?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A simple, selective and precise method based on HPTLC has been developed for the simultaneous determination of aliskiren and hydrochlorothiazide in a fixed-dose tablet formulation and human plasma. The chromatography was performed on silica gel 60 GF254 plates, with a mobile phase consisting of methanol–chloroform (6:4, v/v). Densitometric analysis of the analytes was carried out at 225 nm. Under optimized conditions, the Rf values were 0.26 ± 0.02 and 0.71 ± 0.02, and the resulting regression plots were linear (r2 ≥ 0.9997) in the concentration ranges of 1.00–10.0 and 0.10–1.00 μg band−1 for aliskiren and hydrochlorothiazide. The limit of detection and limit of quantitation of the validated method were 0.206 and 0.624 μg band−1 for aliskiren and 0.015 and 0.046 μg band−1 for hydrochlorothiazide, respectively. The % expected content of aliskiren and hydrochlorothiazide in the commercial tablet formulation was 99.2% and 101.3%, respectively. For spiked plasma sample preparation, the analytes and nebivolol internal standard were extracted from 500 μL of plasma sample by solid-phase extraction on LiChrosep® DVB-HL cartridges. The mean extraction recovery of aliskiren and hydrochlorothiazide from human plasma was 87.2% and 76.5%, respectively. In addition, the stability of the analytes in plasma was established under different storage conditions.
1 Introduction
Hypertension is one of the major causes of many cardiovascular diseases including stroke, myocardial infarction and congestive heart failure. Thus, efficient control and management of hypertension is essential to minimize cardiovascular mortality and morbidity [Citation1–Citation3]. Generally, the target blood pressure (BP) is below 140/90 mmHg in hypertension patients. Reduction in blood pressure significantly reduces the risk of organ damage, vascular diseases and death. Currently, several therapies are available to lower blood pressure, including mono and combination therapy with β-blockers, diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB) and calcium channel antagonists [Citation4]. It has been observed that combination therapies using agents that have complementary mechanisms of action as inhibitors of the renin-angiotensin-aldosterone system (RAAS) are essential for patients at high cardiovascular and renal risk.
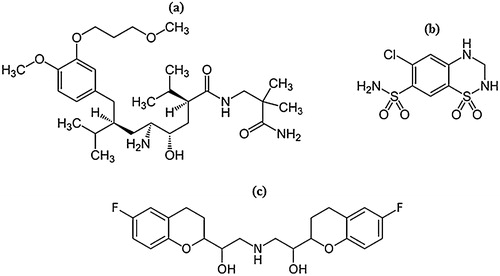
Aliskiren (ALS, a) is the first orally active non-peptide inhibitor that directly targets renin, and it was approved by the US Food and Drug Administration in March 2007 [Citation3]. ALS inhibits RAAS at its rate-limiting step by reducing the plasma renin activity (PRA). ALS effectively controls blood pressure and is generally well tolerated as either a monotherapy or in combination with other antihypertensive drugs such as hydrochlorothiazide (HCTZ, b), amlodipine and valsartan [Citation4,Citation5]. HCTZ is a diuretic drug of the thiazide class that inhibits active chloride reabsorption and thus increases the excretion of sodium chloride and water. It has been demonstrated that a fixed dose combination of ALS with HTCZ in hypertensive patients provides long-term efficacy and safety in blood pressure control compared to monotherapy [Citation5].
Several methods have been reported for the determination of ALS as an isolated sample or together with other antihypertensive drugs in more complicated matrices such as pharmaceutical formulations and biological fluids. These methods have been based on spectrophotometry [Citation6–Citation8], spectrofluorometry [Citation9,Citation10], electrophoresis [Citation11,Citation12], liquid chromatography (LC) [Citation8,Citation13–Citation21], LC–MS/MS [Citation22–Citation25] and UPLC–MS/MS [Citation26]. Similarly, a number of analytical methods have been presented for the estimation of HCTZ as a single analyte [Citation27] and in the presence of different antihypertensive agents [Citation8,Citation12,Citation14,Citation16,Citation18,Citation19,Citation28–Citation39] in pharmaceutical preparations and biological matrices using spectrophotometry [Citation29,Citation32], capillary electrophoresis [Citation28], HPTLC [Citation30,Citation31], LC [Citation33–Citation35], UPLC [Citation36], LC–MS/MS [Citation37,Citation38] and UPLC–MS/MS [Citation39]. However, there are no reports for the simultaneous determination of aliskiren and hydrochlorothiazide by HPTLC. This technique affords parallel analyses of multiple samples in a single run while using only small amounts of solvents, thereby reducing the time and cost of analysis. Moreover, it provides accurate and precise results and is much easier to carry out compared to HPLC or LC–MS. In the present work, both drugs were analysed in their fixed dose binary formulation (Tekturna HCT®, Novartis Pharmaceuticals) and in human plasma by HPTLC. The methods developed were validated according to ICH guidelines for linearity, sensitivity, selectivity, accuracy and precision, recovery, stability and robustness [Citation40].
2 Experimental
2.1 Chemicals and materials
Reference standards of aliskiren hemifumarate (ALS, 99.40%), hydrochlorothiazide (HCTZ, 99.52%) and nebivolol (NBL, 99.8%, c) were purchased from TLC Pharmachem Inc. (Ontario, Canada). HPLC grade chloroform and methanol, acetic acid and ammonia solutions were purchased from E. Merck (Mumbai, India). Aluminium-backed HPTLC pre-coated silica gel Plates 60 GF254 (20 cm × 10 cm, 200 μm layer thickness) were procured from Merck KGaA (Darmstadt, Germany). Pharmaceutical formulation, Tekturna HCT® (300 mg aliskiren and 25 mg hydrochlorothiazide) combination tablets were purchased from Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. LiChrosep® DVB-HL (30 mg, 1 cc) solid-phase extraction (SPE) cartridges were obtained from Merck Pvt. Ltd. (Mumbai, India). Blank human plasma in K3EDTA was obtained from Supratech Micropath (Ahmedabad, India) and stored at −20 °C until use.
2.2 HPTLC equipment and conditions
The HPTLC system (CAMAG, Muttenz, Switzerland) consisted of a TLC Scanner III, Linomat V auto-sprayer connected to a nitrogen cylinder and plate heater. The plates were washed with methanol and activated at 105 °C for 20 min prior to use. The calibration standards, quality controls and samples of pharmaceutical formulation were applied to the plates as 6-mm-wide bands with 6-mm spacing between each band using a Hamilton microliter syringe and Linomat V sample applicator. The sample application speed was set at 10 mm s−1. The distance from the plate side edge and the bottom of the plate was 10 mm. After the plates were air dried, linear ascending chromatography was carried out using methanol–chloroform (6:4, v/v) as the mobile phase in a 20 cm × 10 cm twin-through glass chamber (CAMAG), which was pre-saturated with mobile phase (for 15 min), at 25 ± 1 °C and 60 ± 5% relative humidity. The development distance was 8.0 cm, and the development time was 20 min. Densitometric scanning was performed in the absorbance–reflectance mode at 225 nm after scanning between 200 and 400 nm using a deuterium lamp. The slit dimensions were 5 mm in length and 0.45 mm in width, with a scanning rate of 20 mm s−1. Each track was scanned three times, and baseline correction was used. The winCATS software version 1.4.2 (CAMAG) was used to control the operating parameters during the entire experiment.
2.3 Preparation of standard solutions, calibration standards and quality control samples
Separate standard stock solutions of ALS (2.0 mg mL−1, free base form) and HCTZ (1.0 mg mL−1) were prepared by dissolving appropriate amounts in methanol.
To prepare calibration plots, 1.0, 2.0, 3.0, 4.5, 6.0, 8.0 and 10.0 μL of ALS working solution (1000 μg mL−1) and 1.0, 2.0, 3.0, 4.5, 6.0, 8.0 and 10.0 μL of HCTZ working solution (100 μg mL−1) were applied by co-spotting on TLC plates to achieve a concentration in the ranges of 1.00–10.0 μg band−1 for ALS and 0.10–1.00 μg band−1 for HCTZ. The plates were developed and scanned as described above. Similarly, quality control (QC) samples were prepared at 2.50/0.25, 5.00/0.50 and 9.00/0.90 μg band−1 for ALS/HCTZ.
To prepare spiked plasma calibration samples, a suitable aliquot from the respective stock solutions was added to 0.5 mL of drug-free plasma (5% in blank human plasma) to obtain concentrations in the ranges of 1.00–10.0 μg band−1 for ALS and 0.10–1.00 μg band−1 for HCTZ. Likewise, QC samples were prepared at 2.50/0.25, 5.00/0.50 and 9.00/0.90 μg band−1 for ALS/HCTZ. In addition, a stock solution of nebivolol (1.0 mg mL−1) was prepared in methanol and used as an internal standard.
2.4 Solid phase extraction of plasma samples
NaOH (50 μL, 0.01 N) was added to spiked plasma samples (CSs and QCs), and the samples were briefly vortexed for 15 s. The samples were then loaded on LiChrosep® DVB-HL extraction cartridges, which were preconditioned with 1.0 mL of methanol followed by 1.0 mL of 0.1% acetic acid in water. The samples were washed with 2 × 1.0 mL of 0.1% acetic acid in water. The analytes and IS were eluted using 750 μL of 2% ammonia solution in methanol and collected in pre-labelled vials. The samples were then evaporated to dryness under a gentle stream of nitrogen at 30 °C. The dried samples were reconstituted with 500 μL of methanol and briefly vortexed, and 10 μL was used for band spotting on TLC plates.
2.5 Analysis of tablet formulation
To determine the contents of ALS and HCTZ in Tekturna HCT® tablet formulation (300 mg of aliskiren and 25 mg of hydrochlorothiazide), 20 tablets were weighed and ground to a fine powder. An amount equivalent to 300 mg of ALS and 25 mg of HCTZ was transferred into a 100-mL volumetric flask containing 50 mL of methanol, sonicated for 30 min and diluted with additional methanol. After filtration, a working solution having concentrations of 300 μg mL−1 ALS and 25 μg mL−1 HCTZ was prepared by diluting the stock solution with methanol, and 10 μL was applied to the plates in six replicates. After chromatographic development, the peak areas of the bands were measured at 225 nm, and the amount of each drug present in the tablet was estimated from the regression equations.
3 Results and discussion
3.1 Method development
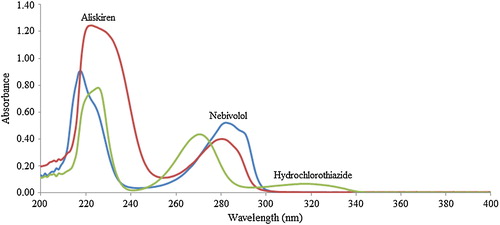
To select the analytical wavelength for the quantification of the drugs, standard solutions were prepared, and their UV absorption spectra were acquired using a spectrophotometer (). Upon assessment of their overlain spectra, it was observed that the selected drugs exhibited considerable absorbance at 225 nm, and hence, this wavelength was selected for further analysis.
Fig. 2 Zero-order spectra of aliskiren (25 μg mL−1), hydrochlorothiazide (5 μg mL−1) and nebivolol (IS, 5.0 μg mL−1) in methanol.
3.1.1 Optimization of chromatography
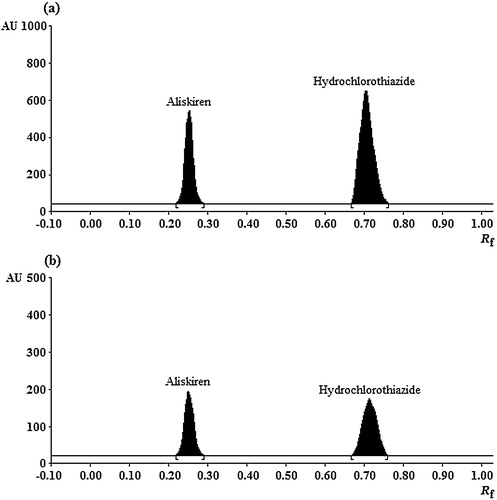
For chromatographic elution of analytes and IS, several trials were carried out using toluene, n-hexane, ethyl acetate, chloroform, dichloromethane, acetonitrile and methanol as single mobile phase solvents and their binary combinations. Initial experiments with single solvents revealed negligible migration of NBL (retention factor, Rf < 1.0) in toluene, n-hexane, ethyl acetate and chloroform, while Rf was greater than 0.6 for ALS and HCTZ with minimal resolution. In pure methanol and acetonitrile, ALS and HCTZ had limited resolution (Rf ∼ 3.5–4.0), while NBL gave a diffused spot and migrated near the solvent front. Thus, optimization of the mobile phase was performed by testing combinations of these solvents. After several tests, it was found that the methanol–chloroform solvent mixture provided better resolution, with relatively lower diffusion and tailing of the ALS band. Further experiments varied their ratio of the two solvents, and finally, the mobile phase of methanol–chloroform (6:4, v/v) provided good retention, peak symmetries and adequate resolution between the drugs. The Rf values under the optimized conditions were 0.26 ± 0.02, 0.71 ± 0.03, and 0.56 ± 0.02 for ALS, HCTZ and NBL, respectively ( and ). Several antihypertensive drugs, namely, amlodipine, ramipril, candesartan, propranolol and nebivolol, were tested as internal standards. The results showed that all drugs except nebivolol were unsuitable either due to different wavelength of absorption or limited chromatographic resolution from the analytes.
3.1.2 Optimization of extraction process
To the best of our knowledge, there are no methods for the simultaneous determination of ALS and HCTZ in human plasma. Reported procedures for the estimation of ALS either alone [Citation22–Citation24] or in combination with other antihypertensive drugs [Citation20,Citation25] have used protein precipitation (PP) with acetonitrile [Citation20], liquid–liquid extraction (LLE) with methyl tert-butyl ether [Citation22] or solid phase extraction (SPE) from human serum [Citation23], urine [Citation25] or saliva [Citation24]. Thus, to develop a simple method, initial trials were performed using acetonitrile as protein precipitant as reported by Mannemala and Nagarajan [Citation20]. Although the recovery for ALS was quantitative (∼90%), the recovery of HCTZ was poor (35–40%). Thus, further trials were conducted using SPE with LiChrosep® DVB-HL extraction cartridges. Washing and elution steps were carefully optimized for precise recovery at all QC levels. Selective use of 0.1% acetic acid in water for washing and 2% ammonia solution in methanol for elution of analytes resulted in consistent recovery for the analytes as well as the IS.
3.2 Method validation
The developed method was validated for linearity, accuracy (recovery) and precision, selectivity, limit of detection (LOD) and limit of quantitation (LOQ), robustness and stability as per ICH [Citation40] and USFDA [Citation41] guidelines. Linearity was determined by analysis of five linear curves in the concentration range of 1.00–10.0 μg band−1 for ALS and 0.10–1.00 μg band−1 for HCTZ. The calibration plots for ALS and HCTZ were constructed by plotting the area under the peak against analyte concentration and the regression equations were computed from these plots. The calibration curves showed good linearity (r2 ≥ 0.9997) over the concentration range for both of the analytes, as shown in . For plasma samples, peak area ratio (analyte/IS) versus the concentration was plotted with r2 ≥ 0.9998 for ALS and HCTZ.
Table 1 Linear regression and statistical data for aliskiren and hydrochlorothiazide.
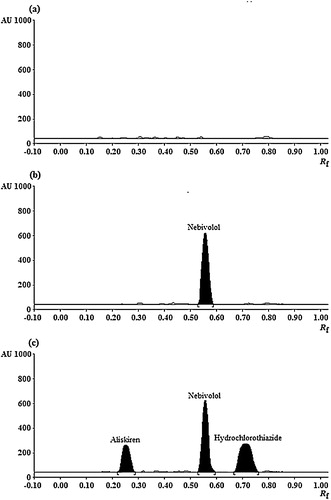
The specificity of the method was assessed by analysing the bands (in terms of Rf values) obtained for the samples with those of standards, and the results obtained were in good correlation. For the bioanalytical method, the specificity in the measurement of the Rf values (six replicates) was in the range of 1.24–1.87%, with the value expressed as % RSD. Similarly, the peak purity of ALS and HCTZ was determined by comparing three different positions of the chromatograms: peak start, peak apex and peak end. The results found were in close agreement for both of the analytes. Further, for plasma samples, the selectivity was evaluated by analysing blank plasma, plasma spiked with IS and together with the analytes and IS, as shown in . The densitograms showed no interference of plasma components at the Rf values of the analytes or IS. At the same time, there was no change in the Rf values in the plasma for both of the analytes. The LOD and LOQ for the analytes were calculated from the slope of the calibration lines and the standard deviation of the intercept. The results for neat solutions (aqueous samples) and in plasma are summarized in .
Fig. 4 HPTLC densitograms of (a) blank human plasma, (b) blank plasma spiked with nebivolol (1.00 μg band−1) and (c) plasma sample spiked with aliskiren (5.00 μg band−1), hydrochlorothiazide (0.50 μg band−1) and nebivolol (1.00 μg band−1).
To study the intra-day (repeatability) and inter-day precision (intermediate precision) of the method, six replicates of the mixed standards of ALS/HCTZ at 2.50/0.25, 5.00/0.50, 9.00/0.90 μg band−1 were analysed on the same day and on three consecutive days, respectively. The intra- and inter-day precision (% RSD) for neat samples and in plasma was 0.46–1.75% and 0.75–5.31%, respectively, for both the analytes (). The accuracy (recovery) for neat samples was determined by a standard addition technique. This was performed at three levels: 80%, 100% and 120% of pre-analysed samples. For plasma samples, the absolute recovery was determined by comparing the peak areas of freshly prepared (extracted) samples with unextracted standard solutions having identical concentration at three QC levels [Citation41]. The mean recovery of NBL was 89.3%. The detailed results are presented in .
Table 2 Intra- and inter-day accuracy and precision data for aliskiren and hydrochlorothiazide.
Table 3 Recovery of drugs in dosage forms by standard addition method and in human plasma (n = 6).
The stability of the stock solution and working solution of analytes and IS were checked for short-term stability at room temperature and long-term stability at 5 °C. The samples at room temperature were stable for a minimum period of 36 h, while the samples at 5 °C were stable for at least 30 days. Plasma sample stability was evaluated by measuring the area ratio response (analyte/IS) of samples against freshly prepared comparison standards with identical concentrations at two QC levels. Bench-top stability at 25 ± 1 °C, freeze–thaw stability, processed sample stability, dry extract stability at 5 ± 3 °C and long-term stability (at −70 °C) was performed at both QC levels in six replicates. The results indicate no significant change in the stability of the analytes under any condition ().
Table 4 Stability data of aliskiren and hydrochlorothiazide under different conditions (n = 6).
Method robustness was tested under five different conditions by changing the composition of mobile phase components (±0.2 mL for each), the volume of methanol–chloroform (6.2:4.1, 5.8:3.9 and 6.3:4.2 (v/v)), the chamber saturation time (±3.0 min) and the time from band application to chromatography and from chromatography to band scanning (±10.0 min). The results shown in establish that alteration of the analytical conditions does not affect the performance of the method significantly, as evident from the precision values in the measurement of peak area of the analytes. This confirms the robustness of the developed method. Finally, the method was used to analyse a commercially available tablet formulation, Tekturna HCT® (300 mg aliskiren and 25 mg hydrochlorothiazide). The average assay % (± SD) for ALS was 99.6 ± 0.74% and 100.2 ± 0.53% for HCTZ, which proves that the method is suitable for the analysis of ALS and HCTZ from the pharmaceutical formulation without any interference from the excipients.
Table 5 Results for robustness of the method (n = 5).
4 Conclusion
A selective and robust HPTLC method has been developed for the determination of ALS and HCTZ in tablet formulation and spiked human plasma following ICH guidelines. This is possibly the first method for the simultaneous estimation of this drug combination in human plasma by any technique. Further, the statistical analysis proves that the method is suitable for analysing ALS and HCTZ in their pure form and in pharmaceutical preparations without any interference from the excipients. The validation data suggest that the proposed densitometric method is reproducible, accurate and precise, and it can be readily applied for pharmacokinetic/bioequivalence studies.
Conflict of interest
None declared.
Acknowledgements
The authors are thankful to the Department of Zoology, Gujarat University and Veeda Clinical Research Pvt. Ltd for providing infrastructure facility to carry out some experiments.
Notes
Peer review under responsibility of Taibah University.
References
- S.VaidyanathanV.JarugulaH.A.DieterichD.HowardW.P.DoleClinical pharmacodynamics and pharmacokinetics of aliskirenClin. Pharmacokinet.472008515531
- S.MorgantiC.LonatiAliskiren: the first direct rennin inhibitor available for clinical useJ. Nephrol.242011541549
- S.SenS.SabirliT.OzyigitY.UresinAliskiren: review of efficacy and safety data with focus on past and recent clinical trialsTher. Adv. Chronic Dis.42013232241
- M.B.HovaterE.A.JaimesOptimizing combination therapy in the management of hypertension: the role of the aliskiren, amlodipine, and hydrochlorothiazide fixed combinationIntegr. Blood Press. Control620135967
- M.BurnierFixed combinations in the pragmatic management of hypertension: focus on aliskiren and hydrochlorothiazide as a single pillIntegr. Blood Press. Control320105762
- M.Wrasse-SangoiL.T.SecrettiI.F.DiefenbachC.M.B.RolimM.S.SangoiDevelopment and validation of an UV spectrophotometric method for the determination of aliskiren in tabletsQuim. Nova33201013301334
- M.A.RamadanM.B.AbuiribanDevelopment and validation of a spectrophotometric method for determination of aliskiren in tablets using o-phthalaldehydeInt. J. Pharm. Sci. Rev. Res.212013333337
- M.I.EzzeldinE.ShokryM.A.FouadR.I.ElbagaryApplication of chromatographic and spectrophotometric methods for the analysis of aliskiren and hydrochlorothiazide antihypertensive combinationInt. J. Adv. Chem.120131320
- Z.AydoğmuşSpectrofluorimetric determination of aliskiren in dosage forms and urineLuminescence272012489494
- Z.AydogmusF.SariS.T.UluSpectrofluorimetric determination of aliskiren in tablets and spiked human plasma through derivatization with dansyl chlorideJ. Fluoresc.222012549556
- M.S.SangoiM.Wrasse-SangoiP.R.OliveiraC.M.B.RolimM.SteppeSimultaneous determination of aliskiren and hydrochlorothiazide from their pharmaceutical preparations using a validated stability-indicating MEKC methodJ. Sep. Sci.34201118591866
- M.M.SalimW.M.EbeidN.El-EnanyF.BelalM.WalashG.PatonaySimultaneous determination of aliskiren hemifumarate, amlodipine besylate, and hydrochlorothiazide in their triple mixture dosage form by capillary zone electrophoresisJ. Sep. Sci.37201412061213
- G.K.SwamyJ.V.L.N.S.RaoJ.M.R.KumarU.A.KumarD.V.R.N.BikshapathiD.V.KumarAnalytical method development and validation of aliskiren in bulk and tablet dosage form by RP-HPLC methodJ. Pharm. Res.42011865867
- M.S.SangoiM.Wrasse-SangoiP.R.OliveiraV.TodeschiniC.M.B.RolimRapid simultaneous determination of aliskiren and hydrochlorothiazide from their pharmaceutical formulations by monolithic silica HPLC column employing experimental designsJ. Liq. Chromatogr. Relat. Technol.492011170175
- M.Wrasse-SangoiM.S.SangoiP.R.OliveiraL.T.SecrettiC.M.B.RolimDetermination of aliskiren in tablet dosage forms by a validated stability-indicating RP-LC methodJ. Chromatogr. Sci.492011170175
- F.BelalM.WalashN.El-EnanyS.ZayedSimultaneous determination of aliskiren and hydrochlorothiazide in tablets and spiked human urine by ion-pair liquid chromatographyPharmazie682013933938
- F.BelalM.WalashN.El-EnanyS.ZayedHighly sensitive HPLC method for assay of aliskiren in human plasma through derivatization with 1-naphthyl isocyanate using UV detectionJ. Chromatogr. B93320132429
- V.R.B.VemulaP.K.SharmaI.SinghviA validated RP-HPLC method for determination of aliskiren and amlodipine in tablet dosage formInt. J. Pharm.32013601606
- R.I.El-BagaryG.PatonayA.A.ElzahrE.F.ElkadyW.A.EbeidIon-pair LC method for simultaneous determination of aliskiren hemifumarate, amolodipine besylate and hydrochlorothiazide in pharmaceuticalsChromatographia772014257264
- S.S.MannemalaJ.S.NagarajanDevelopment and validation of a HPLC-PDA bioanalytical method for the simultaneous estimation of aliskiren and amlodipine in human plasmaBiomed. Chromatogr.292015346352
- F.A.OzdemirA.AkyüzSimultaneous determination of amlodipine and aliskren in tablets by high-performance liquid chromatographyJ. Chromatogr. Sci.522014685690
- V.AdireddyN.R.PilliV.R.DerangulaS.R.SatlaC.V.GanguriV.PonneriLiquid chromatography-tandem mass spectrometric assay for aliskiren, a novel renin inhibitor in micro-volumes of human plasma: a pharmacokinetic application in healthy South Indian male subjectsBiomed. Chromatogr.27201310621069
- B.B.BurckhardtS.RamusovicJ.TinsS.LaeerDetermination of aliskiren in human serum quantities by HPLC-tandem mass spectrometry appropriate for pediatric trialsBiomed. Chromatogr.272013477486
- B.B.BurckhardtJ.TinsS.LaeerLiquid chromatography-tandem mass spectrometry of aliskiren in saliva and its application to a clinical trial with healthy volunteersJ. Pharm. Biomed. Anal.962014118126
- B.B.BurckhardtJ.TinsS.LaeerSimultaneous quantitative and qualitative analysis of aliskiren, enalapril and its active metabolite enalaprilat in undiluted human urine utilizing LC–ESI–MS/MSBiomed. Chromatogr.28201416791691
- S.MagieraFast, simultaneous quantification of three novel cardiac drugs in human urine by MEPS-UHPLC-MS/MS for therapeutic drug monitoringJ. Chromatogr. B93820138695
- F.LiuY.XuS.GaoJ.ZhangQ.GuoDetermination of hydrochlorothiazide in human plasma by liquid chromatography/tandem mass spectrometryJ. Pharm. Biomed. Anal.44200711871191
- M.G.QuagliaE.DonatiG.CarlucciP.MazzeoS.FanaliDetermination of losartan and hydrochlorothiazide in tablets by CE and CECJ. Pharm. Biomed. Anal.292002981987
- R.M.MaggioP.M.CastellanoT.S.KaufmanA multivariate approach for the simultaneous determination of losartan potassium and hydrochlorothiazide in a combined pharmaceutical tablet formulationAnal. Bioanal. Chem.391200829492955
- B.H.MehtaS.B.MorgeHPTLC-densitometric analysis of candesartan cilexetil and hydrochlorothiazide in tabletsJ. Planar Chromatogr.212008173176
- S.S.KadukarS.V.GandhiP.N.RanjaneS.S.RanherHPTLC analysis of olmesartan medoxomil and hydrochlorothiazide in combination tablet dosage formsJ. Planar Chromatogr.222009425428
- M.A.HegazyF.H.MetwalyM.AbdelkawyN.S.AbdelwahabSpectrophotometric and chemometric determination of hydrochlorothiazide and spironolactone in binary mixture in the presence of their impurities and degradantsDrug Test. Anal.22010243251
- S.J.JoshiP.A.KarbhariS.I.BhoirK.S.BinduC.DasRP-HPLC method for simultaneous estimation of bisoprolol fumarate and hydrochlorothiazide in tablet formulationJ. Pharm. Biomed. Anal.522010362371
- H.LiJ.HeQ.LiuZ.HuoS.LiangY.LiangY.ItoSimultaneous determination of hydrochlorothiazide and reserpine in human urine by LC with a simple pre-treatmentChromatographia732011171175
- I.SalamaSimultaneous HPLC-UV analysis of telmisartan and hydrochlorothiazide in human plasmaBull. Faculty Pharm. Cairo Uni.4920111924
- B.SatheeshS.K.PulluruK.NitinD.SaravananSimultaneous determination of eprosartan, hydrochlorothiazide and their related compounds in pharmaceutical dosage forms by UPLCJ. Liq. Chromatogr. Relat. Technol.34201118851900
- D.V.BharathiK.K.HothaP.K.ChatkiV.SatyanarayanaV.VenkateswarluLC–MS/MS method for simultaneous estimation of candesartan and hydrochlorothiazide in human plasma and its use in clinical pharmacokineticsBioanalysis4201211951204
- A.G.JangidR.H.TaleV.V.VaidyaA single, selective and simple validated method for simultaneous estimation of amiloride and hydrochlorothiazide in human plasma by liquid chromatography–tandem mass spectrometryBiomed. Chromatogr.26201295100
- X.QiuZ.WangB.WangH.ZhanX.PanR.XuSimultaneous determination of irbesartan and hydrochlorothiazide in human plasma by ultra high performance liquid chromatography tandem mass spectrometry and its application to a bioequivalence studyJ. Chromatogr. B9572014110115
- ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2 (R1), Geneva, November 2005.
- Guidance for Industry, Bioanalytical Method ValidationMay 2001US Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM)