Abstract
A series of new calamitic liquid crystals, 4-[3-(pyridin-4-yl)prop-2-enoyl]phenyl 4-alkyloxybenzoates, comprising a pyridyl core, ester–chalcone central linkage and terminal alkyloxy chain were synthesized and characterized. This series consists of four members that differ by the length of the alkyloxy chain (CnH2n+1O–, where n = 10, 12, 14, 16). The structures of the title compounds were elucidated using spectroscopic and spectrometric techniques, such as FT-IR, NMR (1H and 13C) and EI-MS. The mesomorphic properties were studied using differential scanning calorimetry and optical polarizing microscopy. The decyloxy-containing compound was found to be non-mesogenic, whilst the compounds containing n-dodecyloxy to n-hexadecyloxy chains exhibited an enantiotropic smectic A phase with a fan-shaped texture. From the structure–property relationship study, it was proposed that the number of carbons in the alkyloxy chain must be at least 12 (n ≥ 12) to generate the smectic phase in the corresponding substituted ArCOOArCOCH=CHC5H4N compounds.
Keywords:
1 Introduction
The electronically conducting behaviour of liquid crystals (LCs) has received overwhelming attention since the discovery of high hole mobility in the hexagonal columnar phase of hexapentyloxy-triphenylene liquid crystals [Citation1–Citation5]. Recently, calamitic LCs have been identified as another potential material for application in electronic devices due to the presence of some ordered mesophases, such as SmA, SmC, SmB, SmE, among others. The two-dimensional arrangement (i.e., layers) in smectics is more tolerant with regard to defects than the one-dimensional ordered discotic columns, resulting in enhanced electronic performance [Citation6]. The electronically conducting behaviour of calamitic liquid crystals was first investigated in the SmA phase of 2-phenylbenzothiazole derivatives. The presence of layer structures in all smectic liquid crystalline materials induces π–π stacking interactions that can facilitate the hopping of electrons, thereby increasing charge carrier mobility [Citation7–Citation9].
Our previous work focused on the synthesis of a few homologous series of smectic LCs containing a benzothiazole moiety [Citation10–Citation13]. Incorporation of a benzothiazole ring into calamitic liquid crystals as a mesogenic core enabled the resulting molecules to easily exhibit mesophases due to the more polarizable nature of the heteroatoms sulphur and nitrogen [Citation14]. In this paper, we report a new homologous series of heterocyclic liquid crystals comprising a pyridyl unit (). Introduction of a nitrogen atom into the aromatic core results in a more complex mesomorphic behaviour. In a few cases, highly ordered smectic phases (e.g., G, H) are observed at or near room temperature, which are properties that are of interest, if the materials also possess useful charge mobilities for application in real devices.
Liquid crystals containing a chalcone central linkage are relatively rare. In the literature, there are several reports of mesogenic compounds having chalcone linkages. Many years ago, Chudgar and Shah [Citation15] and Yeap et al. [Citation16] reported a homologous series of mesogenic compounds containing ester-chalcone linkages. Recently, Thaker et al. [Citation17] also synthesized mesomorphic compounds containing a Schiff base–chalcone linkage. The emergent scientific interest in the synthesis of heterocyclic liquid crystals and the outstanding liquid crystalline behaviour of chalcone-containing compounds has prompted us to synthesize a series of mixed pyridyl–chalcone liquid crystals. The presented molecules consist of three core units (one pyridyl and two phenyl rings) connected via enone and ester linkers that can increase the molecular broadness and anisotropy.
2 Experimental
2.1 Characterization
Infrared spectroscopy was performed on a Perkin-Elmer System 2000 FT-IR Spectrometer. All compounds were analyzed using KBr discs with a measurement range of 4000 to 400 cm−1. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded in CDCl3 using a JEOL LA-400 MHz NMR spectrometer with tetramethylsilane as the internal standard. EI-MS (70 eV) was measured with a Finnigan MAT95XL-T Mass Spectrometer at a source temperature of 200 °C.
Phase-transition temperatures and associated enthalpy changes were measured using a Mettler Toledo Differential Scanning Calorimeter DSC823e at heating and cooling rates of 10 °C/min and −10 °C/min, respectively. A polarizing optical microscope (Carl Zeiss) equipped with a Linkam hotstage was used for temperature dependent studies of the liquid crystal textures. A video camera (Video Master coomo20P) installed on the microscope was attached to a video capture card (Video Master coomo600), allowing real-time video capture and image saving. The textures exhibited by the compounds were observed using polarized light with crossed polarizers. Samples were prepared as thin films sandwiched between a glass slide and a cover slip. Phase identification was made by comparing the observed textures with those reported in the literature [Citation18,Citation19].
2.2 Synthesis
All solvents and reagents were purchased commercially and used without any further purification. 4-Dimethylaminopyridine (DMAP), 4-hydroxyacetophenone, pyridine-4-carbaldehyde, 1-bromoalkane (CnH2n+1Br, where n = 10, 12, 14, 16), potassium hydroxide and potassium carbonate were obtained from Merck (Germany). Ethyl 4-hydroxybenzoate and N,N′-dicyclohexylcarbodiimide (DCC) were purchased from Acros Organics (USA).
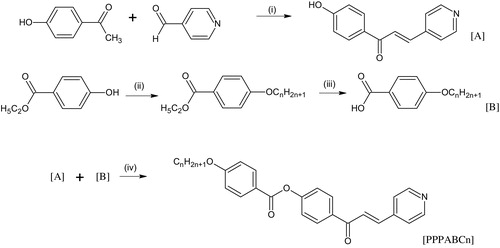
The synthetic route for the formation of the title compounds, 4-[3-(pyridin-4-yl)prop-2-enoyl]phenyl 4-alkyloxybenzoates (PPPABCn, where n = 10, 12, 14, 16), is illustrated in . Intermediate compounds 1-(4-hydroxyphenyl)-3-(pyridin-4-yl)prop-2-en-1-one and 4-alkyloxybenzoic acid were prepared according to the method described by Ha and Low [Citation20] and Kadkin et al. [Citation21], respectively.
2.2.1 Synthesis of mixed pyridyl–chalcone liquid crystals
1-(4-Hydroxyphenyl)-3-(pyridin-4-yl)prop-2-en-1-one (2 mmol, 0.45 g), the appropriate 4-alkyloxybenzoic acid (2 mmol) and DMAP (1 mmol, 0.12 g) were dissolved in a 10-mL mixture of DCM and DMF, and the solution was stirred at 0 °C. DCC (2 mmol, 0.41 g) dissolved in 10 mL of DCM was added to the mixture dropwise, and the solution was continuously stirred for an hour at 0 °C. The mixture was then stirred at room temperature for 48 h. Finally, the mixture was filtered and the solvent was removed by evaporation. All of the crude products were purified by repeated recrystallizations using a 2:1 ratio of methanol and chloroform until constant melting points were obtained. The purity of all of the compounds was verified by thin layer chromatography (Merck 60 F254) and visualized under short-wave UV light.
The IR, NMR (1H and 13C) and mass spectral data for the representative compound, PPPABC12, are summarized as follows.
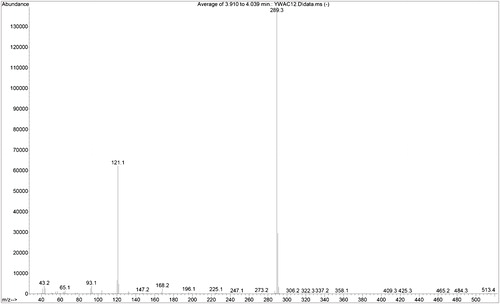
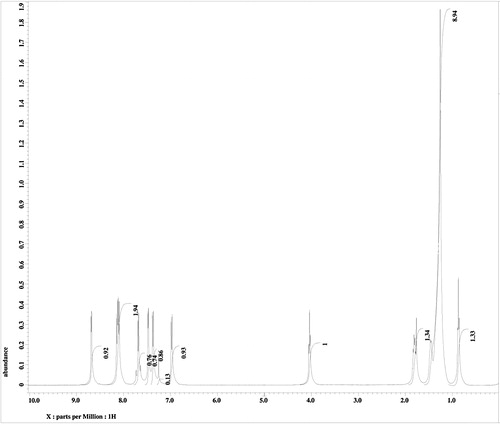
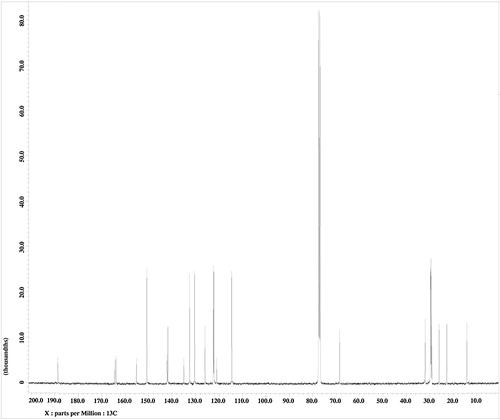
4-[3-(Pyridin-4-yl)prop-2-enoyl]phenyl 4-dodecyloxybenzoates (PPPABC12): IR νmax (KBr, cm−1): 2922, 2852 (C–H aliphatic); 1728 (C=O ester); 1662 (C=O keto), 1273 (C–O, aromatic ether). 1H NMR (400 MHz, CDCl3, δppm): 0.9 (t, 3H, CH3–), 1.3–1.5 (m, 18H, CH3–(CH2)9–(CH2)2–O–), 1.8 (p, 2H, –CH2–CH2–O–), 4.0 (t, 2H, –CH2–O–), 7.0 (d, 1H, olefinic–H), 7.4 (d, 2H, Ar–H), 7.5 (d, 1H, olefinic), 7.6–7.7 (m, 4H, Ar–H), 8.1–8.2 (m, 4H, Ar–H), 8.7 (d, 2H, Ar–H). 13C NMR (100 MHz, CDCl3, δppm): 14.23 (CH3–), 22.79, 26.06, 29.44, 29.68, 32.01 for methylene carbons (CH3–(CH2)10–), 64.49 (–CH2O–), 122.38, 141.77 for olefinic carbons, 114.52, 120.94, 122.13, 125.94, 130.37, 132.52, 134.96, 142.11, 150.75, 155.21, 163.95 for aromatic carbons, 164.45 (–COO–), 188.71 (C=O keto). EI-MS m/z (rel. int. %): 513 (<1) [M+], 289 (100).
3 Results and discussion
3.1 Synthesis and spectroscopic studies
The synthetic route used for the preparation of PPPABCn is shown in . The Claisen–Schmidt condensation method was used in the reaction of 4-hydroxyacetophene and pyridine 4-carbaldehye to yield intermediate A. Etherification of ethyl-4-hydroxybenzoate in acetone with appropriate 1-bromoalkane and acidification using concentrated hydrochloric acid afforded intermediate B. Intermediates A and B were then subjected to Steglich esterification in the presence of DCC and DMAP to yield the title compounds PPPABCn.
Scheme 1 Synthetic route for the target compounds. (i) KOH dissolved in EtOH:H2O (1:1), 2 M HCl (ii) CnH2n+1Br, K2CO3, CH3COCH3 (iii) H2O:EtOH (1:1), KOH, conc. HCl (iv) DCC, DMAP, DCM, DMF.
The structure of the title compound was elucidated using EI-MS, IR and NMR spectroscopic techniques. shows the EI-MS spectrum of representative compound PPPABC12. The prominent molecular ion peak at 513 m/z in the mass spectrum established its molecular formula as C33H39NO4, supporting the proposed structure.
Infrared data of PPPABC12 show the absorption bands assignable to the stretching of aliphatic C–H bonds in the frequency range of 2852 to 2946 cm−1. The band appearing at 1728 cm−1 can be ascribed to the stretching of the C=O bond belonging to the ester group. Another C=O bond, observed at a lower frequency of 1662 cm−1, is attributed to the keto group. Formation of the aromatic ether bond (C–O–Ar) was confirmed based on the absorption band at 1273 cm−1.
and show the 1H and 13C NMR spectra, respectively, for PPPABC12. The 1H NMR spectrum of PPPABC12 shows two triplets assignable to the methyl (CH3) group of the ether linkage and methylene protons attached to the ether oxygen (–CH2–O–) at δ 0.9 and δ 4.0, respectively. Triplet splitting was observed due the presence of two neighbouring protons in both cases. The other methylene protons of CH3–(CH2)9–(CH2)2–O– group in the alkyl chain were confirmed by the presence of multiplet signals at δ = 1.3–1.5. A pentet signal assignable to the methylene protons of the CH2CH2CH2O– group appears at δ 1.8. The presence of two olefinic protons is confirmed with the presence of doublets at chemical shifts of δ 7.0 and δ 7.4. Resonances attributable to the eight aromatic and four pyridyl protons were observed in the range of δ 7.4–8.7.
The molecular structure of PPPABC12 was further substantiated by 13C NMR spectroscopy. The presence of the C=O ester group can be corroborated by the 13C signal observed at δ 164.45. The most downfield signal at δ 188.71 is assigned to the C=O of the enone group. Signals attributable to the olefinic carbons can be found δ 122.38 and 141.77. The peaks belonging to the aromatic and pyridyl carbons gave 13C signals between δ 114.52 and δ 163.95. The methylene carbon attached to the ether oxygen (–CH2O–) possessed the highest chemical shift (δ 64.49) among the methylene carbons. The remaining carbons on the alkyl chain exhibited signals between δ 14.23 and δ 32.01.
3.2 Mesomorphic behaviour
The mesophases of all of the compounds were observed under an optical polarizing microscope during heating and cooling cycles. The optical photomicrographs of PPPABC12 and PPPABC14 are depicted in as a representative illustration. Upon cooling from an isotropic liquid, the co-existence of fan-shaped and homeotropic (dark area) textures of the smectic A (SmA) phase was observed for PPPABC12 (a) and PPPABC14 (b). All observed liquid crystalline textures are typical according to the literature [Citation18,Citation19].
The phase transition temperatures and corresponding enthalpy changes of the compounds PPPABCn were determined by differential scanning calorimetry (DSC). The data obtained from the DSC analysis are summarized in . The DSC thermogram () of PPPABC14 shows two endotherms upon heating that can be attributed to the isotropic–mesophase and mesophase–crystal transitions. Such transitions were also supported by the enthalpy values of the respective compounds. It is clear from that PPPABC14 and PPPABC16 exhibited enantiotropic properties, as the mesophases were observed during both heating and cooling cycles. All of the compounds exhibited a smectic A (SmA) phase, except for the n-decyloxy derivative (i.e., PPPABC10).
Fig. 4 Optical photomicrographs (100×) of (a) PPPABC12 and (b) PPPABC14 exhibiting smectic A phase with fan-shaped and homeotropic textures.
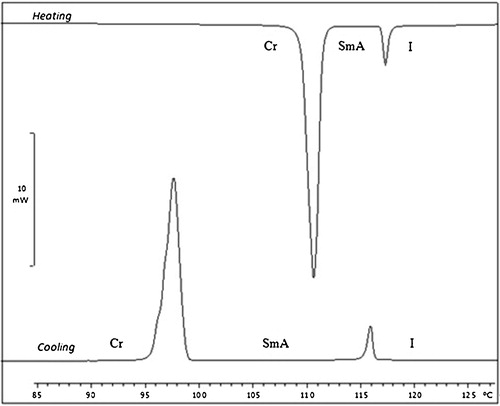
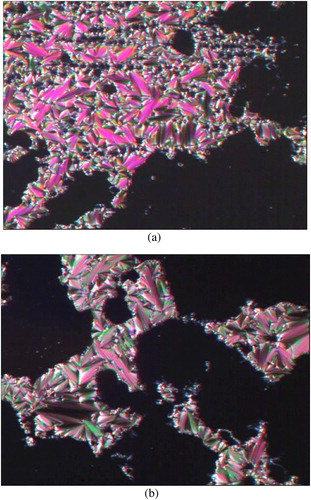
Table 1 Phase transition and transition enthalpy changes for PPPABCn upon heating and cooling.
The linking group is one of the determining factors that influences whether the target compound will exhibit a smectic phase instead of a nematic phase. The ester linking group generally favours lamellar packing due to the dipole–dipole interaction, which ultimately generates the smectic phase [Citation22]. In the case of PPPABCn, conjugation over the entire molecule was achieved between the enone linking group and pyridyl core system, which led to expression of the smectic phase. This suggests that the heteroatom of pyridyl group at the terminal position has a substantial effect on influencing certain phases. It has been reported that the inclusion of heteroatoms can significantly change the polarity, polarisability and occasionally the geometric shape of the molecule, thus influencing the type of mesophase, phase transition temperatures, dielectric and other properties of the mesogens [Citation14]. Because of the presence of nitrogen in the pyridyl ring at the terminal position, these compounds may also serve as a new type of donor group for hydrogen bonding studies (e.g., –COOH⋯N–) with acceptor molecules (for example, 4-alkyloxybenzoic acids) to form new binary liquid crystals [Citation23–Citation26].
As the length of the terminal chain increased, the phase changed from non-mesogenic to enantiotropic smectic A phase. The smectic character was absent in PPPABC10, but was present in the compounds containing longer alkyl chains (PPPABC12, 14, 16). PPPABC10, containing an n-decyloxy chain, is too rigid to be mesogenic, while longer terminal alkyloxy chains may favour a lamellar arrangement in smectic layer structures due to higher Van der Waals interactions and the possibility of intertwining between alkyloxy chains. It can therefore be proposed that to generate the smectic phase in the substituted ArCOOArCOCH=CHC5H4N compounds, the number of carbons in the alkyloxy chain (CnH2n+1O–) must be at least 12 (n ≥ 12). Lastly, the SmA phase range (ΔSmA) was reduced as the chain length increased from C12 to C16, which could be due to dilution of the mesogenic core [Citation27,Citation28].
4 Conclusion
In this paper, we have described the synthesis and mesomorphic behaviour of a new homologous series of 4-[3-(pyridin-4-yl)prop-2-enoyl]phenyl 4-alkyloxybenzoates comprising an ester–chalcone central linkage and a pyridyl core. Members of the homologous series containing C12 alkyl chains or longer (i.e., C14 and C16) exhibited liquid crystalline behaviour in which the SmA phase was observed. Therefore, it can be concluded that the current core system requires a specific alkyloxy chain length (n ≥ 12) to produce a smectic phase. The length of the terminal alkyloxy chain affects the melting and clearing temperatures as well as the mesophase ranges. In our future work, molecular modifications will be carried out on this homologous series to yield liquid crystalline compounds with high thermal stabilities and wider mesophase ranges.
Acknowledgements
The authors would like to thank Universiti Tunku Abdul Rahman for the research facilities and financial support through UTAR Research Fund (Project No. IPSR/RMC/UTARRF/2014-C1/H01 with the Vote No. 6200/H21).
Notes
Peer review under responsibility of Taibah University.
References
- D.AdamF.ClossT.FreyD.FunhoffD.HaarerH.RingsdorfP.SchuhmacherK.SiemensmeyerTransient photoconductivity in a discotic liquid crystalPhys. Rev. Lett.701993457460
- M.R.HuangX.G.LiJ.WangLiquid crystalline polyaniline and its derivativesTongji Daxue Xuebao (in Chinese)312003848852
- X.G.LiM.R.HuangW.DuanY.L.YangNovel multifunctional polymers from aromatic diamines by oxidative polymerizationsChem. Rev.102200229253030
- X.G.LiQ.F.LuM.R.HuangSelf-stabilized nanoparticles of intrinsically conducting co-polymers from 5-sulfonic-2-anisidineSmall4200812011209
- X.G.LiM.R.HuangFacile optimal synthesis of inherently electroconductive polythiophene nanoparticlesChem. Eur. J.15200964466455
- W.PisulaM.ZornJ.Y.ChangK.MullenR.ZentelLiquid crystalline ordering and charge transport in semiconducting materialsMacromol. Rapid Commun.30200911791202
- B.P.ChandraN.PeriasamyJ.N.DasTriboluminescence, a new tool to investigate fracture-initiation time of crystals under stressPramana81977395401
- M.FunahashiJ.HannaFast hole transport in a new calamitic liquid crystal of 2-(4′-heptyloxyphenyl)-6-dodecylthiobenzothiazolePhys. Rev. Lett.78199721842187
- A.OhnoA.HaruyamaK.KurotakiJ.HannaCharge-carrier transport in smectic mesophases of biphenylsJ. Appl. Phys.1022007083711
- S.T.HaT.M.KohH.C.LinG.Y.YeapY.F.WinS.T.OngY.SivasothyL.K.OngHeterocyclic benzothiazole-based liquid crystals: synthesis and mesomorphicLiq. Cryst.362009917925
- S.T.HaT.M.KohS.T.OngY.SivasothySynthesis and mesomorphic behavior of benzothiazole-based liquid crystals having terminal methoxyl groupChin. Chem. Lett.20200914491452
- S.T.HaT.M.KohG.Y.YeapH.C.LinS.L.LeeY.F.WinS.T.OngMesogenic Schiff base esters with benzothiazole core: synthesis and phase transition studiesPhase Transit.832010195204
- T.M.KohS.T.HaG.Y.YeapH.C.LinNew mesomorphic benzothiazoyl derivatives: synthesis and characterizationChin. Chem. Lett.242013926928
- L.L.LaiC.H.WangW.P.HsienH.C.LinSynthesis and characterization of liquid crystalline molecules containing the quinoline unitMol. Cryst. Liq. Cryst.2871996177181
- N.K.ChudgarS.N.ShahNew fluorescent mesogens with a chalcone central linkageLiq. Cryst.41989661668
- G.Y.YeapI.SusantiB.S.TeohW.A.K.MahmoodW.T.A.HarrisonSynthesis and phase transition in new chalcone derivatives: crystal structure of 1-phenyl-3-(4′-undecylcarbonyloxyphenyl)-2-propen-1-oneMol. Cryst. Liq. Cryst.4422005133146
- B.T.ThakerP.H.PatelA.D.VansadiyaJ.B.KanojiyaSubstitution effects on the liquid crystalline properties of thermotropic liquid crystals containing Schiff base Chalcone linkagesMol. Cryst. Liq. Cryst.5152009135147
- D.DemusL.RichterTextures of Liquid Crystals1978Verlag ChemieNew York
- I.DierkingTextures of Liquid Crystals2003Wiley-VCHWeinheim
- S.T.HaY.W.LowSynthesis and phase transition behaviors of new chalcone derivativesJ. Chem. (Hindawi)2013 article ID 943723
- O.N.KadkinH.HanY.G.GalyametdinovSynthesis, computational modelling and liquid crystalline properties of some [3]ferrocenophane-containing Schiff's bases and β-aminovinylketone: molecular geometry–phase behaviour relationshipJ. Organometall. Chem.692200755715582
- S.T.HaK.L.FooR.T.SubramaniamM.M.ItoS.S.SastryS.T.OngHeterocyclic benzoxazole-based liquid crystals: synthesis and mesomorphic propertiesChin. Chem. Lett.22201111911194
- G.SangameswariN.P.S.PrabuM.L.N.M.MohanBinary mixtures of hydrogen-bonded ferroelectric liquid crystals: thermal span enhancement in Smectic X* phaseZeitschrift für Naturforschung A702015757774
- A.Martínez-FelipeC.T.ImrieThe role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimersJ. Mol. Struct.11002015429437
- J.H.LeeS.J.LeeJ.Y.JhoRoom temperature supramolecular columnar liquid crystals formed by hydrogen bonding of isoquinoline derivativesPhase Trans.872014656665
- M.D.MirandaF.V.ChávezT.M.R.MariaM.E.S.EusebioP.J.SebastiãoM.R.SilvaSelf-assembled liquid crystals by hydrogen bonding between bipyridyl and alkylbenzoic acids: solvent-free synthesis by mechanochemistryLiq. Cryst.41201417431751
- G.W.GrayMolecular Structure and Properties of Liquid Crystals1962Academic PressLondon
- P.BerdagueJ.P.BayleM.S.HoB.M.FungNew laterally aromatic branched liquid crystal materials with large nematic rangesLiq. Cryst.141993667674