Abstract
The present study aimed to screen for antibacterial activities in soft corals of the eastern Red Sea coast. Specimens of different species were surveyed, collected, preserved, extracted and assayed according to standard protocols. Bioactive materials were extracted using a dichloromethane/methanol mixture, and the activity was determined using the well diffusion and microdilution assay methods. All extracts demonstrated variable activity against marine bacteria previously isolated from the sea water (in situ near the soft corals). Among soft corals, Sarcophyton spp. and Sinularia polydactyla showed the highest activity (MIC = 30–50 μg/ml), while the crude extract of Xenia spp. showed the lowest activity (MIC = 200–250 μg/ml) against the isolated marine bacterial strains.
Antibacterial activity was observed for five out of the seven soft coral extracts (72%) against two Gram-positive (Staphylococcus aureus and Bacillus spp.) bacterial pathogens, while none of the extracts inhibited Gram-negative (Escherichia coli) bacteria. The most potent (MIC = 40-75 μg/ml) crude extracts were obtained from S. polydactyla and Sarcophyton spp., while the crude extract of Xenia spp. was the least effective (MIC = 200 μg/ml) against the tested Gram-positive bacteria. The results from the current study suggest that soft corals of the Red Sea (Yunbu, SA) are a potential source of novel antibiotics.
1 Introduction
The growing needs for drugs to control new illnesses in the early sixties and to combat very resistant strains of microorganisms encouraged the search for new sources of marine bioactive natural products. The marine environments are among the richest and most complex ecosystems in terms of biological and chemical diversity [Citation1]. In 2012, approximately 1241 new biochemical compounds were reported, which undoubtedly identifies the marine environment as a rich source of bioactive molecules [Citation2,Citation3].
Bioactive natural products have been isolated from marine macro- or micro-organisms. All of the progressive improvements in the past 50 years of exploration of the marine environment have resulted in the isolation of approximately 24,662 structurally unique new bioactive compounds from marine organisms [Citation4]. Marine invertebrates are the major producers of marine natural products [Citation5]. Sponges (phylum Porifera) have long been recognized as the most interesting group of marine invertebrates for the discovery of new drugs.
The biotechnological potential of other groups of marine invertebrates such as cnidarians (jellyfishes, sea anemones and corals) has attracted the attention of researchers because of their ability to produce powerful toxins and venoms [Citation6]. Over 3000 marine natural product compounds have been described from cnidarians alone, mostly in the last decade. Soft corals (Octocorallia, Alcyonacea) are known to contain a rich variety of marine secondary metabolites and are considered an extremely diverse group of marine organisms. The biologically active substances from corals have not only great significance in chemical ecology but also express various biological activities such as antitumor, antibacterial, antiviral and antifungal [Citation7].
The unique characteristics of the Red Sea make it one of the most promising areas as a source of medicinal and nutritional natural products. Recently, biomedical leads from Red Sea marine invertebrates were reviewed [Citation8]. The taxonomy of Red Sea soft corals was discussed by [Citation9–Citation11], and many other studies in the last decade discussed the biological activities of Red Sea soft corals [Citation12–Citation15]. Most of these studies focused on testing crude extracts and their subsequent purified compounds against human pathogens. More recently, marine bacteria associated with the soft coral Sarcophyton glaucum from Hurghada, Egypt were tested for their antimicrobial activities [Citation16]. To the best of our knowledge, few studies have tested the antibacterial activity against bacteria isolated from the waters surrounding the corals, except for [Citation17], which also tested it against clinical bacterial pathogens. Therefore, the aim of the present study was to screen and test the antibacterial activities of soft corals of the eastern Red Sea against a range of marine and clinical pathogenic bacteria.
2 Materials and methods
2.1 Study area
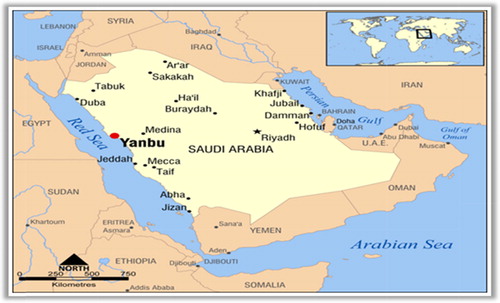
The Yanbu region, extending from Sharm al Khawr near Ras Jarbu in the north to Masturah in the south, covers a linear distance of approximately 160 km. The region contains extensive shallow coastal marine habitats and a complex offshore barrier reef system. The coastline consists of alluvial sand flats and low-lying hills that extend inland for more than 10 km, with salt marshes found in several locations in the intertidal zone. Yanbu's industrial city ( and ) covers approximately 15 km of the coastline, occupying an area of 158 km2. The city contains the largest oil shipping complex in the Red Sea, as well as more than 20 hydrocarbon, petrochemical and mineral facilities.
2.2 Collection and identification of soft corals
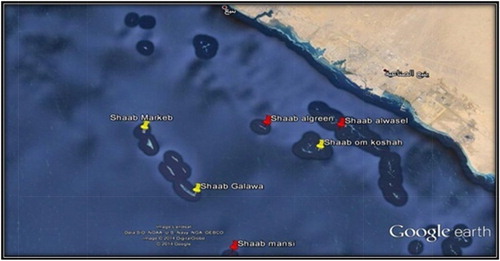
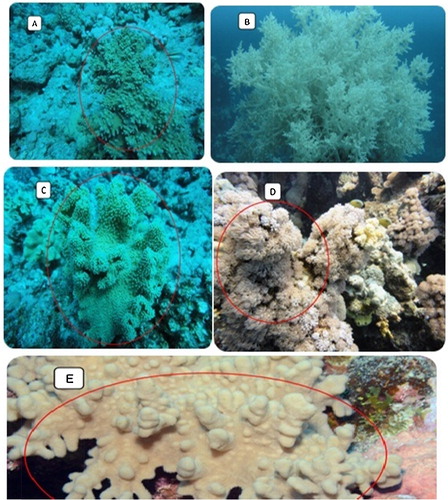
Five species of marine soft corals (A–E) were collected from Yanbu (Red Sea) by self-contained underwater breathing apparatus (SCUBA) diving as well as snorkelling from different sites during the autumn of 2012. Two colonies from each species were sampled, one for the purpose of extraction and bioactivity testing and the other for identification. Every sample was photographed in situ. The coral morphology and the surrounding environment and habitat of the collected soft corals were recorded on an underwater slate. The samples were collected by means of a scalpel or a pair of scissors. In some cases, pieces of rocks to which the animal was attached were removed and placed in zip lock plastic bags. The collected soft coral specimens for systematic study were fixed in 4% formalin in sea water for 24 h and then rinsed in fresh water and kept in 70% ethanol as a final preservative. Upon arriving in the laboratory, sodium hypochlorite (household bleach) was used to dissolve the soft coral tissue, and the remaining sclerites were carefully rinsed with double-distilled water.
Fig. 3 Shows the collected soft corals in its habitat. A: Sinularia polydactyla. B: Litophyton spp., C: Sarcophyton spp. D: Xenia spp., E: Sinularia leptoclados.
The collected soft coral species were kindly identified by Dr. Tarek A. Temraz, Marine Science Department, Suez Canal University, Ismailia, Egypt. For every sample, a method adopted from [Citation18–Citation20] was applied, where small squares of approximately 1 cm were cut from the colony with a scalpel and then mounted on a glass slide with 2 drops of bleach. Once the bubbles have ceased, the sclerites were spread out by stirring, and the specimen was examined under a microscope equipped with a camera and a stage micrometre. When the sclerites were very dark and difficult to distinguish from the tissue remains, clearing was carried out using a mixture of phenol-xylene.
2.3 Preparation of soft coral crude extracts
The samples were frozen immediately after collection and maintained at −20 °C prior to the extraction. The frozen samples of soft corals were left to defrost, broken down into small pieces and extracted at room temperature. The macerated tissues were soaked in a mixture of methanol/methylene chloride (1:1) three times until no color was obtained to ensure complete extraction. The combined extracts were filtered through Whatman no.1 filter paper and dried at 40 °C using a rotary evaporator (Rotavapor R-200, BUCHI). The residues were put into vials and stored at −20 °C for further analysis.
2.4 Bacterial isolation and characterization
Two Gram-negative clinical bacterial isolates (Pseudomonas aeruginosa and Escherichia coli) obtained from Prince Mohammad Bin Abdulaziz Hospital in Medina and two Gram-positive bacterial isolates (Staphylococcus aureus and Bacillus sp.) obtained from the Microbial Biotechnology Laboratory, Biology Department, Taibah University were used in this study. They were stored on nutrient agar plates and were propagated in nutrient broth (OXOID) medium. Batches of medium (10 ml) were inoculated with colonies from nutrient agar (OXOID) plates containing freshly grown S. aureus, Bacillus sp., P. aeruginosa and E. coli cells and incubated overnight at 37 °C.
The used marine bacteria were isolated from the waters surrounding the corals. Following the technique of [Citation17], standard serial dilution and plating techniques was used on Marine agar (18 g Difco Marine Broth (MB), 9 g NaCl and 18 g Difco Agar per 1 L of deionized water), and incubated at 25 °C. The colony characteristics were recorded, and the bacterial isolates were morphologically characterized using Gram staining. Identification to generic level was achieved by the recommended schemes in Bergey's Manual of Systematic Bacteriology [Citation21], and the metabolic fingerprints were determined using the API 50-STREP system (Biomerieux, Paris, France).
2.5 Antibacterial activity testing
2.5.1 Well diffusion method
The well diffusion method was conducted according to [Citation22] using Marine Agar plates. For the antibacterial activity, 20 ml of sterile nutrient agar was poured into Petri dishes, allowed to set at 37 °C and then inoculated uniformly with 0.1 ml of a 24 h broth culture of test bacteria. Once the inoculated agar plates (9-cm diameter) and the medium had solidified, wells (4-mm diameter) were cut out of the agar, and 20 μl (containing 400 μg and 200 μg of the stock and single dilution, respectively) of the antibacterial extract dissolved in dimethyl sulphoxide (DMSO) plus a control well of DMSO were placed into each well. The assay plates were incubated at 37 °C for 24 h, and the endpoint was determined by visualizing the clear zone of inhibition of growth around the wells.
2.5.2 Microtiter diffusion method
To determine the potential effect of the antibacterial extract, a micro dilution assay [Citation23,Citation24] was performed in 96-well microtiter plates. In brief, the extracts were diluted in dimethyl sulphoxide (DMSO) to various concentrations (by the dry weight of the extract) and incubated with a suspension of an actively growing cells in appropriate fresh medium diluted to a starting concentration of 3 × 105 cells per well. For the assays, DMSO only was used as a negative control. Four replicates were used for every data point. The antibacterial activity was determined at the point when the optical density of the growth control (bacteria plus media) reached an absorbance at 630 nm of approximately 0.30, and the extracts were regarded as inactive if the optical density (OD) of the bacterial cultures was more than 90% of that of the control cultures after 24 h.
3 Results
3.1 Antibacterial activity testing against marine bacteria
Five species of dominant Red Sea soft corals were tested for their antibacterial activities, including Sinularia leptoclados, Sinularia polydactyla, Litophyton spp., Sarcophyton spp. and Xenia spp. A total of seven extracts were assayed against four strains of bacteria (Gram-positive) isolated from the water surrounding the corals. The crude extracts from the five soft coral species showed variable activity against marine bacteria.
The obtained crude extracts inhibited the bacterial growth for at least two bacterial isolates with activities ranging from moderate to high (). Three out of the seven (43%) soft coral extracts showed mild activity against at least three bacterial isolates of the four examined strains. Of the active soft coral species examined, Sarcophyton spp. (cap) was the most effective inhibitor of all tested marine bacteria.
Table 1 Antimicrobial activities of extracts of Yanbu soft corals against isolated bacteria from the environment of the corals at a concentration of 400 μg/well.
3.2 Antibacterial activity testing against human pathogenic bacteria
The antibacterial activities of the seven extracts of soft corals against three clinical isolates of pathogenic bacteria are shown in . The bacterial strains exhibited variable sensitivity to the different soft coral extract concentrations.
Table 2 Antimicrobial activities by well diffusion assay against human pathogenic bacteria at two concentrations (Conc1: 400 μg/well, Conc2: 200 μg/well).
The results showed antibacterial effects for five out of the seven soft coral extracts (72%) against two Gram-positive (Bacillus spp. and S. aureus) bacterial pathogens, while none of the extracts inhibited the Gram-negative (E. coli) bacteria. Of the active soft coral species examined, Sarcophyton spp. and S. polydactyla exhibited the highest antibacterial activity, while the extract of S. leptoclados was the least effective inhibitor.
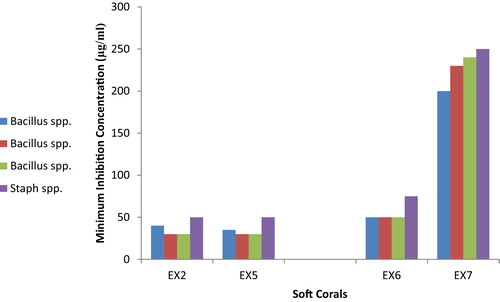
3.3 MIC values for antibacterial activity against marine bacteria
Antibacterial bioassays using the micro dilution method indicated that the S. polydactyla and Sarcophyton spp. extracts were the most active (MIC = 30–50 μg/ml), while those obtained from Xenia spp. were the least active (MIC = 200–250 μg/ml), as presented in . The micro dilution assay results corresponded with the data obtained from the agar diffusion assays.
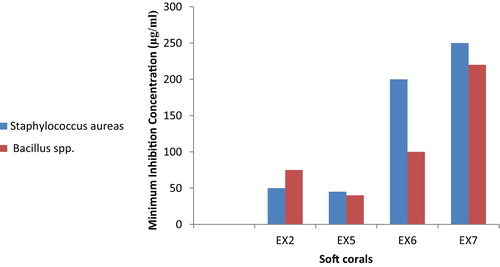
3.4 MIC values for antibacterial activity against human pathogenic bacteria
Of the studied extracts, again the two species S. polydactyla and Sarcophyton spp. showed good activity against all isolates of clinical bacteria (Bacillus spp. and S. aureus), with minimum inhibition concentrations (MIC) in the range of 40–75 μg/ml (). Of the active soft coral species examined, Xenia spp. exhibited the lowest antibacterial activity (MIC ≥ 200 μg/ml).
4 Discussion
As a consequence of the large proportion of bioactive metabolites with different biological activities provided by marine organisms, coral reef ecosystems have become sources of great interest to natural product discovery [Citation25]. The current study was intended to screen the organic extracts of five soft coral species collected from the coral reef ecosystems at Yanbu, Saudi Arabia on the Red Sea coast and test their potential to inhibit the growth of clinical and natural marine bacteria.
Our results indicate that all of the tested soft coral extracts possessed antibacterial activity against marine bacteria isolated from the seawater surrounding the corals. These results agree with the results of [Citation17], who observed that the majority (83%) of crude extracts from six soft corals from Aqaba, Red Sea were active against marine bacteria. This observation also supports the results of [Citation16], who observed that among the isolated strains, 80% showed activity against at least one indicator microbe.
It was noted that the extract of the soft coral S. polydactyla has effective inhibitory activity compared to other soft corals, inhibiting all Gram-positive bacterial isolates. Similar results were obtained by [Citation26], who found that the genus Sinularia produced antibacterial compounds that were stronger than similar compounds obtained from other genera of soft corals. The same study reported that more than 60% of the studied soft coral species of Sinularia contained terpenoid compounds. Furthermore, antagonistic activity was reported for bacterial symbionts of the soft coral S. polydactyla against Streptococcus equi and tuberculosis (TB) bacteria [Citation27].
The present study also showed that the crude extracts of Sarcophyton sp. (cap) and Sarcophyton sp. (stalk) had a considerable effect in the inhibition of tested marine and human pathogenic bacteria. Sarcophyton is well known as a producer of sarcophine derivatives [Citation28–Citation30]. Sarcophine was isolated from S. glaucum collected from the Red Sea, with yields up to 3% of the animal dry weight [Citation31]. The negative or weak activity detected in the whole specimens of the genus Sarcophyton may be linked to extracts gained from two different Sarcophyton species. The high activity allocated to the capitulum and stalk portions of Sarcophyton can be explained as these portions being the most valuable and having the greatest probability of being attacked or eaten following the optimal defence theory [Citation32,Citation33]. Various activities were displayed by the diterpene sarcophytolide isolated from the soft coral S. glaucum collected from Hurghada, Red Sea, Egypt [Citation29]. Two hundred and five terpenes had been previously isolated from the genus Sarcophyton (16 species), demonstrating various biological features, such as antiviral, antifouling, antifeedant, and anti-inflammatory activities [Citation34].
Appreciable and variable antibacterial activities were previously detected against different potentially pathogenic fish and human bacteria for crude extracts of ten collected Red Sea soft corals from Egypt [Citation35]. Terpenoids were the dominant bioactive molecules in various Red Sea soft corals including S. glaucum, Sarcophyton trocheliophorum, S. polydactyla, Sooglossus gardineri, Litophyton arboreum, Lobophyton spp., Xenia spp. and Cladiella pachyclados [Citation36–Citation39].
In conclusion, soft corals of Red Sea have two values, an ecological value that helps the organism live and survive in its natural habitat and a medicinal value of the antibacterial substances produced by the soft corals. Therefore, the current study could provide evidence that soft corals of the Red Sea (Yunbu, SA) are of interest as a source of new bioactive molecules.
The current study recommends the proper molecular identification of the genus Sarcophyton as well as its associated marine bacteria at the species level in addition to the isolation and structural determination of its active secondary metabolites.
Acknowledgments
I would like to thank the Scientific Research Deanship of Taibah University for financial support. Many thanks to Fahd Al Ahmary and Motaz Berry for their help in collecting the samples and to Dr Wael Samir for helping in the bacteria categorization. I also appreciate the help of the technicians in the Biology Department.
Notes
Peer review under responsibility of Taibah University.
References
- B.HaefnerDrugs from the deep: marine natural products as drug candidatesDrug Discov. Trends Pharmacol. Sci.202003196198
- J.W.BluntB.R.CoppM.H.MunroP.T.NorthcoteM.R.PrinsepMarine natural productsNat. Prod. Rep.312014160258
- A.MartinsH.VieiraH.GasparS.SantosMarketed marine natural products in the pharmaceutical and cosmeceutical industries: tips for successMar. Drugs122201410661101
- J.W.BluntB.R.CoppR.A.KeyzersM.H.MunroM.R.PrinsepMarine natural productsNat. Prod. Rep.322015116211
- D.SipkemaM.C.R.FranssenR.OsingaJ.TramperR.H.WijffelsMarine sponges as pharmacyMar. Biotechnol.72005142162
- T.TurkW.R.KemThe phylum Cnidaria and investigations of its toxins and venoms until 1990Toxicon54200910311037
- J.C.CollThe chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia)Chem. Rev.921992613631
- M.E.F.HegazyT.A.MohamedM.A.AlhammadyA.M.ShaheenE.H.RedaA.I.ElshamyP.W.ParéMolecular architecture and biomedical leads of terpenes from red sea marine invertebratesMar. Drugs135201531543181
- J.VerseveldtReport on the Octocorallia (Stolonifera and Alyonacea) of the Israel South Red Sea expedition 1962, with notes on other collections from the Red SeaSea Fish. Res. Stn. Haifa Bull.4019652848
- J.VerseveldtReport on some Octocorallia (Alcyonacea) from the northern Red SeaIsr. J. Zool.191970209229
- Y.BenayahuXeniidae (Cnidaria: Octocorallia) from the Red Sea with description of a new speciesZool. Med. Leiden641990113120
- S.S.Al-LihaibiW.M.AlarifA.Abdel-LateffS.N.AyyadA.B.Abdel-NaimF.F.El-SendunyF.A.BadriaThree new cembranoid-type diterpenes from Red Sea soft coral Sarcophyton glaucum: isolation and anti-proliferative activity against HepG2 cellsEur. J. Med. Chem.812014314322
- A.ElkhateebA.A.El-BeihA.M.Gamal-EldeenM.A.AlhammadyS.OhtaP.W.ParéM.F.HegazyNew terpenes from the Egyptian soft coral Sarcophyton ehrenbergiMar. Drugs12201419771986
- R.F.Abou El-EzzS.A.AhmedM.M.RadwanN.A.AyoubM.S.AfifiS.A.RossP.T.SzymanskiH.FahmyS.I.KhalifaBioactive cembranoids from the Red Sea soft coral Sarcophyton glaucumTetrahedron Lett.542013989992
- M.F.HegazyT.A.MohamedaF.F.Abdel-LatifM.S.AlsaidA.A.ShahatP.W.ParéTrochelioid A and B, new cembranoid diterpenes from the Red Sea soft coral Sarcophyton trocheliophorumPhytochem. Lett.62013383386
- A.ElAhwanyH.A.GhozlanH.A.ElSharifS.A.SabryPhylogenetic diversity and antimicrobial activity of marine bacteria associated with the soft coral Sarcophyton glaucumJ. Basic Microbiol.5512015210
- Y.KelmanE.KashmanA.RosenbergY.LoyaAntimicrobial activity of Red Sea coralsMar. Biol.1492006357363
- J.VerseveldtY.BenayahuOn two old and fourteen new species of alcyonacea (Coelenterates, Octocorallia) from the Red SeaZool. Verh.20811983133
- J.VerseveldtA revision of the genus Sarcophyton lesson (Octocorallia, Alcyonacea)Zool. Verh.19211982191
- J.VerseveldtA revision of the genus Sinularia may (Octocorallia, Alcyonacea)Zool. Verh.17919801128
- G.HoltN.KriegP.SneathJ.StanelyS.WilliamsBergey's Manual of Determinative Bacteriology1994Williams & WilkinsBaltimore
- S.S.MagaldiC.Mata-EssayagC.HartungdeM.T.PerezO.CarolinaO.YudithWell diffusion for antifungal susceptibility testingInt. J. Inf. Dis.820043945
- T.HaugA.K.KjuulO.B.StyrvoldE.SandsdalenQ.M.OlsenK.StensvagAntibacterial activity in Strongylocentrotus droebachiensis (Echinoidea), Cucumaria frondosa (Holothuroidea), and Asterias rubens (Asteroidea)J. Invertebr. Pathol.81200294102
- A.J.LawrenceR.AfifiM.AhmedS.KhalifaT.PagetBioactivity as an options value of sea cucumbers in the Egyptian Red SeaConserv. Biol.2412010217225
- D.J.FaulknerMarine pharmacologyAntonie Van Leeuwenhoek772000135
- M.K.KhalesiR.K.BeeftinkR.N.WijffelsThe soft coral Sinularia flexibilis: potential for drug developmentR.J.LeewisM.JanseAdvances in Coral Husbandry in Public Aquariums2008Burgers’ ZooArnhem, the Netherlands4760
- O.K.RadjasaT.MartensH.P.GrossartT.BrinkoffA.SabdonoM.SimonAntagonistic activity of a marine bacterium Pseudoalteromonas luteoviolacea TAB4.2 associated with coral Acropora sp.J. Biol. Sci.72007239246
- T.A.TemrazW.E.HoussenM.JasparsD.R.Woolleyet alA pyridinium derivative from Red Sea soft corals inhibited voltage-activated potassium conductance's increased excitability of rat cultured sensory neuronsBMC Pharmacol.62006119
- G.N.MohamedS.KawtherA.AhmedA.AidaH.ZainabM.M.HagerK.A.M.ShadenA.HapipahE.R.HeshamAntibacterial effect of the Red Sea soft coral Sarcophyton trocheliophorumNat. Prod. Res.201610.1080/14786419.2015.1040991
- A.N.BadriaW.A.GuirguisS.PerovicR.SteffenW.E.G.MüllerC.S.HeinzSarcophytolide: a new neuroprotective compound from the soft coral Sarcophyton glaucumToxicology2–3161998133143
- S.SawantD.YoussefA.MayerP.SylvesterV.WaliM.ArantK.El SayedAnticancer and anti-inflammatory sulfur-containing semisynthetic derivatives of sarcophineChem. Pharmaceut. Bull.548200611191123
- R.A.ZangerlC.E.RutledgeThe probability of attack and patterns of constitutive and induced defense: a test of optimal defense theoryAm. Nat.1471996599608
- B.G.TothO.LanghamerH.PaviaInducible and constitutive defenses of valuable seaweed tissues: consequences for herbivore fitnessEcology862005612618
- L.LiangG.Yue-WeiTerpenes from the soft corals of the genus Sarcophyton: chemistry and biological activitiesChem. Biodivers.1012201321612196
- H.A.IbrahimS.Z.MohamedM.A.El-RegalA.Z.FarhatAntibacterial activity of some Red Sea soft corals, EgyptBlue Biotechnol. J.142012497516
- E.A.AboutablS.M.AzzamC.G.MichelN.M.SelimM.F.HegazyA.A.M.AliA.A.HusseinBioactive terpenoids from the Red Sea soft coral Sinularia polydactylaNat. Prod. Res.27201322242226
- M.F.HegazyA.M.Gamal EldeenA.A.ShahatF.F.Abdel-LatifT.A.MohamedB.R.WhittleseyP.W.ParéBioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucumMar. Drugs102012209222
- M.F.HegazyA.A.El-BeihbA.Y.MoustafadA.A.HamdyM.A.AlhammadyR.M.SelimM.Abdel-RehimbP.W.ParéCytotoxic cembranoids from the Red Sea soft coral Sarcophyton glaucumNat. Prod. Commun.6201118091812
- H.M.HassanM.A.KhanfarA.Y.ElnagarR.MohammedL.A.ShaalaD.T.A.YoussefM.S.HifnawyK.A.El SayedPachycladins A–E, prostate cancer invasion and migration inhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachycladosJ. Nat. Prod.732010848853