Abstract
This paper uses a statistical approach to evaluate the degree of metal pollution, trace element concentrations, and seasonal evolutions of various physicochemical parameters of Moroccan drinking water sludge. Principal component analysis (PCA) was used to analyze these physicochemical parameters (including water temperature (Tw), volatile suspended solids (VSS), and suspended matter (SM)) in liquid raw sludge, the conductivity (Cond(s) and pH(s)) in addition to the trace element content of the supernatant (e.g., Cd(s), Pb(s), Cr(s)), pH and heavy metal and trace element contents (e.g., Pb, Cr, Cd, Fe, Al, Cu, Zn, P, N, K and C) in dried hydroxide sludge. The measured quantities of Cd and Cr in the supernatant did not exceed their recommended limits in wastewater treated for irrigation; however, the amount of Zn, Pb and Cu measured in dried hydroxide sludge exceeded their limits as defined by international standards. PCA reveals that the principal component F1 indicates that 25.20% of all variance can be mostly attributed to Zn content and conductivity, while the principal component F2 demonstrates that 21.00% of all variance is likely caused by the previously mentioned physicochemical parameters, most significantly C and pH(s). Finally, this paper analyses the merits of this analytical approach and discusses its important applications for solving crucial environmental issues.
1 Introduction
Heavy metal pollution is one of the most serious environmental issues that has accompanied rapid global economic development [Citation1]. For example, heavy metal pollution in aquatic systems poses a significant risk to human health because heavy metals are not biodegradable and may thus affect the ecological food chain [Citation1,Citation2]. In general, heavy metal pollution occurs when industrial processes (including water purification or treatment) generate waste that is released into the environment. This waste includes large quantities of organic pollutants, including heavy metals, which are then absorbed by sludge; in this way, sludge becomes a reservoir for heavy metals [Citation2,Citation3].
Drinking water treatment plants generate hydroxide sludge after water passes through filters and sludge blanket decanters [Citation3–Citation5]. The process of clarification removes suspended matter (SM) and colloids from treated water in three successive operations: coagulation, flocculation and sedimentation. All sludge is defined as being a highly wet waste with liquids of insignificant value [Citation6]; hydroxide sludge has a very similar constitution but differs only in its suspended matter concentration. Sludge settling ponds mostly consist of suspended solids in raw water with a body of metal hydroxide produced by coagulation [Citation3,Citation4,Citation7,Citation8]. These ponds may also contain low CaO contents if interacting with clay derived from a limestone, as has been seen in the sludge of the Bouregreg drinking water production unit. Washing water filters essentially contain the same residues as those found in sludge settling tanks, although with much lower concentrations [Citation3,Citation9].
Of all water pollutants, heavy metals are one of the longest lasting and most dangerous. Heavy metals can be present in sludge in variable concentrations, depending on the origin of the sludge (i.e., industrial or residential), and they remain a major obstacle in sludge valorization. Metals found in sludge typically exist in soluble, exchangeable and adsorbed forms; they are associated mainly with organic matter and are bound to carbonates and sulphides in crystalline residues. Therefore, heavy metals in sludge can be influenced by the presence of complexing agents, precipitating ligands, and physicochemical parameters including pH, temperature and redox potential [Citation10–Citation12]. It is thus necessary to assess what heavy metals are present in the sludge as well as their relative concentrations.
The aim of the present work is to use a statistical approach to perform a detailed quantitative analysis of the distribution of heavy metals in the dried sludge and supernatant from the drinking water treatment plant of Bouregreg (Rabat, Morocco), as well as the trace elements and physico-chemical parameters affecting raw sludge. These results will then be interpreted and discussed, with a particular focus on the valorization of the sludge and potential applications of this research.
2 Materials and methods
The treatment of drinking water at the Bouregreg processing station begins with a pre-treatment, which consists of a screening followed by a period of disinfection or peroxidation, in which chlorine gas is added in a process called pre-chlorination. At the Bouregreg processing station, the most commonly used coagulant is aluminium sulphate Al2(SO4)3, and the most commonly used flocculants are polyelectrolyte or alginate. Decantation then occurs, primarily in the decanter sludge bed. Finally, water undergoes filtration through a series of pools, including the sand layer, and filters are washed by reversing the flow direction of water.
2.1 Characterization
Liquid raw drinking water sludge was collected every month for one year from the drinking water decantation plant of the Bouregreg drinking water treatment plant in Rabat, Morocco. These collected samples of raw sludge underwent a settling period of 2 h to recover each sample’s supernatant as well as its corresponding hydroxide sludge. The supernatant was stored at 4 °C, and the dried sludge was allowed to recover in the open air for 8 days. Physico-chemical parameters for each sample were determined using a variety of techniques.
First, the liquid portion of each sample was allowed to homogenize for 10 min before a pH-metre of mark W.T.W. was used to measure its pH [Citation13]. The conductivity (measured in Cond(s)) of the supernatant was then measured by a W.T.W. Mark conductometer. The filtrate of each sample then passed through a glass micro-fibre filter, and its residue was dried at 105 °C before being weighed again; the resulting measured difference in mass represents the suspended matter (SM) content in the filtered sample volume. The quantity of volatile suspended solids (VSS) was similarly obtained by determining the difference between the weight of the ash residue recorded at 550 °C and its weight following a period of drying at 105 °C [Citation14].
A Flash Thermoanalyser EA 112 was used to measure quantities of C, H, N, S, and O in the samples. Samples first underwent total combustion at 1050 °C under a stream of oxygen and pressure; then, the resulting products were separated by a chromatographic column and measured by a thermal conductivity detector.
Quantities of heavy metals were measured by an Ultima 2 Jobin Yvon ICP-AES (France) atomic emission spectrometer with a spectral range of 120–800 nm. Prior to this analysis, samples underwent dilution and acidification to increase the solubility of the heavy metals in the dried hydroxide sludge.
2.2 Statistical treatment
The most common technique for summarizing patterns among variables in multivariate datasets is principal component analysis (PCA). This analysis is primarily used to identify patterns in variables as well as to highlight similarities and differences within datasets. The main advantage of PCA is that, once these patterns have been identified, datasets can be compressed to reduce the existing number of dimensions without a corresponding loss of information [Citation15,Citation16].
PCA has been used in many other environmental studies [Citation17–Citation19]. In this study, PCA is used to interpret chemical data and to extrapolate from additional pollution-related data gathered from hydroxide sludge generated by the Bouregreg drinking water treatment plant. Variables analyzed by PCA include the physico-chemical parameters of liquid raw sludge (i.e., water temperature (Tw), volatile suspended solids (VSS), and suspended matter (SM)), the heavy metal and trace element contents of dried hydroxide sludge (i.e., Pb, Cr, Cd, Fe, Al, Cu, Zn, P, N, K, C and pH), and the characteristics of the supernatant (i.e., Cd(s), Pb(s), Cr(s), conductivity (defined as Cond(s)) and pH(s)).
3 Results and discussion
3.1 Analysis of spatiotemporal rating parameters
presents the results of physicochemical analysis on the spatiotemporal rating parameters of liquid raw sludge, dried hydroxide sludge, and supernatants for all samples. presents the heavy metal and trace element contents measured in hydroxide sludge and supernatants.
Table 1 Physico-chemical parameters in sludge hydroxides and supernatant.
Table 2 Measured heavy metals and nutrients in sludge hydroxides and supernatants.
3.1.1 Air temperature (Ta) and water temperature (Tw)
A maximum temperature of 20 °C was recorded during the warm season (August 2011) and a minimum temperature of 11 °C was recorded during the cold season (January 2012). This variation in temperature reflects the influence of the regional climate. The recorded water temperature can generally be considered moderate, as it falls between 14 °C and 19 °C. Both of these different temperatures fall below 30 °C, a temperature that represents the limiting value of direct discharge into the receiving environment. Likewise, temperatures under 35 °C generally represent limits for irrigation water [Citation20].
The measured water temperature (Tw = 11 °C) was relatively high compared to the measured air temperature (Tw = 16 °C), most likely due to the increased water runoff and torrential rain of the winter season.
3.1.2 pH of the dried hydroxide Sludge (pH) and pH of the supernatant (pH(s))
The measured pH of each sample reflects its concentration of H3O+ ions. We can classify the pH of the supernatant and dried hydroxide sludge as follows, using the methodology of Gagnard et al. [Citation21]:
| - | The recorded pH of the supernatant (pH(s)) is slightly alkaline (pH = 7.2–7.5) during summer, autumn and winter and is more alkaline (7.5–8.7) during the spring. According to the World Health Organization (WHO) [Citation22], the pH of drinking water must be between 6.5 and 8.5. | ||||
| - | The recorded pH of the dried hydroxide sludge (pH) is close to neutral (6.8–7.2), except during the months of November and April, in which the pH is slightly alkaline (7.2–7.5). | ||||
These results demonstrate that the dried hydroxide sludge from Bouregreg has a pH between 6.8 and 7.3 and is thus slightly alkaline. This pH content is likely caused when the salt of a strong acid (such as aluminium sulfate) is used to trigger the process of coagulation and flocculation of water, which causes heavy metals to precipitate and become adsorbed by the sludge. The growth of microorganisms in water is affected by a pH value of less than 5 or greater than 8.5.
3.1.3 Conductivity of the supernatant (Cond(s))
The values of conductivity recorded in the supernatant range from 540 μs/cm in February 2012 to 766 μs/cm in June 2012 (). These values do not exceed the maximum permitted conductivity value of drinking water (1055 μs/cm). These values are also, on average, less than the direct discharge limit in the receiving environment (2700 μs/cm) [Citation23]. Additionally, these supernatants record average values between 666 μs/cm and 1000 μs/cm, indicating that they have undergone significant mineralization [Citation24].
3.1.4 SM and VSS of liquid raw sludge
In this study, sludge records a mean SM concentration of 39 g/L, with a minimum concentration of 6.7 g/L and a maximum concentration of 100.7 g/L. These values fall well above the Moroccan SM limit of 50 mg/L [Citation20]. High VSS contents were also recorded during summer and autumn, most likely due to the concurrent flooding of the Sidi Mohammed ben Abdallah dam. The high SM values can similarly be attributed to a period of intense watershed erosion following sudden rainstorms.
To select an appropriate water treatment procedure, it is necessary to determine the moisture rate. Other studies have analyzed the supercritical water gasification (SCWG) of biomass with moisture contents ranging from 76.2 to 94.4 wt% and have determined that total gas yields decrease with a corresponding decrease in moisture content; the CO2 yield decreases most significantly, whereas the H2 and CH4 yields decrease only slightly. Other studies have demonstrated that the carbonization process is accelerated in samples with lower moisture contents. Therefore, the significant reduction of the CO2 yield can be attributed to the carbon’s transformation into coke [Citation25].
The measured VSS/SM ratio elucidates the organic nature of the sludge. The mean VSS/SM values vary between 20.3 and 35% (); these values represent the percentage of sludge with high mineral contents. However, the VSS/SM ratio falls between 50% and 75% for sludge that is high in organics [Citation26].
3.2 Evaluation of heavy metals and trace elements in dried hydroxide sludge and the supernatant
In this study, we measured concentrations of Cd, Pb and Cr in both the dried hydroxide sludge and the supernatants for samples of the Bouregreg drinking water treatment plant. The results of these analyses can be used to determine the relative concentrations of Cd, Pb and Cr for all sludge samples and their supernatants. In the supernatant, [Crs] > [Pbs] > [Cds]; in the dried hydroxide sludge, [C] > [P] > [N] > [Al] > [Fe] > [K] > [Zn] > [Cu] > [Pb] > [Cr] > [Cd] (). Mean concentrations of elements are significantly higher in the dried hydroxide sludge than they are in the supernatant. Metal contents in the supernatant ranged from 0.003 to 0.042 mg/L cadmium, 0.003–0.079 mg/L lead, and 0.082–0.051 mg/L chromium (). These mean values are lower than the recommended limits of trace elements in wastewater that has been treated for irrigation () [Citation27].
Table 3 Limiting contents of metals in supernatants (mg/L).
To the best of our knowledge, there is currently no regulation of pollutant loads in the drinking water sludge produced by water treatment facilities. Therefore, in this study, we use the thresholds suggested by French standards [Citation28] as reference values (). The mean measured values of cadmium and chromium are very low compared to the concentrations suggested by French standards, indicating that the sludge measured here is not significantly polluted. Measured quantities of copper, lead and zinc in the dried hydroxide sludge are higher than the reference values of the French standards, suggesting that the sludge here may have been moderately polluted by these metals. This is significant because high concentrations of Cu and Zn in sludge pose a major problem to the cement industry, according to Schmill et al. [Citation29].
Table 4 Limiting contents of metals in sludge (mg/kg).
High concentrations of iron measured in these samples can be attributed to the structure of the silicates that represent the major constituents of dried hydroxide sludge [Citation30]. Additionally, the presence of iron in sludge can promote the proliferation of certain strains of bacteria that reside in corroded iron pipes [Citation31].
Furthermore, phosphorous can exist in various oxidized forms, including meta HPO3, ortho H3PO4, and pyro H4P2O7. In an aqueous medium, the ortho form is more common than the meta and pyro forms because it is the most stable phosphorous form between pH 5–8 [Citation32,Citation33]. The presence of ortho-phosphate in natural waters is primarily associated with characteristics of the nearby land as well as the decomposition of phosphate from organic matter. Excess phosphorus in water can also be hazardous; for example, high levels of PO43− can cause eutrophication, which greatly increases algae content in tanks and large pipes. These algae may in turn severely influence the passage of light and oxygen consumption in tanks, which can eventually result in serious risks to wildlife [Citation34].
3.3 Typology of variables studied in water of the Bouregreg drinking water treatment plant
Significant correlations exist between the different physico-chemical parameters, trace elements and heavy metals of liquid raw sludge, supernatants and dried hydroxide sludge (). For example, Cu and N measured in the dried hydroxide sludge show a significant correlation, supported by a high correlation coefficient of r = 0.722, whereas Cu and Zn correlate poorly, with a correlation coefficient of r = 0.545.
Table 5 Correlation matrix between physico-chemical parameters, heavy metals and trace elements.
Additionally, the measured conductivity of the supernatant (Cond(s)) correlates strongly with metals in the supernatant, such as Cd(s) (r = −0.725) and Pb(s) (r = −0.503), as well as with those in the dried hydroxide sludge, such as Cr (r = −0.644), Fe (r = 0.620), Cu (r = 0.583) and Zn (r = 0.706). These correlations suggest that these metals strongly influence the conductivity of the samples. Furthermore, the conductivity of each sample also indicates the degree of mineralization in each water sample because each ion has a specific conductivity [Citation35].
The degree of correlation between the conductivity of the supernatant and parameters such as pH(s) and temperature is low (r = −0.02 and r = 0.05, respectively), suggesting that the electrical conductivity of the water is not dependent on these parameters. Contrastingly, the recorded pH(s) of the supernatant is positively correlated with the Fe content of the dried hydroxide sludge (r = 0.529). This correlation suggests that pH can likely influence the distribution of Fe in dried hydroxide sludge.
Temperature also plays a very important role in determining the solubility of salts in a given water system, as well as its gas content and pH [Citation16]. In this study, the measured water temperature (Tw) shows a moderately positive correlation with the carbon content of the dried hydroxide sludge (r = 0.637), suggesting that biological factors can influence the content and distribution of carbon in these systems.
Furthermore, Fe is moderately correlated with Al (r = −0.46) in dried hydroxide sludge; this correlation may be because other silicates and oxy-hydroxides generally prefer other metals to Fe and Al. A moderately significant positive correlation was also noted between N and Pb (r = 0.630) in the dried hydroxide sludge; however, a lower correlation (r = −0.291) was recorded between N and Pb(s), likely due to the greater accumulation of N in sludge.
Additionally, there exists a significant correlation between the calculated contents of Cd and Cr in hydroxide sludge (r = 0.815), indicating a strong affinity between these elements, likely due to a common origin. In the supernatant, Cd(s) behaves relatively similarly to Pb(s) (r = 0.59), suggesting that Cd demonstrates a higher chemical affinity in the sludge.
The measured suspended matter (SM) of raw sludge shows a very significant correlation with the measured volatile suspended solids (VSS) in raw sludge (r = 0.997). SM and VSS both also display significant correlations with lead and nitrogen in dried sludge; lead and nitrogen represent elements that were likely trapped in organic particles (in both suspended and dissolved forms) and settled to the bottom of the water sample.
PCA allows us to analyze all of the water data collected from the Bouregreg drinking water treatment plant and to describe the structure using two main gradients, F1 and F2. For these analyses, we constructed a data matrix consisting of 12 samples (1 station × 12 campaigns) to analyze a series of 21 variables (Cd(s), Pb(s), Cr(s), Cd, Pb, Cr, Fe, Al, Cu, Zn, P, N, K, C, pH(s), pH, Tw, Ta, Cond(s), SM and VSS). The values of the two components F1 and F2, as well as their contributions to the total amount of inertia, are presented in A.
Fig. 1 Graphical approach to the PCA of physicochemical parameters and heavy metals according to F1XF2.
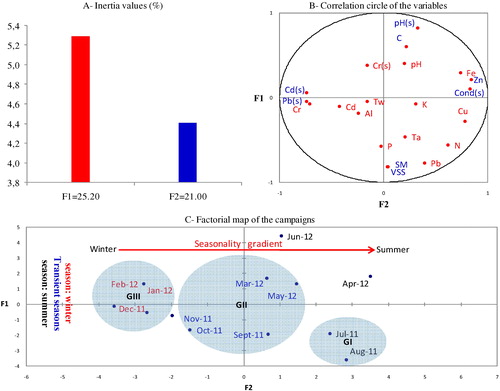
The results of this PCA (A) allow us to perform an initial typological analysis of these different variables according to their individual affinities, as well as to determine their contributions to the first two principal components. The F1 and F2 axes represent 46.20% of the total information (25.20% for the F1 axis and 21.00% for the F2 axis). B and C display the PCA values along with the circle of correlation, factorial maps, and campaigns.
The F1 Axis is determined by Zn content (r = 0.83) and conductivity (r = 0.82). It defines a gradient of mineralization, as opposed to the contamination gradients defined by Pb (r = −0.742) and Cd (r = 0.741), which reflect anthropic contributions. This result conflicts with data from previous studies suggesting that increasing conductivity maintains higher values of Pb in sludge than in water, where it is much more soluble. The high levels of Pb found in this study could have been contributed by exhaust gas from nearby vehicles [Citation36]. In exhaust gas, Pb exists in a state that prefers to exist in water; as El Morhit et al. [Citation36] explained, this situation is caused by the remobilization and release of heavy metals.
Contrastingly, Zn displays a different trend than Pb: its presence in dried hydroxide sludge is represented by low water mineralization but by strong agricultural activity. This same phenomenon has been observed in previous studies [Citation37] and has been explained by the fact that increasing conductivity promotes the release of Zn in the supernatant.
The F2 axis is represented by pH(s) (r = 0.81) and C (r = 0.59) (B). This axis defines a carbon gradient which opposes the contamination gradients of suspended matter (r = 0.81) and volatile matter (r = 0.82) contributed from natural origins. This trend is characterized by the presence of ferro-magnesium minerals within the geological context of the area. However, there seems to be a less noticeable influence of seasonality, particularly temperature, in this system. For example, there is no apparent relationship between temperature and conductivity. The water of the Bouregreg drinking water treatment plant furthermore indicates that pH(s) varies with fluctuations in the carbon content of organic matter.
Global analysis is used to define a typology dominated by the individualization of three campaign groups: GI, GII and GIII (C). This method of spatial organization defines the exact location of each campaign relative to their situation:
GI: combines two periods (July 2011 and Aug 2011) which have Zn and Cu pollution and whose waters are mediocre to poor.
GII: contains five periods (Nov 2011, Oct 2011, Sept 2011, Mar 2012 and May 2012), which have poor water quality but are relatively less contaminated than waters in the G1 and GIII groups. The difference in the levels of water contamination between these groups is likely due to the increased amount of wastewater created by enhanced activity during the periods of interest at the drinking water treatment plant of Bouregreg.
GIII: contains three periods (Dec 2011, Feb 2012 and Jan 2012) during which highly contaminated water was characterized by very high concentrations of Cd(s) and Pb(s) contributed by pollution.
The highest concentrations of Cd and Pb were recorded during the winter season. This suggests they share an origin of domestic wastewater within the Rabat region. However, significantly higher values of conductivity and Zn were measured during the summer season.
A previous study reported that Cd deposition rates were significantly higher in the summer and winter than they were in the rainy season and that Pb deposition rates were significantly higher in the rainy and summer seasons than they were in winter [Citation38]. This study is supported by another one [Citation39] which suggested that average levels of all the metals in a set comprising Fe, Mn, Zn, Cr, Cu, Pb and Cd were relatively higher in summer than in winter season. The reason might be the leaching of the metals into the reservoir from the roadside and agricultural runoffs during wet summer season.
4 Conclusions
This study highlights the significant impact of drinking water sludge production on the environment. This produced sludge serves as a reservoir for heavy metals, which can then be removed by rainwater to contaminate soils and nearby streams. Here, the statistical tool of principal component analysis (PCA) is used to assess the extent of the pollution caused by these heavy metals and to analyze the impacts of various physico-chemical parameters. PCA confirms the existence of significant correlations between Cu, N and Zn; VSS and SM; N, and Pb; C and Tw; Cond(s), Cd(s), Pb(s), Cr, Fe, Cu, Zn and pH(s); pH(s) and Fe; N and Pb; Cd and Cr; and Cd(s) and Pb(s). In particular, the existence of a significant correlation between Cd and Cr hydroxides in sludge suggests that there is a high chemical affinity between these two metals in the hydroxide sludge. SM and VSS also display a significant correlation with lead and nitrogen hydroxide sludge, suggesting that these metals are transported as particles suspended within dissolved organic matter and minerals, which are then deposited and gradually accumulate in the dry hydroxide sludge. This accumulation of heavy metals in hydroxide sludge is often caused by adsorption and/or precipitation. Therefore, this study contributes to the current understanding of the possible causes of environmental pollution; the results of this study can eventually be used to control this pollution. Finally, the constituents present in the studied sludge potentially represent raw materials that could be used in cement following the appropriate thermal treatment, as has already been discussed in our previous study [Citation3,Citation4,Citation40].
Notes
Peer review under responsibility of Taibah University.
References
- Z.ZhouT.HuangY.LiW.MaS.ZhouS.LongSediment pollution characteristics and in situ control in a deep drinking water reservoirJ. Environ. Sci.201610.1016/j.jes.2016.05.006
- K.M.Al-QahtaniWater purification using different waste fruit cortexes for the removal of heavy metalsJ. Taib. Univ. Sci.201510.1016/j.jtusci.2015.09.001
- M.DahhouM.El MoussaouitiA.BenlallaA.El HamidiM.TaibiM.A.ArshadStructural aspects and thermal degradation kinetics of water treatment plan, sludge of Moroccan capitalWaste Biomass Valor.201610.1007/s12649-016-9513-5
- A.BenlallaM.ElmoussaouitiM.DahhouM.AssafiUtilization of water treatment plant sludge in structural ceramics bricksAppl. Clay Sci.1182015171177
- N.Husillos-RodríguezS.Martínez-RamírezM.T.Blanco-VarelaM.GuillemJ.PuigE.LarrotchaJ.FloresEvaluation of spray-dried sludge from drinking water treatment plants as a prime material for clinker manufactureCem. Concr. Compos.332011267275
- A.KoohestanianM.HosseiniZ.AbbasianThe separation method for removing of colloidal particles from raw waterAm. Eurasian J. Agric. Environ. Sci.42008266273
- Z.ZhouY.YangX.LiY.ZhangX.GuoCharacterization of drinking water treatment sludge after ultrasound treatmentUltrason. Sonochem.2420151926
- S.IshikawaN.UedaY.OkumuraY.lidaK.BabaRecovery of coagulant from water supply plant sludge and its effect on clarificationJ. Mater. Cycles Waste Manage.92007167172
- M.DahhouM.El MoussaouitiN.KhachaniM.AssafiL.A.HsainS.MostahsineK.BouqallabaPhysico-chemical characterization of sludge from a unit water drinking productionMATEC Web Conf.201210.1710.1051/matecconf/20120201017
- M.AhmaruzzamanIndustrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metalsAdv. Colloid Interface16620113659
- P.R.LokeshkumarR.G.ParagTreatment of water containing heavy metals using a novel approach of immobilized modified sludge biomass based adsorbentsSep. Purif. Technol.1632016215227
- R.ShahabaldinP.MohanadossT.AmirrezaM.Shaza-EvaM.D.Mohd-FadhilT.Shazwin-MatS.FarzanehS.Fadzlin-MdPerspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewaterJ. Environ. Manage.1632015125133
- Quebec assessment centre in environmental analysis, Determination of pH: electrometric method, MA. 100 – pH 1.1, Rev. 3, Ministry of Sustainable Development, Environment, Wildlife and Parks in Quebec2014http://www.ceaeq.gouv.qc.ca/methodes/chimie_inorg.htm
- Quebec assessment centre in environmental analysis, Determination of total solids and total volatile solids: gravimetric method, MA. 100 – S. T. 1.1, Rev. 4, Department of Sustainable Development, Environment and the Fight against Climate Change2015http://www.ceaeq.gouv.qc.ca/methodes/chimie_inorg.htm
- S.SivakumarA.ChandrasekaranR.RavisankarS.M.RavikumarJ.Prince Prakash JebakumarP.VijayagopalI.VijayalakshmiM.T.JoseMeasurement of natural radioactivity and evaluation of radiation hazards in coastal sediments of east coast of Tamilnadu using statistical approachJ. Taib. Univ. Sci.82014375384
- M.El MorhitM.YagoubiA.BelmakkiM.ZouhdiMonthly physicochemical characterization of a hospital effluent according to technical and care activities (Avicenna Rabat-Morocco)World J. Pharm. Sci.442015247267
- J.LiuC.F.WeiQ.XieW.H.ZhangCapacities of soil water reservoirs and their better regression models by combining “merged groups PCA” in Chongqing, ChinaActa Ecol. Sin.3420145365
- S.G.DalalP.V.ShirodkarT.G.JagtapB.G.NaikG.S.RaoEvaluation of significant sources influencing the variation of water quality of Kandla creek, Gulf of Katchchh, using PCAEnviron. Monit. Assess.16320104956
- A.S.VeyheD.HofossS.HansenY.ThomassenT.M.SandangerJ.Øyvind OdlandE.NieboerThe Northern Norway Mother-and-Child Contaminant Cohort (MISA) Study: PCA analyses of environmental contaminants in maternal sera and dietary intake in early pregnancyInt. J. Hyg. Environ. Health2182015254264
- O.El RhaouatI.El KherratiF.El khayyatH.ChiguerK.EzzianiA.Ibeda1M.FarehY.SaidiK.El KharimD.BelghytiPhysic-chemical evaluation of urban wastewater of the town of Sidi KacemComput. Water Energy Environ. Eng.320143035
- G.GagnardC.HuguetJ.P.RyserAnalysis of soil and vegetation in the conduct of fertilization. Control of fruit qualityGeneral Secreteriat OILB/SROPvol. 831998
- WHO3rd ed.Guidelines for Drinking-water Qualityvol. 12006WHO (World Health Organization)Geneva
- Moroccan StandardsOfficial Bulletin of Morocco, Ministry of Environment of Morocco, Rabat, No. 50622002
- J.RodierThe Analysis of Natural Water, Waste Water, Sea Water8th ed.1996DenodParis
- C.HeC.L.ChenA.GiannisY.YangJ.Y.WangHydrothermal gasification of sewage sludge and model compounds for renewable hydrogen production: a reviewRenew. Sustain. Energy Rev.39201411271142
- J.C.BoeglinProgress in the clarification of wastewater and sludge drying machine by the use of organic polymers synthesisXXIVth International Congress of Pure and Applied ChemistryGermany, vol. 3431974
- A.K.BiswasRole of wastewater reuse in planning and managementA.K.BiswasA.ArarTreatment and Reuse of Sewage Effluent for Irrigation1987Butterrsworth Scientific GuildfordU.K.
- OTVTreatment and enhancement of the sludge; Book collection of OTV, Ed 19971997 ISBN: 978-2951105904
- L.SchmillV.WezemazlA.G.PartnerCement and Environment: A Durable Bond2011Ed HolcimSwitzerland
- R.D.WelkenK.WeillerBehaviour of iron and manganese in comparison to other in Elbs estuary. Heavy metal environmentalinterConf21989227229
- E.TorvinenS.SuomalainenM.J.LehtolaI.T.MiettinenO.ZacheusL.PaulinM.L.KatilaP.J.MartikainenMycobacteria in water and loose deposits of drinking water distribution systems in FinlandAppl. Environ. Microbiol.70201419731981
- P.C.M.BoersThe influence of pH on phosphate release from lake sedimentsWater Res.251991309311
- H.S.JensenF.Q.AndersenImportance of temperature, nitrate and pH for phosphate release from aerobic sediments of four shallow europhic lakesLimnol. Oceanogr.371992577589
- S.GarrasS.BacroumeM.RahoutiS.E.BarchaA.BellaouchouA.FekhaouiTotal phosphorus behaviour modelling in the Smir Lake Reservoir (Tetouan, Morocco)J. Mater. Environ. Sci.6201516841691
- M.El MorhitM.FekhaouiA.SerghiniS.El blidiA.El abidiR.BennaakamA.YahyaouiM.JbilouImpact of water resources and water quality sediment of the Loukkos estuary (Atlantic Coast, Morocco)Bull. Sci. Inst. Earth Sci. Sec.3020083947
- M.El MorhitM.FekhaouiA.El AbidiA.YahyaouiA.HamdaniImpact of human activities on the degradation of the quality of sediments of the estuary of Loukkos (Morocco)Frecnh J. Ind. Ecol.201161
- M.El MorhitM.FekhaouiA.SerghiniS.EL BlidiA.EL AbidiA.YahyaouiM.HachimiStudy of the spatiotemporal evolution of hydrological parameters characterizing the water quality of the estuary Loukkos (Morocco)Bull. Sci. Inst. Earth Sci. Sec.3422012151162
- R.K.SharmaM.AgrawalF.M.MarshallAtmospheric deposition of heavy metals (Cu, Zn, Cd and Pb) in Varanasi City, IndiaEnviron. Monit. Assess.14212008269278
- J.IqbalM.H.ShahOccurrence, risk assessment, and source apportionment of heavy metals in surface sediments from Khanpur lake, PakistanJ. Anal. Sci. Technol.5282014112
- R.M.R.ZamoraO.C.AlfaroN.CabirolF.E.AyalaA.D.MorenoValorization of drinking water treatment sludges as raw materials to produce concrete and mortarAm. J. Environ. Sci.42008223228
