Abstract
Efficient nucleophilic addition reactions of indole derivatives with various azo-linked aldehydes were carried out using Fe3+-montmorillonite K10 under solvent-free conditions to afford the corresponding diindolylmethanes in short reaction times and excellent yields.
1 Introduction
It is well known that diindolylmethanes (DIMs) and related compounds exhibit a wide range of biological activities, such as antibacterial, antitumor and antileishmanial properties [Citation1,Citation2]. Diindolylmethanes are the most active cruciferous substances for promoting beneficial estrogen metabolism in women and men [Citation3]. DIMs increase the body's natural metabolism of hormones and promote the production of good estrogen (2-hydroxyestrogen). This indole antioxidant is patented for alleviating symptoms of fibromyalgia [Citation4]. Thus, the synthesis of indole and its derivatives has been a popular topic for research. Several synthetic methods for the preparation of DIMs have been reported using catalysts such as HOAc [Citation5], molecular iodine [Citation6], montmorillonite clay K10 [Citation7,Citation8], indium trichloride and triflate [Citation9], aluminum chloride [Citation10], ion exchange resin [Citation11], lithium perchlorate [Citation12], copper (II) bromide [Citation13], molibdatophosphoric acid [Citation14], scandium (III) trifluoromethanesulfonate [Citation15], bentonite [Citation16], silica supported sodium hydrogen sulphate and amberlyst-15 [Citation17]. Most of these methods suffer from disadvantages such as long reaction periods, use of expensive Lewis acids, harsh reaction conditions, excess toxic solvents, low yield and a cumbersome product isolation procedure. Due to these problems, it is desirable to develop a green, more efficient, mild and versatile method for the synthesis of diindolylmethanes in improved yields. In recent years, science and technology has been moving toward the use of eco-friendly, solvent-free conditions in organic synthesis. This has led in some cases to improved results and more benign synthetic methods, including the use of inexpensive and eco-friendly materials as a catalyst [Citation18]. In addition, prior research has focused on solvent-free syntheses [Citation19].
Azo dyes are compounds that contain azo groups linked to methane or an aromatic sp2-hybridized C-atom. Azo dyes are a well-known class of organic photoactive materials due to their excellent optical switching properties, good chemical stabilities and high solution process abilities [Citation20,Citation21]. Amaranth is a common azo dye colorant and is implicated in adverse reactions, such as chronic urticarial and angio-edema in adults and children [Citation20,Citation22]. These materials are also widely used in the textile and heat transfer printing industries [Citation23], as well as the photo-refractive polymer and optical data storage industries [Citation24,Citation25].
Because of the importance of diindolylmethanes owing to their wide range of pharmacological activities, we were interested in the synthesis of azo-linked diindolylmethanes. Although it is not the first synthesis of azo-linked diindolylmethanes [Citation26], we have optimized the reaction time, yield and reusability of the catalyst. This is the first report of the synthesis of azo-linked diindolylmethanes using synthetic Fe3+-montmorillonite K10 under solvent-free conditions.
2 Results and discussion
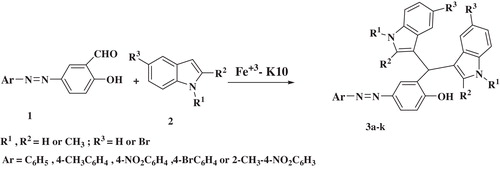
Recent development in diindolylmethane chemistry and our continued interest in the development of green and efficient syntheses of pharmaceutical and heterocyclic compound [Citation27–Citation30] motivated us to develop an efficient method for the synthesis of azo-linked diindolylmethanes. Various azo-linked aldehydes and indole derivatives were used as starting materials, and Fe3+-montmorillonite K10 was employed as the catalyst under solvent-free conditions ().
At the onset of this work, we investigated a variety of conditions with the model reaction of 2-hydroxy-5-(phenyldiazenyl) benzaldehyde and indole using Fe3+-montmorillonite K10 as a promoter (). First, we examined different solvents such as toluene, CHCl3, CH3CN, CH3CH2OH and H2O. The results show that a solvent-free reaction is superior to reactions with solvent, irrespective of the particular solvent used (, entries 1–6).
Table 1 The solvent and catalyst amount optimization on the synthesis of 3a.
Next, we evaluated the loading amount of Fe3+-montmorillonite K10. No reaction was observed in the absence of Fe3+-montmorillonite K10 even after 12 h; both starting materials were recovered in quantitative yields. To optimize the catalyst loading, 0.01 g, 0.03 g, 0.05 g, 0.1 g and 0.15 g of montmorillonite Fe3+-K10 was tested, 0.1 g of Fe3+-montmorillonite K10 was sufficient to push the reaction forward; amounts of 0.01 g, 0.03 g and 0.05 g of montmorillonite Fe3+-K10 were insufficient. Higher amounts of Fe3+-montmorillonite K10 did not lead to a significant change in the reaction yields (, entries 6–10).
A series of azo-linked aldehydes with either electron-donating or electron-withdrawing groups attached to aromatic ring were investigated. The substitution groups on the aromatic ring had no obvious effect on the yield. As expected, satisfactory results were observed and the results are summarized as .
Table 2 Synthesis of azo-linked diindolylmethane derivatives (3a–k) using montmorillonite Fe3+-K10 under solvent free-conditions.
Apart from the mild conditions of the process and its excellent results, the simplicity of product isolation and the ability to recycle the catalyst offer significant advantages. Because K10 is insoluble in acetone/chloroform and the other desired products are soluble in it, the catalyst can be easily separated by filtration. The remaining reaction solution can be evaporated to furnish the crude product. It is shown that the catalyst can be recycled after six rounds while retaining catalytic efficiency; indeed, it is not decreased even in the seventh round.
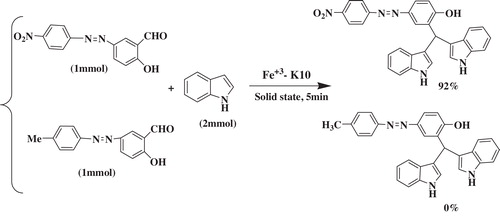
Following these results, we further investigated the potential of Fe3+-montmorillonite K10 to catalyze the selective synthesis of azo-linked diindolylmethanes of different types of azo-linked salicylaldehydes. The results showed that Fe3+-montmorillonite K10 is able to discriminate between aromatic compounds containing electron-donating and electron-withdrawing groups, a transformation that is difficult to accomplish via conventional methods ().
The structures of the synthesized compounds were elucidated by IR, NMR and elemental analysis. In the 1H NMR spectra of azo-linked diindolylmethanes, the benzylic C–H proton resonated at 6.1–6.2 ppm, and in the 13CNMR spectra of azo-linked diindolylmethanes, the benzylic C–H carbon resonated at 32 ppm.
3 Experimental
Chemicals were purchased from Merck and Fluka and used as purchased. Melting points were measured on an Electro-thermal 9100 apparatus. 1HNMR spectra were obtained on a Bruker DRX 500, 250 in DMSO-d6 as solvent and with TMS as internal standard. FT-IR spectra were recorded on a Shimadzu FT-IR-8400S spectrometer. Elemental analyses were recorded on a Carlo-Erba EA1110CNNO-S analyser.
3.1 General procedure for the solid state synthesis of azo-linked dihydropyridines 3a–k
A mixture of indole (2.0 mmol), azo-linked aldehyde (1.0 mmol) and catalyst Fe3+-K10 (0.1 g) was added to a mortar and the mixture was pulverized with a pestle. A spontaneous reaction took place (4–12 min, , monitored by TLC, 4:1, hexane/acetone). After completion of the reaction, acetone (10 mL) was added, and insoluble reagents were removed by filtration. The filtrate was evaporated under reduced pressure, and the resulting crude material was purified by recrystallization from ethanol to afford pure products.
3.1. 2-(Di(1H-indol-3-yl)methyl)-4-(phenyldiazenyl)phenol (3a): m.p. 263–265 °C; FT-IR (KBr): 3460, 3400, 2337, 1649, 1519, 1465, 1342 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 6.27 (s, 1H), 6.82 (d, 2H, J = 1.9 Hz), 6.85 (t, 2H, J = 7.8 Hz), 7.00–7.12 (m, 3H), 7.32 (d, 2H, J = 8.2 Hz), 7.32 (d, 2H, J = 8.3 Hz), 7.33 (d, 1H, J = 7.3 Hz), 7.48 (t, 2H, J = 7.6 Hz), 7.65 (d, 1H, J = 2.8 Hz), 7.65–7.79 (m, 3H), 10.8 (s, 1H), 10.94 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ: 32.5 (benzylic CH), 112.4, 116.6, 118.3, 119.1, 119.7, 121.7, 121.8, 122.9, 124.5, 126.7, 127.6, 130.7, 131.1, 132.8, 137.5, 145.9, 152.9, 159.0. MS: m/z (%) = 442 (14), 326 (100). Anal. Calcd. for C29H22N4O: C, 78.71; H, 5.01; N, 12.66. Found: C, 78.84; H, 5.13; N, 12.59.
3.2. 2-(Bis(2-methyl-1H-indol-3-yl)methyl)-4-(phenyldiazenyl)phenol (3b): m.p. 265–267 °C; FT-IR (KBr): 3404, 3355, 3149, 2736, 1583, 1363, 1269 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 2.3 (s, 3H), 2.5 (s, 3H), 6.3 (s, 1H), 6.84–6.89 (m, 4H), 7.05 (t, 2H, J = 7.6 Hz), 7.15 (d, 1H, J = 8.7 Hz), 7.32 (d, 2H, J = 7.7 Hz), 7.35 (d, 2H, J = 8.4 Hz), 7.59 (d, 1H, J = 8.7 Hz), 7.77 (d, 1H, J = 6.6 Hz), 7.83 (s, 1H), 8.07 (d, 1H, J = 7.8 Hz), 8.9 (s, 1H), 10.5 (s, 1H), 10.8 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ: 12.79 (CH3), 33.7 (benzylic CH), 111.2, 112.4, 116.4, 118.8, 119.0, 120.8, 120.9, 122.8, 128.4, 129.0, 130.0, 131.1, 132.3, 132.7, 135.9, 145.8, 152.7, 159.8 ppm. MS: m/z (%) = 470 (12), 340 (100). Anal. Calcd. for C31H26N4O: C, 79.12; H, 5.57; N, 11.91. Found: C, 79.13; H, 5.63; N, 11.87.
3.3. 2-(Di(1H-indol-3-yl)methyl)-4-((4-nitrophenyl)diazenyl)phenol (3c): m.p. 150–152 °C; FT-IR (KBr): 3624, 3413, 3168, 2931, 1591, 1512, 1454, 1338, 1278 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 6.3 (s, 1H), 6.84 (d, 2H, J = 1.7 Hz), 6.82 (t, 2H, J = 7.2 Hz), 7.07 (t, 2H, J = 7.6 Hz), 7.17 (d, 1H, J = 8.7 Hz), 7.23 (d, 2H, J = 7.7 Hz), 7.38 (d, 2H, J = 8.4 Hz), 7.70–7.82 (m, 2H), 7.88 (d, 2H, J = 8.6 Hz), 8.31 (d, 2H, J = 8.7 Hz), 10.3 (s, 1H), 10.8 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ: 32.4 (benzylic CH), 112.4, 116.8, 118.1, 119.2, 121.8, 122.8, 123.4, 124.5, 125.5, 127.5, 127.5, 133.6, 137.5, 146.4, 148.4, 156.4, 160.4 ppm. MS: m/z (%) = 487 (11), 371 (100). Anal. Calcd. for C29H21N5O3: C, 71.45; H, 4.34; N, 14.37. Found: C, 71.41; H, 4.48; N, 14.47.
3.4. 2-(Bis(2-methyl-1H-indol-3-yl)methyl)-4-((4-nitrophenyl)diazenyl)phenol (3d): m.p. 320–321 °C; FT-IR (KBr): 3508, 3433, 3056, 2923, 1662, 1591, 1421, 1348, 1271 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 2.10 (s, 6H), 6.18 (s, 1H), 6.65 (t, 2H, J = 7.8 Hz), 6.80–6.89 (m, 4H), 6.8 (d, 1H, J = 8.7 Hz), 7.17 (d, 2H, J = 8.3 Hz), 7.77 (t, 2H, J = 6.9 Hz), 7.83 (d, 2H, J = 8.9 Hz,), 8.29 (d, 2H, J = 8.9 Hz), 10.5 (s, 1H), 10.8 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ: 12.1 (CH3), 33.7 (benzylic CH), 111.2, 112.1, 116.7, 118.9, 120.4, 121.9, 125.8, 129.2, 129.4, 132.7, 132.8, 135.8, 146.6, 156.3, 161.0 ppm. MS: m/z (%) = 515 (12), 385 (100). Anal. Calcd. for C31H25N5O3: C, 72.22; H, 4.89; N, 13.58. Found: C, 72.31; H, 4.51; N, 13.65.
3.5. 2-(Bis(5-bromo-1H-indol-3-yl)methyl)-4-((4-nitrophenyl) diazenyl)phenol (3e): m.p. 320–322 °C; FT-IR (KBr): 3417, 2860, 1593, 1452, 1512, 1338, 1271, 1081 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 6.18 (s, 1H), 6.92 (d, 2H, J = 2.0 Hz), 7.11 (d, 1H, J = 8.6 Hz), 7.18 (dd, 2H, J = 2.2, 8.9 Hz), 7.35 (d, 2H, J = 8.9 Hz), 7.43 (d, 2H, J = 2.2 Hz), 7.77 (d, 1H, J = 2.2 Hz), 7.79 (dd, 1H, J = 2.4, 8.8 Hz), 7.84 (d, 2H, J = 8.7 Hz), 8.33 (d, 2H, J = 8.7 Hz), 10.80 (s, 1H), 11.00 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ 32.2 (benzylic CH), 111.4, 114.5, 117.0, 117.5, 121.6, 123.8, 123.9, 124.3, 125.7, 126.2, 126.6, 129.9, 132.5, 136.2, 146.8, 148.5, 156.3, 160.3 ppm. MS: m/z (%) = 646 (7), 644 (14), 642 (7), 449 (100). Anal. Calcd. for C29H19Br2N5O3: C, 53.98; H, 2.97; N, 10.85. Found: C, 53.82; H, 2.81; N, 10.21.
3.6. 2-(Di(1H-indol-3-yl)methyl)-4-(p-tolyldiazenyl)phenol (3f): m.p. 320–321 °C; FT-IR (KBr): 3413, 3055, 2858, 1600, 1485, 1452, 1342, 1272 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 2.4 (s, 1H), 6.23 (s, 1H), 6.79 (s, br, 2H), 6.9 (s, br, 2H), 7.11 (s, br, 3H), 7.23 (t, 4H, J = 8.2 Hz), 7.35 (d, 3H, J = 7.6 Hz), 7.62 (d, 3H, J = 6.5 Hz), 10.38 (s, 1H), 10.77 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ 21.76 (CH3), 32.5 (benzylic CH), 112.3, 116.5, 118.3, 119.6, 119.7, 121.4, 121.7, 122.1, 124.2, 126.2, 127.2, 130.9, 134.1, 137.5, 141.1, 145.0, 152.7, 158.7 ppm. MS: m/z (%) = 456 (23), 340 (100). Anal. Calcd. for C30H24N4O: C, 78.92; H, 5.30; N, 12.27. Found: C: 78.95; H, 5.41; N, 12.41.
3.7. 2-(Bis(5-bromo-1H-indol-3-yl)methyl)-4-(p-tolyldiazenyl)phenol (3g): m.p. 258–260 °C; FT-IR (KBr): 3417, 3130, 2921, 2853, 1587, 1452, 1352, 1217, 1087 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 2.45 (s, 3H), 6.4 (s, 1H), 6.8 (s, 2H), 7.11 (d, 1H, J = 7.7 Hz), 7.18 (d, 2H, J = 7.6 Hz), 7.29 (d, 2H, J = 6.9 Hz), 7.39 (d, 2H, J = 8.2 Hz), 7.48 (s, 2H), 7.60–7.69 (m, 4H), 10.5 (s, 1H), 11.0 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ 21.7 (CH3), 32.2 (benzylic CH), 111.8, 114.5, 116.2, 117.9, 121.8, 122.1, 122.9, 124.3, 126.0, 126.2, 129.3, 130.0, 132.2, 136.5, 141.2, 146.1, 150.5, 158.6 ppm. MS: m/z (%) = 612 (18), 418 (100). Anal. Calcd. for C30H22Br2N4O: C, 58.65; H, 3.61; N, 9.12. Found: C, 58.70; H, 3.72; N, 9.05.
3.8. 4-((4-Bromophenyl)diazenyl)-2-(di(1H-indol-3-yl)methyl)phenol (3h): m.p. 262–263 °C; FT-IR (KBr): 3419, 3398, 2923, 1614, 1411, 1485, 1205, 1116 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 6.3 (s, 1H), 6.72 (d, 2H, J = 2.2 Hz), 6.89 (t, 2H, J = 7.5 Hz), 7.05 (dd, 4H, J = 7.8, 12.2 Hz), 7.31 (d, 2H, J = 8.0 Hz), 7.36 (d, 2H, J = 8.3 Hz), 7.39 (d, 2H, J = 8.3 Hz), 7.64–7.80 (m, 3H), 10.7 (s, 1H), 10.79 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ 32.5 (benzylic CH), 112.3, 116.6, 118.27, 119.8, 119.9, 121.7, 122.2, 124.5, 124.9, 126.8, 127.5, 132.7, 133.1, 137.2, 151.8, 154.1, 159.1 ppm. MS: m/z (%) = 525 (6), 523 (12), 521 (6), 404 (100). Anal. Calcd. for C29H21BrN4O: C, 66.80; H, 4.06; N, 10.75. Found: C, 66.34; H, 4.08; N, 10.76.
3.9. 2-(Bis(5-bromo-1H-indol-3-yl)methyl)-4-((4-bromophenyl) diazenyl)phenol (3i): m.p. 263–264 °C; FT-IR (KBr): 3421, 2860, 1554, 1452, 1272, 1087 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 6.3 (s, 1H), 6.96 (d, 2H, J = 1.9 Hz), 7.12 (d, 1H, J = 8.8 Hz), 7.19 (dd, 2H, J = 2.2, 8.6 Hz), 7.39 (d, 2H, J = 8.6 Hz), 7.46 (d, 2H, J = 2.4 Hz), 7.69 (s, 4H), 7.67 (d, 1H, J = 2.6 Hz), 7.70 (dd, 1H, J = 2.2, 8.8 Hz), 10.8 (s, 1H), 11.0 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ 32.3 (benzylic CH), 111.8, 114.5, 116.8, 117.7, 121.8, 122.6, 124.0, 124.5, 126.3, 126.3, 129.3, 132.3, 133.2, 136.2, 145.9, 151.8, 159.3 ppm. MS: m/z (%) = 679 (12), 677 (11), 675 (4), 481 (100). Anal. Calcd. for C29H19Br3N4O: C, 51.28; H, 2.82; N, 8.25. Found: C, 51.34; H, 2.73; N, 8.23.
3.11. 2-(Di(1H-indol-3-yl)methyl)-4-((2-methyl-4-nitrophenyl) diazenyl)phenol (3j): m.p. 320–322 °C; FT-IR (KBr): 3413, 3056, 2856, 1591, 1517, 1451, 1423, 1340, 1271 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 2.4 (s, 3H), 6.26 (s, 1H), 6.89 (s, 2H), 6.92 (d, 2H, J = 8.6 Hz), 7.07 (t, 2H, J = 7.7 Hz), 7.12 (d, 1H, J = 8.4 Hz), 7.32 (d, 2H, J = 7.8 Hz), 7.36 (d, 2H, J = 8.0 Hz), 7.56 (d, 1H, J = 8.8 Hz), 7.74 (d, 1H, J = 6.8 Hz), 7.81(s, 1H), 8.03 (d, 1H, J = 7.4 Hz), 8.2 (s, 1H), 10.2 (s, 1H), 10.5 (s, 2H) ppm. 13C NMR (125 MHz, DMSO-d6): δ 17.7 (CH3), 32.81 (benzylic CH), 112.8, 116.0, 117.3, 118.9, 119.6, 119.7, 121.7, 122.5, 122.9, 124.5, 126.5, 127.6, 127.7, 133.0, 137.5, 138.6, 146.6, 148.2, 154.7, 160.3 ppm. MS: m/z (%) = 501 (9), 385 (100). Anal. Calcd. for C30H23N5O3: C, 71.84; H, 4.62; N, 13.96; O, 9.57. Found: C, 71.71; H, 4.65; N, 13.91.
3.12. 2-(Bis(5-bromo-1H-indol-3-yl)methyl)-4-((2-methyl-4-nitrophenyl)diazenyl)phenol (3k): m.p. 259–260 °C; FT-IR (KBr): 3417, 3130, 2921, 2853, 1587, 1452, 1352, 1217, 1087 cm−1; 1H NMR (500 MHz, DMSO-d6): δ: 2.6 (s, 3H), 6.2 (s, 1H), 6.97 (s, 2H), 7.13 (d, 1H, J = 8.6 Hz), 7.19 (d, 2H, J = 7.8 Hz), 7.39 (d, 2H, J = 8.2 Hz), 7.48 (s, 2H), 7.57 (d, 1H, J = 8.6 Hz), 7.70–7.79 (m, 2H), 8.04 (d, 1H, J = 7.8 Hz), 8.23 (s, 1H), 10.2 (s, 1H), 11.0 (s, 2H) ppm. MS: m/z (%) = 661 (6), 659 (12), 657 (6), 463 (100). Anal. Calcd. for C30H21Br2N5O3: C, 54.65; H, 3.21; N, 10.62. Found: C, 54.71; H, 3.32; N, 10.53.
4 Conclusion
We developed an efficient, green, fast and convenient procedure for the synthesis of azo-linked diindolylmethanes through electrophilic substitution of azo-linked aldehydes and indole derivatives over a montmorillonite catalyst in the solid state. This procedure offers advantages such as reduced reaction time, mild reaction conditions, higher yield, ease of execution and economic viability. This simple process combined with the ease of recovery and recyclability of the catalyst make this an economic, environmentally benign and waste-free chemical process for the synthesis of azo-linked diindolylmethanes.
Acknowledgement
Financial support by Rasht Branch, Islamic Azad University Grant No. 4.5830 is gratefully acknowledged.
Notes
Peer review under responsibility of Taibah University.
References
- B.S.DrasarM.J.HillHuman Intestinal Flora1974Academic PressNew York
- B.S.BharateJ.B.BharateSh.I.KhanB.L.TekwaniM.R.JacobR.MudududdlaR.R.YadavB.SinghP.R.SharmaS.MaityB.SinghI.A.KhanR.A.VishwakarmaEur. J. Med. Chem.632013435443
- M.A.ZeligsDiet and estrogen status: the cruciferous connectionJ. Med. Food119986782
- J.J.MichnoviczH.L.BradlowinM.J.HuangT.OsawaC.T.HoR.T.RosenFood Phytochemicals for Cancer Prevention 1 Washington, DC1994282
- W.NolandM.VenkiteswaranC.RichardsReactivity of nitrovinylquinones with cyclic and acyl enol ethersJ. Org. Chem.26196142414248
- B.P.BandgarK.A.ShaikhMolecular iodine-catalyzed efficient and highly rapid synthesis of bis(indolyl)methanes under mild conditionsTetrahedron Lett.44200319591961
- M.ChakrabartyN.GhoshR.BasakY.HarigayaDry reaction of indoles with carbonyl compounds on montmorillonite K10 clay: a mild, expedient synthesis of diindolylalkanes and vibrindole ATetrahedron Lett.43200240754078
- A.K.MaitiP.BhattacharyyaMontmorillonite clay-catalysed synthesis of bis(indol-3-yl)-methanes and 1,2-bis(indol-3-yl)ethanesJ. Chem. Res.S1997424425
- G.BabuN.SridharP.T.Perumal A convenient method of synthesis of indole with aldehydes and schiff basesSynth. Commun.30200016091614R.NagarajanP.T.PerumalInCl3 and In(OTf)3 catalyzed reactions: synthesis of 3-acetyl indoles, bis-indolylmethane and indolylquinoline derivativesTetrahedron58200212291232
- A.ChatterjeeS.MannaT.OrangeJ.ShooleryLewis-acid-induced electrophilic substitution in indoles with acetone. Part 2J. Chem. Soc. Perkin Trans.11980553555
- X.FengC.GuanC.ZhaoIon exchange resin catalyzed condensation of indole and carbonyl compounds—synthesis of bis-indolylmethanesSynth. Commun.342004487492
- J.S.YadavB.S.ReddyC.MurthyG.M.KumarC.MadanLithium perchlorate catalyzed reactions of indoles: an expeditious synthesis of bis(indolyl)methanesSynthesis2001783787
- L.P.MoZ.MaZ.H.ZhangCuBr2-catalyzed synthesis of bis(indolyl)methanesSynth. Commun.35200519972004
- M.A.ZolfigolP.SalehiM.ShiriAn efficient procedure for the preparation of mono, and di-bis-indolyl methanes catalyzed by molibdatophosphoric acidPhosphorus Sulfur Silicon Relat. Elem.179200422732277
- S.SatoT.SatoA mild and environmentally friendly scandium(III) trifluoromethanesulfonate-catalyzed synthesis of bis(3′-indolyl)alkanes and bis(3′-indolyl)-1-deoxyalditolsCarbohydr. Res.340200522512255
- G.Penieres-CarrilloJ.G.Garcia-EstradaJ.L.Gutierrez-RamirezC.Alvarez-ToledanoInfrared-assisted eco-friendly selective synthesis of diindolylmethanesGreen Chem.52003337339
- J.RameshR.BanerjeeB.PalB.DasSilica supported sodium hydrogen sulfate and amberlyst-15: two efficient heterogeneous catalysts for facile synthesis of bis- and tris(1H-indol-3-yl)methanes from indoles and carbonyl compoundsAdv. Synth. Catal.3452003557559
- K.AswinS.S.MansoorK.LogaiyaP.N.SudhanR.N.AhmedFacile synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones and indeno[1,2-d]pyrimidines catalyzed by p-dodecylbenzenesulfonic acidJ. Taibah Univ. Sci.82014236247
- S.MandalG.DasH.Askari Amino acid-type interactions of L-3,4-dihydroxyphenylalanine with transition metal ions: an experimental and theoretical investigationJ. Mol. Struct.11002015162173S.MandalG.DasH.AskariPhysicochemical properties of the ternary complexes of Pt(II) with uracil and small peptide moieties: an experimental and computational studyNew J. Chem.201510.1039/c5nj00120jS.MandalG.DasH.Askari Experimental and quantum chemical modeling studies of the interactions of l-phenylalanine with divalent transition metal cationsJ. Chem. Inf. Model.54201425242535
- A.VigK.SirbiladzeH.J.NagyP.AranyosiI.RusznakP.SallayThe light stability of azo dyes and dyeings. V. The impact of the atmosphere on the light stability of dyeings with heterobifunctional reactive azo dyesDyes Pigments7220071622
- M.ManuelaM.M.M.RaposoA.M.R.C.SousaA.M.C.FonsecaG.KirschThienylpyrrole azo dyes: synthesis, solvatochromic and electrochemical propertiesTetrahedron61200582498256
- G.ZhangY.MaMechanistic and conformational studies on the interaction of food dye amaranth with human serum albumin by multispectroscopic methodsFood Chem.1362013442449
- A.K.SlarkP.M.HadgettThe effect of specific interactions on dye transport in polymers above the glass transitionPolymer40199940014011
- G.IftimeF.L.LabarthetA.NatansohnP.RochonK.MurtiMain chain-containing azo-tetraphenyldiaminobiphenyl photorefractive polymersChem. Mater.142002168173
- M.S.HoA.NatansohnP.RochonAzopolymers for reversible optical storage. 7. The effect of the size of the photochromic groupsMacromolecules28199561246127
- M.NikpassandL.ZareM.R.MousaviA comparative study for the aqueous synthesis of new generation of diindolylmethanes using l-proline, K10 and nano-Fe3O4 under ultrasound irradiationLett. Org. Chem.92012375381
- L.ZareM.NikpassandMulticomponent synthesis of dihydropyridines catalyzed by l-prolineChin. Chem. Lett.222011531534
- M.NikpassandL.ZareM.SaberiUltrasound-assisted l-proline catalyzed synthesis of novel derivatives of azo-linked dihydropyridinesMonatsch. Chem.1432012289293
- M.NikpassandL.ZareT.ShafaatiRegioselective synthesis of fused azo-linked pyrazolo [4,3-e] pyridines using nano-Fe3O4Chin. J. Chem.302012604608
- M.NikpassandL.Zare FekriM.GharibO.MarviFe3+-montmorillonite K-10 as a green and reusable catalyst for the synthesis of new generation of dihydropyrimidinonesLett. Org. Chem.92012745748