods that have been described are not very cost-effective in terms of solvent consumption and total analysis run time; therefore, the present study was conducted. The exhaustive literature survey revealed that none of the most recognized pharmacopoeias and journals include these drugs in combination for the simultaneous determination of CFX and DLX, and information regarding the stability of the drugs is not available. Therefore, it seemed essential to develop a liquid chromatographic procedure that serves as a reliable, accurate and stability-indicating HPLC method for the simultaneous estimation of CFX and DLX in tablet dosage form. The present investigation was conducted with the goal of establishing a simple, rapid, and robust stability-indicating RP-HPLC method for the simultaneous estimation of CFX and DLX in bulk and in tablet form.
2 Experimental
2.1 Chemicals and reagents
All reagents and solvent were of analytical grade; they included hydrochloric acid, monobasic sodium phosphate, monobasic potassium phosphate, sodium acetate trihydrate, orthophosphoric acid and methanol, which were purchased from Merck Ltd., Mumbai, India. High-purity deionized water was obtained using a Millipore, Milli-Q (Bedford, MA, USA) purification system. The cefixime, dicloxacillin (Blok Pharma Pvt. Ltd, Kolhapur), acetonitrile (Merck Chemicals) and all the other chemicals used were of analytical grade. Doubly distilled water was used for preparing the mobile phase solutions. The tablets containing cefixime and dicloxacillin (commercial name: Hifen-LXX 200) were obtained from a local market in Atpadi, Maharashtra (India). The 0.45–l m nylon filters were purchased from Advanced Micro Devices Pvt. Ltd., Chandigarh, India.
2.2 Instrumentation
A Cyberlab-chrom-HPLC V 4.0 isocratic high pressure liquid chromatograph (Cyberlabs, USA) with an LC-P-100 pump, an LC-UV 100 variable wavelength programmable UV/Vis detector and Cyberstore version 4-0512-039 were used. Chromatographic separation was performed by a reverse-phase Capcell Pak C18 DDS5 column (4.6 mm × 250 mm with a particle size of 5 μm).
2.3 Chromatographic conditions
The mobile phase, which consisted of 5 mM phosphate buffer (pH 5.4): acetonitrile:methanol (42:55:03, v/v/v), was degassed and filtered using a Millipore vacuum filter system equipped with a 0.45 μm membrane filter. Chromatography was performed at an ambient temperature by pumping the mobile phase at a flow rate of 1.0 mL min−1. The column's effluence was monitored at 225 nm.
2.4 Preparation of the stock solution
Accurately weighed quantities of CFX and DLX (10 mg) were transferred to a 10.0 ml volumetric flask. Then, a small amount of the mobile phase was added and the result was ultrasonicated for 5 min and diluted up to the mark with the mobile phase to obtain a final concentration of 1000 μg mL−1.
2.5 Preparation of the standard working solution
From the CFX and DLX stock solutions, 1 ml was transferred to a 10 ml volumetric flask by pipetting, and the final volume was made up with the mobile phase to obtain a final concentration of 100 μg mL−1.
2.6 Preparation of the working sample solution
The powder from twenty tablets (Hifen-LXX 200), each containing 200 mg CFX and 500 mg DLX, were weighed. A quantity of powder that was equivalent to 10 mg CFX and 25 mg DLX was placed in each of several 10 mL volumetric flasks that contained approximately 5 mL of the mobile phase for analysis, and the result was sonicated for 15 min. After sonication, the volume was made up to the mark using the same solution to obtain the sample stock solution of CFX (1000 μg mL−1) and DLX (2500 μg mL−1). Then, the solution was filtered using a 0.45 μm membrane filter. The filtrate (0.010 mL) was quantitatively transferred to a 10 mL volumetric flask to obtain final concentrations of 10 μg mL−1 for CFX and 25 μg mL−1 for DLX.
2.7 Method validation
The chromatographic conditions described in the present manuscript were found to be appropriate for the quantitative determination. After the analytical conditions had been optimized, certain parameters such as the linearity, precision, accuracy (recovery), selectivity, and robustness were evaluated to validate the method [Citation30,Citation31].
2.7.1 System suitability test
System suitability tests were performed in accordance with USP 24/NF 19 to confirm that the reproducibility of the equipment was adequate for the analysis [Citation31]. The test was performed before the analysis of each batch of samples to ensure the reproducibility of the chromatographic system. The criteria selected were based on the actual performance of the method, as determined during its validation. These parameters include the relative standard deviation (%RSD) of the retention times, tailing factor, theoretical plate and asymmetry for six repetitions of the injection.
2.7.2 Linearity
The linearity was studied using six concentrations, 0.5, 1, 5, 10, 15, 20 and 25 μg mL−1, of CFX and DLX. The linearity experiment was performed six times to ensure that the detector's response was linear for various drug concentrations (0.5–25 μg mL−1). The working standards were prepared by adding CFX and DLX solutions with different concentrations to obtain concentrations in the required range; then, the solutions were injected into the HPLC system. The calibration curves were constructed by plotting the peak area versus the concentrations of CFX and DLX, and the regression equations were determined.
2.7.3 Accuracy (% recovery)
The precision and accuracy of the HPLC method can often be enhanced, which also corrects fluctuations in the detector's response. A study of the accuracy was conducted using the standard addition method. The pre-quantified sample solutions of CFX (2.00 μg mL−1) and DLX (5.00 μg mL−1) were spiked with an extra 0, 50, 100, and 150% of the standard CFX and DLX solutions, respectively. These mixtures were analyzed using the developed method. The experiment was conducted six times. The percentage recovery of the samples, %RSD and the percentage were calculated for each concentration.
2.7.4 Precision
The intra-day and inter-day precision were estimated for three different concentrations (0.5, 15 and 25 μg mL−1) of CFX and DLX six times on each of three days to obtain the relative standard deviation (%RSD).
2.7.5 The LOD and the LOQ
Several approaches for determining the detection (LOD) and quantification (LOQ) limits are described in the ICH guidelines. In this study, the LOD and the LOQ were based on the standard deviation of the response and the slope using the signal-to-noise ratio in accordance with ICH guidelines [Citation30].
2.7.6 Robustness
The robustness of the developed method was measured to evaluate the influence of a small but deliberate variation in the chromatographic conditions. The robustness of the method was measured by changing the flow rate (0.9 and 1.1 mL min−1) of the mobile phase, the pH (5.3 and 5.5) of the phosphate buffer and the amount (41% and 43%) of the buffer in the mobile phase.
2.7.7 Selectivity
The selectivity was verified by comparing the chromatograms obtained for the standard, the sample and the corresponding placebo.
2.8 Forced degradation
The International Conference on Harmonization (ICH) guideline entitled “Stability Testing of New Drug Substances and Products” requires that stress testing be conducted to elucidate the inherent stability characteristics of an active substance [Citation30]. The aim of this work was to study the stress degradation of CFX and DLX using the proposed method [Citation32,Citation33].
2.8.1 Hydrolytic degradation under acidic conditions
Using 20 μg mL−1 and 50 μg mL−1 stock solutions of CFX and DLX, respectively, 5 mL of a stock solution and 1 mL of 0.1 N HCl were added to a 10 mL volumetric flask. Then, the volumetric flask was kept under 60–70 °C reflux conditions for 4 h and neutralized with 0.1 N NaOH and 10 mL of a diluent to obtain 10 μg mL−1 and 25 μg mL−1 concentrations of CFX and DLX, respectively. The solution was cooled to room temperature, filtered using 0.45 μm syringe filters and placed in the vials of the HPLC system.
2.8.2 Hydrolytic degradation under alkaline conditions
Using 20 μg mL−1 and 50 μg mL−1 stock solutions of CFX and DLX, respectively, 5 mL of a stock solution and 1 mL of 0.1 N NaOH were added to a 10 mL volumetric flask. Then, the volumetric flask was kept under 60–70 °C reflux conditions for 4 h and neutralized with 0.1 N HCl. Then, 10 mL of a diluent was added to obtain 10 μg mL−1 and 25 μg mL−1 concentrations of CFX and DLX, respectively. The solution was cooled to room temperature, filtered using 0.45 μm syringe filters and placed in the vials of the HPLC system.
2.8.3 Oxidative degradation
Using 20 μg mL−1 and 50 μg mL−1 stock solutions of CFX and DLX, respectively, 5 mL of a stock solution and 1 mL of 3% (w/v) of hydrogen peroxide were added to a 10 mL volumetric flask. The volumetric flask was then kept under 60–70 °C reflux conditions for 1 h, and the volume was made up to the mark with diluents to obtain final CFX and DLX concentrations of 10 μg mL−1 and 25 μg mL−1, respectively. The solution was cooled to room temperature, filtered using 0.45 μm syringe filters and placed in the vials of the HPLC system.
2.8.4 Thermal induced degradation
Using 20 μg mL−1 and 50 μg mL−1 stock solutions of CFX and DLX, respectively, 5 mL of a stock solution was added to a 10 mL volumetric flask. Then, the volumetric flask was kept at 60–70 °C in a hot air oven for 24 h, and the volume was made up to the mark with diluents to obtain 10 μg mL−1 and 25 μg mL−1 concentrations of CFX and DLX, respectively. The solution was cooled to room temperature, filtered using 0.45 μm syringe filters and placed in the vials of the HPLC system.
2.8.5 Photo degradation
Using 20 μg mL−1 and 50 μg mL−1 stock solutions of CFX and DLX, respectively, 5 mL of a stock solution was added to a 10 mL volumetric flask. The samples were transferred to petri dishes and kept in a photo stability chamber (200 W h/m2 of UV light and 1.2 million l × h of visible light) for 7 days. Then, the volumetric flask was made up to the mark with diluents to obtain 10 μg mL−1 and 25 μg mL−1 concentrations of CFX and DLX, respectively. The solution was cooled to room temperature, filtered using 0.45 μm syringe filters and placed in the vials of the HPLC system.
3 Results and discussion
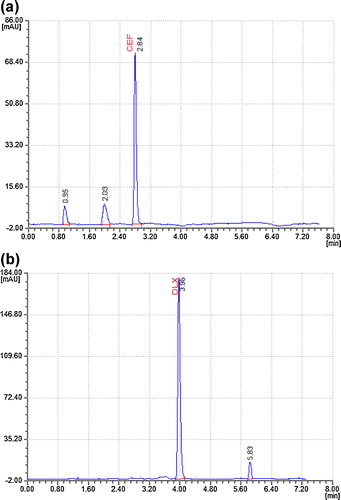
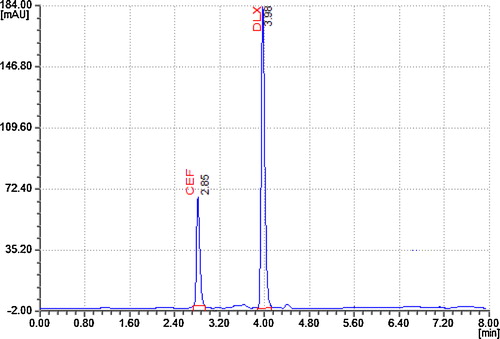
To optimize the RP-HPLC parameters and reach a good resolution and a peak shape for CFX and DLX, several chromatographic conditions were tested. Several mobile phases of different compositions were tested to find one that provided sufficient selectivity for the drugs. The phosphate buffer provided a higher sensitivity and selectivity than other buffers did. Using methanol and acetonitrile as organic components resulted in higher sensitivity, but varying the amounts of methanol and acetonitrile in the mobile phase affected the resolution and run time. Varying the pH of the mobile phase resulted in poor peak shapes; therefore, we introduced orthophosphoric acid into the mobile phase to adjust the pH of the buffer to 5.4. The optimized mobile phase consisted of 5 mM phosphate buffer (pH 5.4): acetonitrile:methanol (42:55:03, v/v/v). The column's effluence was monitored at 225 nm. The optimal injection volume was 10 μL. The column's temperature was maintained at 25 °C (ambient). The Capcell Pak C18 DDS5 column (4.6 mm × 250 mm with a particle size of 5 μm) was used in its isocratic mode with a flow rate of 1 mL min−1. Retention times of approximately 2.85 ± 0.06 and 3.98 ± 0.07 min were consistently observed for CFX and DLX, respectively, in all the analytical runs ().
Fig. 2 A typical chromatogram of a tablet sample solution containing 10 μg mL−1 of CFX and 25 μg mL−1 of DLX.
3.1 Validation of the proposed method
3.1.1 System suitability test
The chromatographic systems used in this analysis must conform to the system suitability limits before sample analysis can commence [Citation31]. The tailing factor (T), theoretical plate number (N), retention time (RT) and asymmetry factor (As) for the principal peak and its degradation product were evaluated using CFX and DLX concentrations of 10 mg mL−1 and 25 mg mL−1, respectively. Typically, at least two of these criteria must be satisfied to demonstrate a system's suitability for the proposed method. Some of the tests were conducted using fresh standard solutions that included drug compounds. The tailing factors were 0.5411 ± 0.0204 for CFX and 0.7340 ± 0.0281 for DLX. The theoretical plate numbers (N) were 5237.50 ± 133.8800 for CFX and 4099.83 ± 354.7429 for DLX. The chromatographic conditions described ensured the adequate retention and asymmetry of the drug compounds. The retention times of the drug CFX and DLX were 2.8416 ± 0.0116 and 3.9433 ± 0.0136 min, respectively. The asymmetry factors were found to be 0.7058 ± 0.0227 for CFX and 0.9286 ± 0.0459 for DLX. The variation in the retention time for six replicate injections of the drug compounds resulted in %RSDs of 0.4113% for CFX and 0.3464% for DLX. The results obtained from the system suitability tests () satisfied the USP and ICH standards [Citation30,Citation31].
Table 1 System suitability test parameter for CFX and DLX having concentration 10 μg mL−1 and 25 μg mL−1.
3.1.2 Specificity
There was no interference from impurities, excipients or additives. The additives in the tablets were practically insoluble in the mobile phase, and the active constituents were soluble.
3.1.3 Linearity
A linear correlation was obtained between the peak area used and the absorbance Vs concentrations of CFX and DLX; the calibration curves were linear for concentrations between 0.5 and 25 μg mL−1. The linearity of the calibration curves was validated by the values of the regression correlation coefficients (r2). The correlation coefficients were 0.9999 for CFX and 0.9999 for DLX. The results of the linearity experiment are listed in .
Table 2 Linear regression analysis of calibration curves (n = 6).
3.1.4 Accuracy (% recovery)
The accuracy experiments were conducted using the standard addition method. The proposed method afforded a recovery of 97.28–100.51% after the additional standard drug solution was spiked with the previously analyzed test solutions. The recovery percentages for CFX and DLX were in the ranges from 97.28 to 100.48% and from 99.53 to 100.51%, respectively. The values of the recovery (%) and %RSD are shown in ; they express the accuracy of the proposed method.
Table 3 Accuracy (% Recovery) determined with developed method (n = 6).
3.1.5 Precision
The intra-day precision of the method ranged from 0.3079 to 2.006%RSD for CFX and DLX. The inter-day precision of the method was found to be between 0.2833 and 2.8293%RSD for CFX and DLX, which indicate that the developed method is precise (). The low values of the RSD (%) indicate that the proposed method is repeatable.
Table 4 Inter-day and intra-days precision of CFX and DLX standards.
3.1.6 LOD and LOQ
The LODs for CFX and CLX were found to be 0.020 and 0.018 μg mL−1, respectively, and the LOQs for CFX and CLX were 0.315–0.205 μg mL−1, respectively ().
3.1.7 Robustness
Deliberate changes in the method, i.e., changes in the composition of the mobile phase, the flow rate and the pH of the phosphate buffer, did not significantly affect the peak tailings, the theoretical plates or the percent assays of CFX and DLX. The results are shown in .
Table 5 Robustness study.
3.2 Stability indication
The ICH guideline entitled “Stability Testing of Drug Substances and Products” requires that stress testing be conducted to elucidate the inherent stability characteristics of an active substance and to rapidly identify differences that might result from changes in the manufacturing process or the sample's source [Citation32]. Formulation drug products were exposed to thermal stress, hydrolytic stress under acidic and basic conditions, oxidative stress, and photolytic stress. An ideal stability-indicating method is one that quantifies the standard drug alone and also resolves its degradation products. As described in Section 2, different types of stress were used: boiling, acid, base hydrolysis, oxidation, and irradiation with UV light. During the degradation study, CFX was degraded in comparison to DLX under all stress conditions. Although unknown degradant peaks were observed in the acid, base, peroxide, photolytic and thermal study, no degradant peaks were reported in the retention time of CFX or DLX. Therefore, CFX and DLX are stable up to specified period (4 h) when the proposed method is used, or they are susceptible to acids, alkali, heat, hydrogen peroxide and photolytic stress.
3.2.1 Degradation of CFX and DLX in 0.1 N HCl at 60–70 °C for 4 h under reflux conditions
The results showed multiple peaks for the degradation products. Major degradation peaks were found at 0.95 and 2.03 min for CFX and at 5.83 min for DLX in both drug products and drug substances. CFX and DLX peaks were observed at retention times of 2.84 min and 3.96 min, respectively. The % drug degradations observed were 10.80% and 3.34% for CFX and DLX, respectively (). No degradant peaks were reported in the retention time of CFX or DLX (a and b).
Table 6 % Degradation of CFX and DLX in different conditions.
3.2.2 Degradation of CFX and DLX in 0.1 N NaOH at 70 °C for 4 h under reflux conditions
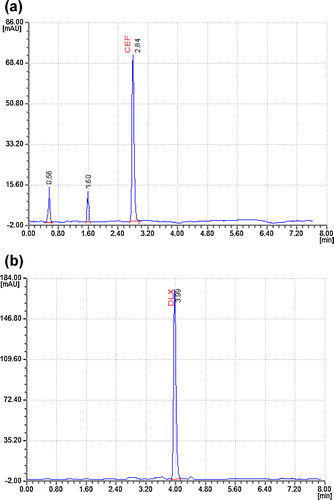
The results showed two peaks, at retention times of 1.35 and 2.54 min, for CFX in both drug products and drug substances. The % drug degradations observed for CFX and DLX were 16.87% and 11.26%, respectively (). This shows that DLX is much more prone to alkaline hydrolysis than CFX, and significant degradation of both drugs was observed. No degradant peaks were reported in the retention time of CFX or DLX (a and b).
3.2.3 Oxidation degradation of CFX and DLX in 3% H2O2 at 70 °C for approximately 1 h under reflux conditions
The sample and drug substances were treated with a 3% solution of hydrogen peroxide and kept in a water bath at 60–70 °C under reflux conditions for approximately 1 h. Major degradation peaks were found at 0.56 and 1.60 min for CFX in drug substances and drug products. The observed % degradations of CFX and DLX were 20.99 and 14.45%, respectively (). Therefore, note that CFX exhibited the maximum amount of degradation under peroxide degradation conditions. No degradant peaks were reported in the retention time of CFX or DLX (a and b).
3.2.4 Thermal degradation of CFX and DLX at 60 °C for approximately 24 h
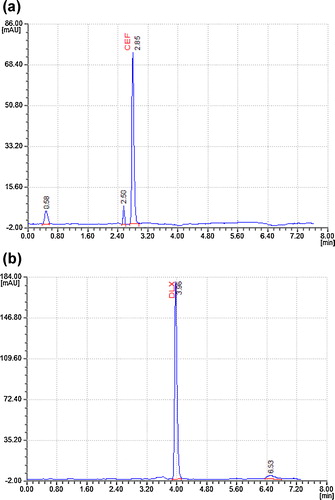
The thermal degradation of CFX and DLX at 60 °C for approximately 24 h in a hot air oven was studied. Degradation peaks were found at 0.88 and 2.05 min for CFX in drug products. The % degradations of CFX and DLX were found to be 3.45 and 2.28%, respectively (). No degradant peaks were reported in the retention time of CFX or DLX (a and b).
3.2.5 Photolytic degradation of CFX and DLX
The sample and drug substances were exposed to the energy of a 1.2 million lux h fluorescent light and to 200 W/m2 of UV light for approximately 7 days. Minor degradation peaks were at 0.58 min and 2.50 min for CFX and at 6.53 min for DLX in both drug substances and drug products. The % degradations of CFX and DLX were found to be 7.26 and 4.72%, respectively (). CFX showed the least degradation under photolytic conditions. No degradant peaks were reported in the retention time of CFX or DLX (a and b).
4 Conclusion
The proposed RP-HPLC method is accurate, precise, rapid, robust, sensitive and selective. The prescribed method adapted the use of an economical and easily available mobile phase, a UV detector, and easy extraction procedures. Washing the column with the same mobile phase made it an excellent method for the quantification of CFX and DLX in bulk drugs and in their pharmaceutical dosage forms. A stability-indicating RP-HPLC method for the estimation of CFX and DLX in their solid dosage forms was established and validated in accordance with the ICH guidelines. The forced degradation experiment and the peak purity data confirmed that there was no merging of the peaks of the active ingredients with those of any other degradation products or other additives. The developed method can be used in routine analyses of drugs in bulk and in different formulations and could help in therapeutic drug monitoring (TDM) and bioavailability studies.
Acknowledgments
The authors are grateful to Prof. Kishore Singh C., President and Prof. Hariprasanna R. C., Principal, RMES's College of Pharmacy, Gulbarga (Karnataka) and Dr C. S. Magdum, Principal, Rajarambapu College of Pharmacy, Kasegaon for providing the facilities necessary for conducting this study.
Notes
Peer review under responsibility of Taibah University.
References
- Martindale: The extra Pharmacopoeia38th ed.2014Royal Pharmaceutical SocietyLondon
- S.D.BhingeS.M.MalipatilL.V.SonawaneR.C.HariprassanaSimultaneous estimation of cefixime and cloxacillin in bulk and tablet formulation by RP-HPLC methodIndian Drugs4920122329
- M.V.DhokaS.J.SandageS.C.DumbreSimultaneous determination of cefixime trihydrate and dicloxacillin sodium in pharmaceutical dosage form by reversed-phase high-performance liquid chromatographyJ. AOAC Int.932010531535
- S.D.BhingeS.M.MalipatilL.V.SonawaneBioanalytical method development and validation for simultaneous estimation of cefixime and dicloxacillin in human PlasmaActa Chim. Slov.612014582586
- D.ZendelovskaT.StafilorP.MilosevskiHigh-performance liquid chromatographic method for determination of cefixime and cefotoxime in human plasmaBull. Chem. Tech. Macedo.2220033945
- M.FangC.XiaoyanZ.YalinZ.DafangSensitive liquid chromatography–tandem mass spectrometry method for the determination of cefixime in human plasma: application to a pharmacokinetic studyJ. Chromatogr. B8192005277282
- L.O.WhiteD.S.ReevesA.M.LoveringA.P.MacGowanHPLC assay of cefixime in serum and CSFJ. Antimicrob. Chemother.311993450451
- C.M.NahataMeasurement of cefixime in serum and cerebrospinal fluid by High-Performance Liquid ChromatographyJ. Liq. Chromatogr.14199137553759
- G.L.LiuR.G.ShaS.GaoY.X.ShenS.X.WangDetermination of cefixime in human plasma and urine using high performance liquid chromatography column switching techniqueYao Xue Xue Bao281993216221
- J.A.McAteerM.F.HiltkeS.B.MichaelD.R.FaulknerLiquid-chromatographic determination of five orally active cephalosporins-cefixime, cefaclor, cefadroxil, cephalexin and cephradine in human serumClin. Chem.33198717881790
- A.KhanZ.IqbalM.I.KhanJ.KhalidA.KhanA.LateefY.ShahN.FazliSimultaneous determination of cefdinir and cefixime in human plasma by RP-HPLC/UV detection method: method development, optimization, validation, and its application to a pharmacokinetic studyJ. Chromatogr. B879201124232429
- E.NemutluS.KirD.KatlanM.S.BeksacSimultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: application to validation and quantification of cefepime, cefixime and cefoperazoneTalanta802009117126
- S.E.JovanovicD.AgbabaD.Zivanov-StakieS.VladimirovHPTLC determination of ceftriaxone, cefixime and cefotaxime in dosage formsJ. Pharm. Biomed. Anal.181998893898
- G.RathinavelP.B.MukherjeeJ.ValarmathyL.SamueljoshuaM.GaneshT.SivakumarT.SaravananA validated RP-HPLC method for simultaneous estimation of cefixime and cloxacillin in tabletsE J. Chem.52008648651
- A.R.WankhedeP.Y.MaliV.KarneA.R.KhaleC.S.MagdumDevelopment and validation of RP-HPLC method for simultaneous estimation of cefixime and cloxacillin in tablet dosage formInt. J. Pharm. Biol. Arch.12012317320
- K.KathiresanR.MuruganS.M.HameedK.G.InimaiT.KanyadharaAnalytical method development and validation of cefixime and dicloxacillin tablets by RP-HPLCRasayan J. Chem.22009588592
- T.G.BarotK.PatidarN.KshartriN.VyasDevelopment and validation of LC method for the determination of ampicillin and dicloxacillin in pharmaceutical formulation using an experimental designE J. Chem.62009955964
- L.K.SorensenL.K.SnorT.ElkaerH.HansenSimultaneous determination of seven penicillins in muscle, liver and kidney tissues from cattle and pigs by a multiresidue high-performance liquid chromatographic methodJ. Chromatogr. B7341999307318
- K.TakebaK.FujinumaT.MiyazakiH.NakazawaSimultaneous determination of b-lactam antibiotics in milk by ion pair liquid chromatographyJ. Chromatogr. A8121998205211
- G.M.GehadF.A.N.EL-DienU.F.EmanSpectrophotometric study of the reaction mechanism between DDQ as π-acceptor and potassium iodate and flucloxacillin and dicloxacillin drugs and their determination in pure and in dosage formsSpectrochim. Acta A6520061119
- F.A.N.EL-DienG.M.GehadY.Z.A.EmanSpectrophotometric determination of flucloxacillin and dicloxacillin in pure and dosage formsSpectrochim. Acta A642006210215
- M.A.EzzatA.A.MohammadA.G.El-RasheedA.K.NashaatSimultaneous determination of amoxycillin and dicloxacillin in capsules by potentiometric titrimetry and High-Performance Liquid ChromatographyTalanta401993811817
- D.V.C.AwangG.LauriaultD.KindackHigh performance liquid chromatographic determination of dicloxacillin in presence of its degradation productsJ. Chromatogr.2831984449452
- P.S.RaoT.J.SunderRajC.H.BhrtiK.RangaRaoG.K.A.NarayanK.ParikhIdentification and characterization of degradation products of dicloxacillin in bulk drug and pharmaceutical dosage formsJ. Pharm. Biomed. Anal.43200714701475
- A.OscarF.G.DinoraL.M.D.RiveroC.T.NellyLiquid chromatographic assay for dicloxacillin in plasmaJ. Chromatogr. B8052004353356
- L.K.SorensenL.K.SnorT.ElkaerH.HansenSimultaneous determination of seven penicillins in muscle, liver and kidney tissues from cattle and pigs by a multiresidue HPLC methodJ. Chromatogr. B7341999307318
- L.K.SorensenB.M.RasmussenJ.O.BoisonL.KengSimultaneous determination of six penicillins in cow's raw milk by a multiresidue high-performance liquid chromatographic methodJ. Chromatogr. B6941997383391
- S.LihlM.PetzHPLC determination of oxacillin, cloxacillin and dicloxacillin in bovine muscle with automated cleanup by on-line solid phase extractionZ. Lebensm Unters Forsch J.1991994229234
- http://www.shvoong.com/medicine-and-health/1608689-rp hplc determination amoxicillin dicloxacillin.
- Q2A ICH Guideline, Validation of Analytical Procedures Text Methodology2003
- The United States Pharmacopoeia 35 National formulary 30, Easton; Rand Mc Nally Taunton; 2012.
- C.H.BhirudS.N.HiremathStability indicating RP-HPLC method for the determination of Atazanavir sulphate in bulk and dosage formDrug Invent. Today520138186
- S.K.JansariN.B.PatelP.R.Patelet alDevelopment and validation of stability indicating method for simultaneous estimation of ciprofloxacin HCl and tinidazole using RP-UPLC methodIOSR J. Pharm.2520121219