?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The anticorrosion effect of 5-hydroxytryptophan (5-HTP) on mild steel (MS) was investigated by gravimetric and electrochemical techniques. Two different concentrations (1 M and 15%) of hydrochloric acid were used to simulate well-acidizing fluid. The results show that 10 × 10−5 M 5-HTP is 96.1% efficient in 1 M HCl and 78.1% efficient in 15% HCl at 30 °C. The efficiency decreases as the temperature increases, reaching 66.9% and 39.8% in 1 M and 15% HCl, respectively, at 90 °C. When 5-HTP is blended with potassium iodide and glutathione, the efficiency increases to above 88% and 78% in 1 M and 15% HCl, respectively, at 90 °C. Increasing the 5-HTP concentration decreases the double-layer capacitance and increases the charge-transfer resistance. 5-HTP behaves as a mixed‐type corrosion inhibitor with anodic predominance and is spontaneously adsorbed on the steel surface. Physisorption of 5-HTP is best described by the Langmuir adsorption model and is also exothermic with a resultant decrease in the entropy of the bulk solution. The results of SEM/EDAX, FTIR and UV–VIS studies support the hypothesis that a protective film of 5-HTP forms on MS facilitated by O, N and C=C functionalities.
1 Introduction
When existing wells deplete and their natural pressure declines, the use of chemistry to maintain production becomes essential. This is achieved through well stimulation, fracturing, secondary and enhanced oil recovery operations. Well acidizing is a common field practice that involves forcing acid through the well bore at high pressure to dissolve formation rocks, enlarge existing flow channels and open new ones. It can also be used for removal or clearing of scale. During these procedures, corrosion of metal structural materials occurs because of their contact with acid. Such materials include line pipes, casings and tubing, as well as storage facilities, which are usually constructed from steel. The consequences of corrosion include rupturing of these materials, spills, failure and loss of integrity of materials and flow problems [Citation1,Citation2]. To avoid the high cost of managing/cleaning spills, down time of shutting down the plant for maintenance, or injury to employees and damage to company integrity, prevention of corrosion by use of corrosion inhibitors (CI) becomes essential. Therefore, very large sums are spent on CIs, as they are a simple, practical and cost effective means of reducing corrosion.
Table 3 Effect of Intensifiers an inhibition efficiency (%) of 10 × 10−5 M 5-HTP on mild steel in a 1 M HCl solution.
Table 4 Effect of Intensifiers on inhibition efficiency (%) of 10 × 10−5 M 5-HTP on mild steel in a 15% HCl solution.
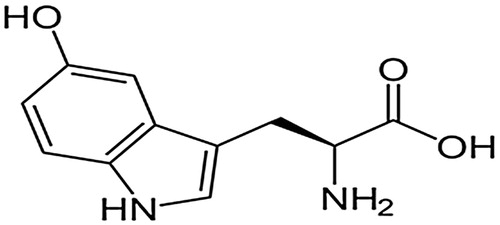
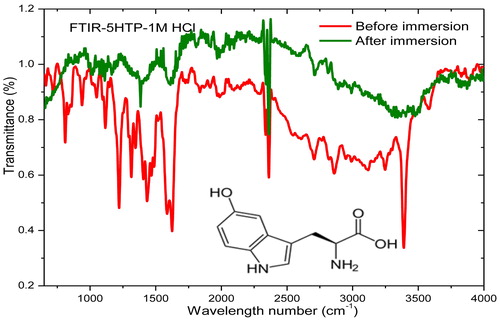
CIs are substances that are added in small amounts to the corroding fluid to retard the speed of corrosive attack on the metal surface it contacts [Citation3]. They offer surface protection by adsorption of their active functionalities on metal surfaces [Citation4]. A thin protective film that acts as a blanket on the metal surface and protects it from the aggressive medium is formed. The inhibitor effectiveness varies with properties, such as its chemical composition, concentration and operating temperature [Citation5]. Many compounds that contain functionalities, such as nitrogen, oxygen, multiple bonds, conjugated double bond systems, heteroatoms and aromatic rings, have been found to be efficient CIs for different metals in various media [Citation6–Citation15]. However, some of them are toxic or very expensive, hence the need to source CIs from cheap, sustainable and non-toxic materials. 5-HTP is not toxic and can be locally sourced in large quantity [Citation16,Citation17] or cheaply synthesized [Citation18,Citation19]. It also contains electron‐rich functionalities, such as those mentioned earlier, in its molecular structure (). These factors motivated us to investigate 5-HTP as an alternative CI for MS.
Hydrochloric acid is widely used in the field for acidizing purposes; therefore, the well-acidizing fluid used in this study is simulated using both 1 M HCl and 15% HCl [Citation20,Citation21]. Mild steel coupons are used to simulate steel pipelines, casings, among others. Standard techniques, such as thermogravimetry or weight loss (TG), electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP), are used to assess the inhibitor efficiency. The morphology of the metal specimen is checked by SEM to observe possible differences with and without the inhibitor. Other techniques, such as FTIR, UV–VIS, and EDAX, are employed to further characterize the inhibition phenomenon. 5-HTP was also blended with some compounds to improve its efficiency at high temperature. Adsorption, kinetic, and thermodynamic models are employed to further explain the interaction of the inhibitor with the metal surface.
2 Experimental
2.1 Preparation of steel specimens
MS sheet was purchased from the Building and Construction Materials Market in Uyo, Akwa Ibom state. It was mechanically press-cut into coupons with dimensions of 2 cm × 2 cm for TG experiments, 1 cm × 1 cm for electrochemical studies and 2 cm × 1 cm for surface analysis. The surface was treated as provided by NACE Recommended Practice RP-0775 and ASTM G-1 & G-4 for surface finishing and cleaning of coupons for weight loss. In addition, the mild steel coupons used for electrochemical studies were abraded to a mirror surface with CC-22F P2000 grade silicon carbide paper. All of the prepared specimens were enclosed in sealed water-proof bags and stored in a moisture‐free desiccator prior to use. The chemical compositions (wt.%) of the MS was C (0.13), Si (0.18), Mn (0.39), P (0.40), S (0.04), Cu (0.025), and Fe (balance).
2.2 Preparation of inhibitor solutions
Analytical grade HCl was diluted to concentrations of 1 M and 15% using double-distilled water. Powdered 5-hydroxytryptophan (HPLC 99.9%, extracted from seeds of Griffonia simplicifolia) was supplied by Shaanxi Kanglai Ecology Agriculture Co., Ltd., China, and was prepared (as received) at five different concentrations (1 × 10−5, 3 × 10−5, 5 × 10−5, 8 × 10−5 and 10 × 10‐5M) in both 1 M and 15% HCl solutions.
2.3 Preparation of inhibitor blends
The additives used in this study were: (i) PEG-4000 (industrial grade), supplied by Richest Group Ltd., Shanghai, China; (ii) sodium gluconate (analytical grade), supplied by Wuhan Yuancheng Gongchuang Technology Co., Ltd., China; (iii) potassium chloride (analytical grade), supplied by Meyer Chemical Technology Co., Lt., Shanghai, China; and (iv) glutathione (industrial grade), supplied by Wuhan Yuancheng Gongchuang Technology Co., Ltd., China. Each compound (as received) was prepared at a concentration of 1 × 10−6 M [Citation3] in the respective acid solutions and blended with 5-HTP at a ratio of 1:1, followed by vigorous stirring.
2.4 Thermogravimetric (weight loss) technique
These measurements were conducted according to the ASTM standard method explained in the literature [Citation20]. A Sartorius CPA225D analytical balance with a sensitivity = ±0.00001 g was used for weight measurement. Pre-weighed steel coupons were immersed in acid solutions without and with test solutions for five hours and maintained at 30 °C in a water bath. The retrieved coupons were cleaned in a 20% NaOH solution containing approximately 200 g/L of zinc dust, dried in air after rinsing in acetone and weighed to determine the weight loss. Experiments were carried out in triplicate, and the mean values of the weight losses (g) were used for computation. The corrosion rate (CR), inhibition efficiency (εWL) and degree of surface coverage (θ) were calculated as follows [Citation22]:(1)
(1)
(2)
(2)
(3)
(3) where ΔWmean is the mean weight loss of MS; CRb and CRi are the corrosion rates (cm h−1) in the absence and presence of the inhibitor, respectively; ρ is the density of iron; A is the average surface area (cm2) of the metal specimens; and t is the immersion time (h). The obtained corrosion rate values were converted to another unit (mmpy) using conversion factors explained in the literature [Citation20]. This procedure was repeated at other temperatures, namely, 45 °C, 60 °C, 75 °C and 90 °C, maintained in a water bath.
2.5 Electrochemical measurements
A Gamry ZRA REF 600-18042 potentiostat/galvanostat was used for electrochemical measurements. The conventional three‐electrode set up was used, which is composed of a saturated calomel electrode (SCE) as the reference electrode, platinum as the counter electrode and one of the different steel coupons as the working electrode. Only three concentrations (1 × 10−5, 5 × 10−5 and 10 × 10−5 M) of the inhibitor were tested. The EIS was conducted at a frequency of 10 kHz to 10 mHz for an open-circuit immersion time of 1800 s at 30 °C. The voltage ranged from −0.15 V to +0.15 V vs. EOC at a scan rate of 0.2 mV/s for PDP measurements [Citation24]. EChem Analyst was used for data analyses/fitting. The charge-transfer resistance was used to compute the inhibition efficiency according to Eq. Equation(4)(4)
(4) . The inhibition efficiency from PDP was calculated from the corrosion current densities using Eq. Equation(5)
(5)
(5) .
(4)
(4) where RctB and RctI are the charge transfer resistances in the absence and presence of the inhibitor, respectively.
(5)
(5) where
and
are the corrosion current densities in the absence and presence of the inhibitor, respectively. The magnitude of the double layer capacitance (Cdl) of the adsorbed film was calculated from the constant‐phase element (CPE) constant (Yo) and charge‐transfer resistance (Rct) using Eq. Equation(6)
(6)
(6) .
(6)
(6) where n is a constant showing the degree of roughness of the metal surface obtained from the phase angle given that (j2 = −1)α and n = 2α⁄(π).
2.6 UV–vis study
UV–vis spectral data were obtained using a 756PG Spectrum (Shanghai Spectrum Instruments Co., Ltd). The UV–vis spectrum was first obtained using a solution containing 10 × 10−5 M 5-HTP prior to immersion of the steel. Another spectrum was obtained with the solution after immersing the steel for 24 h. Only the spectral profiles in 1 M HCl were compared and discussed.
2.7 FTIR study
FTIR spectra of the pure sample and of the 5-HTP film formed on mild steel surface after immersion (both mixed with potassium bromide) were recorded. The spectra were measured using a TENSOR II FTIR Spectrophotometer.
2.8 SEM/EDAX study
SEM images were recorded using an AMETEX S4800 EDAX TSL in vacuum mode before and after immersion in HCl. This was repeated with a coupon immersed in HCl containing a 10 × 10−5 M 5-HTP solution. The instrument was operated at 5 kV. Additionally, the EDAX profiles of the steel surface ab initio and corrosion products in the inhibited and uninhibited solutions were recorded.
3 Results and discussion
3.1 Thermogravimetric study
The corrosion rate, inhibition efficiency and fractional surface coverage obtained from weight-loss measurements for the corrosion of MS in both 1 M and 15% HCl containing different concentrations of 5-HTP are presented in .
Table 1 Corrosion rate, inhibition efficiency and fractional surface coverage data for the inhibition of mild steel corrosion in 1 M and 15% HCl using different concentrations of 5-HTP at 30 °C.
3.1.1 Effect of the inhibitor and acid concentrations
The inhibition efficiency increases as the concentration of 5-HTP increases at a constant temperature (), similar to the trends reported in the literature [Citation23]. A higher inhibition efficiency is obtained if the 5-HTP concentration is further increased. However, the efficiency of 5-HTP decreases as the acid concentration increases. At 30 °C, the inhibition efficiency decreased from 96.01% in 1 M HCl to 78.14% in 15% (approximately 4.4 M) HCl, which represents an approximate 18.61% decrease in inhibition efficiency for a 340% increase in the acid concentration. An efficiency of 78% is still reasonable for a bio-based material and can be improved if blended with synergistic additives.
3.1.2 Effect of temperature and intensifier blends
Temperature is one of the major factors that affect the performance of corrosion inhibitors [Citation25]. Since industries have recently ventured more into production and recovery of hydrocarbons from deep pay zones, this study elucidated the effect of temperature in 5-HTP efficiency. Down the well, the difference in temperature per unit well length has been described as the geothermal gradient. An average geothermal gradient of approximately 25 °C/km (1 °F per 70 feet of depth) is believed to be universal [Citation26], while 28 °C/km is used for the Niger delta fields [Citation27–Citation29].
The results obtained using the 10 × 10−5 M 5-HTP can be seen in . The inhibition efficiency decreases as the temperature increases. In a 1 M HCl solution, the efficiency is reduced from 96.01% at 30 °C to 66.95% at 90 °C. The obtained efficiencies are even lower in 15% HCl compared to 1 M HCl as the temperature increases. This implies that if 5-HTP is used as the corrosion inhibitor in acidizing fluid, it will perform more effectively on the surface than in down hole pipes. To improve on this performance, 5-HTP can be blended with some intensifiers. Intensifiers are desirable because corrosion inhibitors frequently cannot provide adequate protection to steels at high temperatures and long exposure times [Citation3]. The intensifiers used in this study are potassium iodide (KI), polyethylene glycol (PEG), sodium gluconate (NaG), and glutathione (GLU). The inhibition efficiency at 90 °C improved with blends containing PEG, KI and GLU to 88.5, 89.0 and 90.2%, respectively, in 1 M HCl and 78.4, 80.7, and 82.5%, respectively, in 15% HCl (see and ). This demonstrates that the inhibitor blends could be suitable for various oilfield acidizing procedures associated with high temperature operations.
Table 2 Effect of temperature on the inhibition efficiency of 10 × 10−5 M 5-HTP as a corrosion inhibitor for mild steel in acidic solutions.
3.1.3 Adsorption study
Adsorption of corrosion inhibitors can occur through electrostatic interactions between the charged species of the inhibitor and metal surface (physisorption) or by actual chemical interaction between functional groups of the inhibitor and metal orbitals (chemisorption) [Citation30]. To predict which mechanism 5-HTP was adsorbed on MS, surface coverage (θ) data were fitted into different adsorption isotherm models, namely, the Langmuir, Temkin, Freundlich, Flory Huggins and El-Awady et al. The best fit was obtained with the Langmuir isotherm (), with R2 ≥ 0.99985.The expression for the model is given in Eq. Equation(7)(7)
(7) .
(7)
(7) where Cinh is the concentration of inhibitor (M) in the acid solution and Kads is the adsorption–desorption equilibrium constant (M−1), which is used to determine the free energy change of adsorption (ΔGads) according to Eq. Equation(8)
(8)
(8) .
(8)
(8) where 55.5 represents the concentration of water in the solution, R is the universal gas constant and T is the absolute temperature. The values of Kads and ΔGads obtained are given in . Usually, the values Kads relate to the strength of the inhibitor-metal surface binding [Citation31]. The obtained values decrease as temperature increases, implying that the binding strength of 5-HTP decreases as temperature increases due to the desorption of its molecules from the surface. The ΔGads values are between −12.67 kJ mol−1 to −13.53 kJ mol−1 and within the range assigned to the physical adsorption mechanism [Citation32]. Therefore, the adsorptive film has an electrostatic character. The negative values of ΔGads also indicate that 5-HTP was spontaneously adsorbed on MS at all temperatures and acid concentrations.
Fig. 2 Langmuir adsorption isotherms for the inhibition of mild steel corrosion in (i) 1 M and (ii) 15% HCl by different concentrations of 5-HTP.
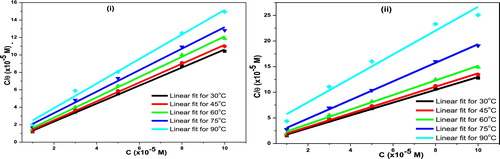
Table 5 Parameters deduced from the Langmuir adsorption isotherm.
3.1.4 Kinetic and thermodynamic studies
Corrosion rate data were fitted into the Arrhenius kinetic model (Eq. Equation(9)(9)
(9) ) to determine the activation energy. Linear plots of log CR (units in mmpy) against the reciprocal of temperature () were constructed.
(9)
(9) where Ea is the activation energy, A is the Arrhenius pre-exponential factor or frequency factor, R is the universal gas constant and T is the absolute temperature. The activation energy deduced was higher with the inhibitor (depending on inhibitor concentration) than the free acid solutions (see ). From the concept of activation and collision theory, it can be considered that for acid molecule collision with MS to result in a corrosive attack that corrodes the metal surface, molecules of the acid must collide with molecules of the metal on the surface. The acid molecules should possess at least enough energy to exceed a minimum threshold, called the activation energy. This implies that the acid molecules must acquire extra (higher) energy in the inhibited solution for corrosion to occur, hence corrosion inhibition. Therefore, addition of 5-HTP increases the activation energy and reduces the tendency of acid molecules to collide and attack the metal surface. The activation energy was lower in 15% HCl than 1 M HCl, implying that the molecules of 15% HCl require a lower energy barrier to cross the activated complex and form corrosion products with mild steel than those of 1 M HCl. The increase in activation energy in the presence of the inhibitor in both acids is consistent with trends reported in the literature and is associated with a physical adsorption mechanism [Citation31].
Fig. 3 Arrhenius plot for the inhibition of corrosion of mild steel in (i) 1 M and (ii) 15% HCl using different concentrations of 5-HTP.
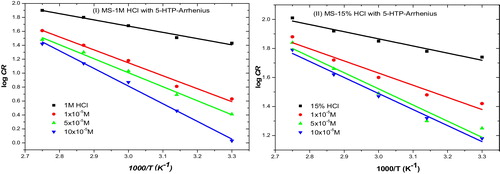
Table 6 Activation parameters for the corrosion of mild steel in both 1 M and 15% HCl containing different concentrations of 5-HTP .
The other activation parameters given in were derived from the transition state equation (Eq. Equation(10)(10)
(10) ). Linear plots of
(in mmpy per Kelvin) against the reciprocal of temperature ().
(10)
(10)
Fig. 4 Transition state plot for the corrosion of mild steel in (i) 1 M and (ii) 15 % HCl in the absence and presence of different concentrations of 5-HTP.
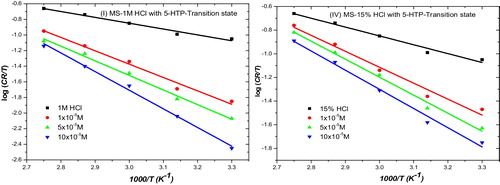
where ΔH* and ΔS* are the enthalpy and entropy change of activation, respectively. The values of ΔH* are all negative, which indicates that the adsorption of 5-HTP on MS is exothermic and involves the evolution of heat. Negative ΔS* values show that at the rate activated complex, there is an association of inhibitor molecules with the steel surface through adsorption [Citation34].
3.2 Electrochemical impedance spectroscopy
The open‐circuit potential scanned for 1800 s afforded a fairly steady potential after 600 s (a). It can also be observed from the figure that the open‐circuit voltage (OCP) is more stable at approximately −480 ± 3 mV, whereas in the presence of inhibitors, the OCP shifts to more negative values. The shift in OCP with 5-HTP from 1 M HCl is not as great as −85 mV, and therefore, the inhibitor cannot be categorized as a cathodic or anodic type [Citation35]. The Nyquist and Bode modulus/Phase angle plots shown in b were obtained for different concentrations of 5-HTP in 1 M HCl. The semicircles in the Nyquist plots are imperfect, and the sizes of the diameters are influenced by the presence of 5-HTP from that of the free acid solution. The imperfection of the shape of the semicircle can be attributed to the surface roughness of the mild steel, while the difference in sizes of their diameters demonstrates that 5-HTP influences the corrosion rate due to inhibition. The diameter increases as the inhibitor concentration increases, following the same trend as the inhibition efficiency.
Fig. 5 (a) Plot of OCP against time for the inhibition of MS corrosion in 1 M HCl. (b) Nyquist and Bode modulus/phase angle plots for inhibition of mild steel corrosion in 1 M HCl using different concentrations of 5-HTP.
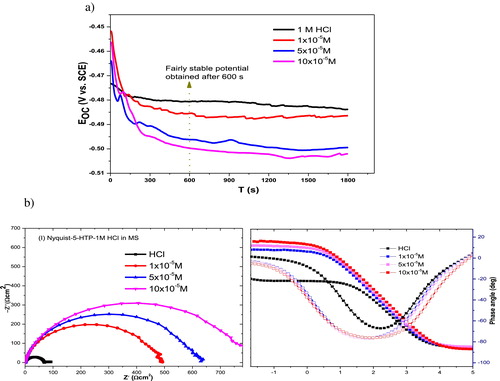
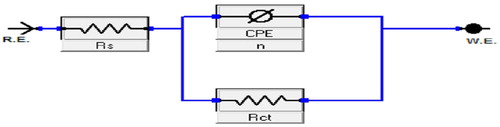
The single capacitive loop obtained indicates that the mechanism of corrosion is mainly controlled by a charge‐transfer process [Citation36]. The shapes of the plots were similar in both the inhibited and free acid solution, indicating that the mechanism of steel corrosion is not influenced by introduction of 5-HTP. The equivalent circuit shown in provides a best fit for analyses of experimental data, with a goodness of fit of less than 0.35 × 10−3. Some associated parameters obtained are given in .
Table 7 Some parameters obtained from the EIS technique used to monitor the inhibition of mild steel corrosion in 1 M HCl containing different concentrations of 5-HTP.
The surface roughness of steel was compensated for by the introduction of a non-integer element dependent on the frequency, called the constant phase element, CPE, which can be estimated using Y0and n. CPE is related to the impedance by:(11)
(11) ZCPE is the impedance of the CPE, Y0 is the CPE constant,wis the angular frequency, j is an imaginary complex number, and (j2 = −1)α is the phase angle of CPE. The value of n indicates a deviation of the CPE and can be used to predict the degree of roughness or inhomogeneity of the mild steel surface. value decreased on addition of extracts, suggesting that the surface roughness of the mild steel is increased by adsorption of the inhibitor molecules on the steel surface active sites [Citation37]. It also indicates that there is relative and/or integrated influence on the CPE rather than only a single resistance, capacitance or inductive element. The decrease in n on addition of inhibitors can also be associated with insulation of the metal/solution interface by formation of a surface film, which is responsible for the increase in the charge‐transfer resistance. The charge‐transfer resistance increases with the increase in inhibitor concentration, showing that the ‘blanketing’ property of the film improves as the inhibitor concentration increases. The inhibition efficiency also increases with the increase in inhibitor concentration, following the same trend as from the gravimetric studies.
This capacitive response shown by an increase in peak heights in the Bode plot suggests the formation of an electrochemical double layer with a capacitance (Cdl) estimated using Eq. Equation(6)(6)
(6) . The Cdl values decrease in the presence of inhibitors, similar to the results reported in the literature [Citation38], which are attributed to a decrease in the local dielectric or an increase in the thickness of the double layer or both, caused by the adsorbed protective film of the inhibitors.
3.3 Potentiodynamic polarization
During corrosion of mild steel in HCl, at least one oxidation and one reduction process takes place. Typical reactions involving iron at the electrodes are:(12)
(12)
(13)
(13)
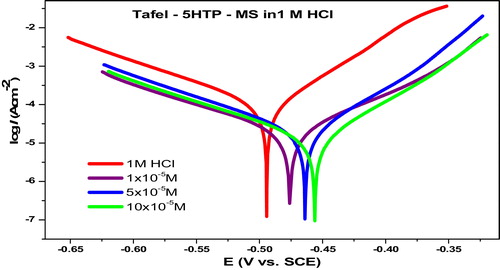
The total cathodic and anodic processes contribute to the compromise or free corrosion potential (Ecorr) and the corresponding current density (Icorr) obtained through analyses of the obtained Tafel plot (). The Tafel cathodic and anodic constants (βcand βa) were also obtained from the slopes of the plots. Some of the PDP parameters determined are shown in . The Icorr values decrease with an increase in inhibitor concentration due to formation of the adsorbed protective film. A shift in Ecorr values to more positive values with the inhibited solutions compared to the free acid solution was observed. Since anodic inhibitors shift the corrosion potential in the positive direction [Citation37], it is implied that 5-HTP has a dominant influence on the partial anodic reaction. However, the highest shift from that of the free acid (δEcorr) was −47 mV, which is less than the −85 mV required to categorize the inhibitor as either the cathodic or anodic type. A similar shift in Ecorr values has been reported in the literature, and the inhibitor is regarded to be a mixed type inhibitor with anodic predominance [Citation38]. This implies that 5-HTP inhibits both the iron dissolution and hydrogen evolution processes, but more actively inhibits the anodic iron oxidation reaction.
Table 8 Some parameters obtained from the PDP technique used to monitor the inhibition of mild steel corrosion by 5-HTP at 30 °C.
The values of βc and βa obtained change with the concentration of inhibitor, but without a definite trend. Olasunkanmi et al also obtained a similar trend and inferred mixed‐type inhibitors with cathodic predominance because the magnitude of the net change was higher in βc than βa compared to the free acid solution [Citation39]. In this study, larger changes in βa values were obtained than in βc values, supporting the hypothesis that the inhibitors show anodic predominance. The calculated inhibition efficiency also increased with the increasing concentration of the inhibitor, similar to the weight loss and EIS results.
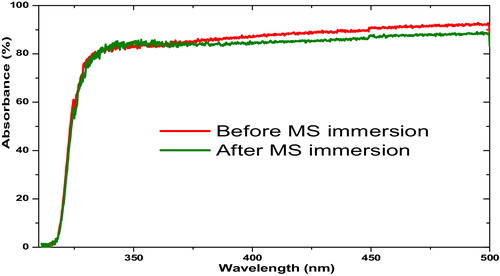
3.4 UV–visible spectroscopy
The absorption spectra shown in reveal that after immersion of the mild steel coupon, the band obtained in the UV-visible region shifted to lower absorbance values. This behaviour may be ascribed to a possible interaction between Fe2+ and the inhibitor compounds in the inhibited solution [Citation39]. This could have been due to some electronic transitions, such as n → π or n → π* (involving the non-bonding electrons of O and N) or π → π* (involving multiple bonds and a conjugated system), and the formation of a surface complex between the inhibitor and metal surface. This complex acts as the adsorbed protective film that reduces direct acid attack on the metal surface, hence its corrosion inhibition.
3.5 FTIR study
This technique was employed to predict the functional group(s) in the inhibitor involved in the adsorption process. Before metal immersion, the prominent spectral peaks provide information on the functional groups in the compound. After immersion, some of the peaks are either lost or are less prominent due to the involvement of the corresponding functional group(s) in adsorption. The FTIR spectra of 5-HTP are shown in . The peaks at approximately 3100–3300 cm−1, 1600–1700 cm−1 and 1200–1300 cm−1 are shown by a black line. The peak at approximately 3100–3300 cm−1 can be assigned to either –OH or –NH vibrations: –OH vibrations are broad in the presence of hydrogen bonding, while –NH2 is characterized by a single peak. These functional groups are available in the molecular structure of 5-HTP, which is embedded in . The peak at approximately 1600–1700 cm−1 is popularly assigned to the C=O of an acid group, which is available in the molecule. The peak at approximately 1200–1300 cm−1 can be assigned to the C–O group, which is also present in the molecule. The disappearance of these peaks after immersion of mild steel (see the red line) demonstrates that these functional groups may be actively involved in the adsorption process.
3.6 SEM/EDAX studies
SEM micrographs of abraded mild steel coupons prior to immersion and those immersed in 1 M HCl with and without 5-HTP for 24 h were recorded by SEM. The result reveals that the surface of the abraded steel coupon (, top left) was considerably smoother with minimal undulation or pitting. The surface of the coupon immersed in the free acid solution (, top middle) was severely pitted by acid corrosion. However, the coupon immersed in the solution containing 5-HTP (, top right) was relatively smooth compared to the free acid solution. This demonstrates that addition of the inhibitor reduces the corrosive pitting that occurs in the free acid solution. The protective layer contained some cracks, which may be due to uneven distribution of 5-HTP molecules over the steel surface. It is also possible that the active sites on the mild steel surface are not equivalent or do not possess similar affinities for the active molecules of the inhibitors. In that case, the adsorption of some molecules on a portion of the surface differentially blankets the acid from attacking that portion by steric hindrance or a micelle-like conformation of adsorbed molecules, as described by [Citation40].
Fig. 10 SEM/EDAX profiles of an abraded mild steel surface (left) and MS immersed in 1 M HCl without (middle) and with 10 × 10−5 M 5-HTP.
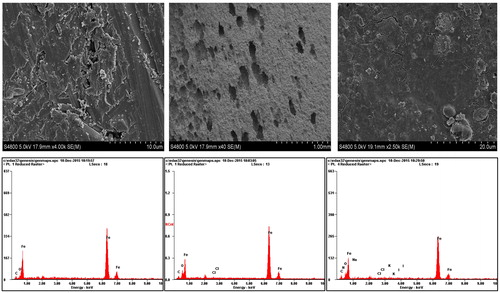
To further establish the reliability of the inferences drawn from the FTIR results, EDS profiles of the surfaces of mild steel were obtained without and with formation of the adsorbed film. The spectral profile of the pure mild steel surface before immersion (, bottom left) shows the presence of Fe, C and a small amount of O. On immersion in 1 M HCl, Fe slightly decreased, O slightly increased and Cl ions are introduced perhaps due to interaction of the acid with Fe (, bottom middle). The profile in the solution containing 5-HTP also shows increased O and N atoms (, bottom right). The EDAX data obtained are summarized in . Thus, adsorption of the inhibitor on the surface mainly involved N and O atoms and resulted in decreased iron exposure due to the protective effect of the inhibitors. This result is in agreement with the observations of the FTIR studies.
Table 9 Summary of atoms obtained on a pure MS surface (case I), corroded MS surface (case II), and MS corrosion inhibited by 5-HTP surface (case IV) investigated by EDAX.
3.7 Mechanism of inhibition
Corrosion inhibitors are believed to act by adsorption on metal surfaces by either physical or chemical means [Citation31]. Based on the results obtained from this study, two mechanisms are proposed for the inhibition of MS corrosion by 5-HTP molecules. First, adsorption of 5-HTP on the MS surface may result in electrostatic interactions between charged –COO− group 5-HTP species and iron ions. Second, it is also possible that since 5-HTP can be easily protonated, Cl− on the surface forms a protective film by interacting with the protonated group (–NH3+) in 5-HTP. The proposed mechanisms are supported by the results obtained from functional group analyses.
4 Conclusion
Corrosion of MS was monitored in laboratory‐simulated well-acidizing fluid with and without different concentrations of 5-HTP to determine its anticorrosive effect. 5-HTP functioned as an effective corrosion inhibitor, with its efficiency increasing with increases in concentration but decreasing as the temperature increased. Potassium iodide, PEG and GLU can improve the efficiency of 5-HTP at high temperatures. 5-HTP is spontaneously physically adsorbed on MS surfaces and obeys Langmuir adsorption. The active functional groups involved in the adsorption process are O, N and C=C. 5-HTP and its blends can serve as eco‐friendly alternative anticorrosive oilfield chemical additives for the well‐acidizing procedure.
Acknowledgements
The authors acknowledge support from the World Bank through the Robert S. McNamara Fellowship programme to conduct laboratory work abroad. We also acknowledge Dr. Shuangqin Sun and the entire Corrosion Protection research group of the Materials Physics and Chemistry Department, China University of Petroleum Qingdao for providing the facilities for carrying out this research. We appreciate the African Centre of Excellence (ACE), Centre for Oilfield Chemicals Research (CEFOR) for their support. EI is grateful to Prof A. P. Udoh, Dr. Li, Dr. Wang, Ubong Jerome, Chen, Chao, Xiang and Min in UPC for their assistance.
Notes
Peer review under responsibility of Taibah University.
References
- J.ArthurB.B.DanielM.LayneHydraulic fracturing considerations for natural gas wells of the Marcellus ShaleGulf Coast Assoc. Geol. Soc. Trans.5920094959
- L.T.PopoolaA.S.GremaG.K.LatinwoB.GuttiA.S.BalogunCorrosion problems during oil and gas production and its mitigationInt. J. Ind. Chem.412013115
- M.FinšgarJ.JacksonApplication of corrosion inhibitors for steels in acidic media for the oil and gas industry: a reviewCorros. Sci.8620141741
- C.G.DarivaA.F.GalioCorrosion inhibitors—principles, mechanisms and applicationsDev. Corros. Prot.201410.5772/57255
- V.S.SastriTypes of corrosion inhibitor for managing corrosion in underground pipelinesM.E.OrazemUnderground Pipeline Corrosion2014Woodhead Publishing166211
- M.El AzzouziaA.AounitiaS.TighadouinaH.ElmsellemaS.RadiaB.HammoutiaA.El AssyrybF.BentisscA.ZarroukSome hydrazine derivatives as corrosion inhibitors for mild steel in 1.0 M HCl: weight loss, electrochemichal, SEM and theoretical studiesJ. Mol. Liq.2212016633641
- D.M.GurudattK.N.MohanaH.C.TandonAdsorption and corrosion inhibition characteristics of some organic molecules containing methoxy phenyl moiety on mild steel in hydrochloric acid solutionMater. Discov.201610.1016/j.md.2016.03.005
- C.XuW.L.JinH.L.WangH.T.WuN.HuangZ.Y.LiJ.H.MaoOrganic corrosion inhibitor of triethylenetetramine into chloride contamination concrete by eletro-injection methodConstr. Build. Mater.1152016602617
- C.VermaM.A.QuraishiE.E.EbensoI.B.ObotA.El Assyry3-Amino alkylated indoles as corrosion inhibitors for mild steel in 1 M HCl: experimental and theoretical studiesJ. Mol. Liq.2192016647660
- M.YadavS.KumarR.R.SinhaI.BahadurE.E.EbensoNew pyrimidine derivatives as efficient organic inhibitors on mild steel corrosion in acidic medium: electrochemical, SEM, EDX, AFM and DFT studiesJ. Mol. Liq.2112014135145
- K.ZakariaA.HamdyM.A.AbbasO.M.Abo-ElenienNew organic compounds based on siloxane moiety as corrosion inhibitors for carbon steel in HCl solution: weight loss, electrochemical and surface studiesJ. Taiwan Inst. Chem. Eng.201610.1016/j.jtice.2016.05.036
- L.MadkourC.Savaş KayaL.GuoQuantum chemical calculations, molecular dynamics simulation and experimental studies of using some azo dyes as corrosion inhibitors for iron. Part 1: mono-azo dye derivativesJ. Taiwan Inst. Chem. Eng.682016461480
- C.VermaP.SinghM.A.QuraishiThermodynamical, electrochemical and surface investigation of Bis (indolyl) methanes as Green corrosion inhibitors for mild steel in 1 M hydrochloric acid solutionJ. Assoc. Arab Univ. Basic Appl. Sci.2120152430
- Z.HuY.MengX.MaH.ZhuJ.LiC.LiD.CaoExperimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HClCorros. Sci.1122016563575
- A.H.El-AskalanyS.I.MostafaK.ShalabiA.M.EidS.ShaabanNovel tetrazole-based symmetrical diselenides as corrosion inhibitors for N80 carbon steel in 1 M HCl solutions: experimental and theoretical studiesJ. Mol. Liq.2232016497508
- A. Adotey, J. Nii, Local production of 5-HTP from the seeds of Griffonia simplicifolia. Diss, 2009.
- P.RibeiroR.A.WebbThe synthesis of 5-hydroxytryptamine from tryptophan and 5-hydroxytryptophan in the cestode Hymenolepis diminutaInt. J. Parasitol.131983101106
- G.FrangatosF.L.ChubbA new synthesis of 5-hydroxytryptophanCan. J. Chem.378195913741376
- R.HaraK.KinoEnhanced synthesis of 5-hydroxy-l-tryptophan through tetrahydropterin regenerationAMB Express320137078
- Z.AhmadPrinciples of Corrosion Engineering and Corrosion Control2006Butterworth-Heinemann
- A.SinghM.A.QuraishiAcidizing corrosion inhibitors: a reviewJ. Mater. Environ. Sci.612015 224–223
- M.K.PavithraT.V.VenkateshaM.K.Punith KumarN.S.AnanthaElectrochemical, gravimetric and quantum chemical analysis of mild steel corrosion inhibition by colchicine in 1 M HCl mediumRes. Chem. Intermed.423201624092428
- S.B.Al-BaghdadiF.T.M.NooriW.K.AhmedA.A.Al-AmieryThiadiazole as a potential corrosion inhibitor for mild steel in 1 M HClJ. Adv. Electrochem.220166769
- S.K.SahaA.DuttaP.GhoshD.SukulP.BanerjeeNovel Schiff base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: experimental and theoretical approachPhys. Chem. Chem. Phys.1820161789817911
- N.I.KairiJ.KassimThe effect of temperature on the corrosion inhibition of mild steel in 1 M HCl solution by Curcuma longa extractInt. J. Electrochem. Sci.8201371387155
- I.AkpabioJ.EjedaweJ.EbeniroE.UkoGeothermal gradients in the Niger Delta Basin from continuous temperature logsGlob. J. Pure Appl. Sci.92003265272
- C.N.NwankwoA.S.EkineGeothermal gradients in the Chad Basin, Nigeria, from bottom hole temperature logsInt. J. Phys. Sci.42009777783
- J.AdedapoA.IkpokonteK.SchoeneichE.KurowskaAn estimate of oil window in Nigeria Niger Delta Basin from recent studiesGas520141218
- G.EmujakporueA.EkineDetermination of geothermal gradient in the Eastern Niger Delta Sedimentary Basin from bottom hole temperaturesJ. Earth Sci. Geotech. Eng.432014109114
- V.RajeswariD.KesavanM.GopiramanP.ViswanathamurthiK.PoonkuzhaliT.PalvannanCorrosion inhibition of Eleusine aegyptiaca and Croton rottleri leaf extracts on cast iron surface in 1 M HCl mediumAppl. Surf. Sci.3142014537545
- E.B.ItuenO.AkarantaA.O.JamesProtection of J55 steel surface in acidic well treatment fluids using green anticorrosive oilfield chemicals from 5-hydroxytryptophanAm. Chem. Sci. J.201610.9734/ACSJ/2016/28976
- S.A.UmorenBiomaterials for corrosion protection: evaluation of mustard seed extract as eco-friendly corrosion inhibitor for X60 steel in acid mediaJ. Adhes. Sci. Technol.30201618581879
- A.S.FoudaS.K.ShalabiG.Y.ElewadyH.F.MerayyedChalcone derivatives as corrosion inhibitors for carbon steel in 1 M HCl solutionsInt. J. Electrochem. Sci.9201470387058
- A.HamdyN.S.El-GendyThermodynamic adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid mediumEgypt. J. Petrol.2220131725
- P.SinghA.SinghM.QuraishiThiopyrimidine derivatives as new and effective corrosion inhibitors for mild steel in hydrochloric acid: electrochemical and quantum chemical studiesJ. Taiwan Inst. Chem. Eng.602015 588–561
- K.K.AnupamaK.RamyaK.M.ShainyA.JosephAdsorption and electrochemical studies of Pimenta dioica leaf extracts as corrosion inhibitor for mild steel in hydrochloric acidMater. Chem. Phys.16720152841
- L.O.OlasunkanmiM.K.MwadhamE.E.EbensoQuinoxaline derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium: electrochemical and quantum chemical studiesPhysica E: Low-dimens. Syst. Nanostruct.762016109126
- X.WangL.LiuP.WangW.LiJ.ZhangY.YanHow the inhibition performance is affected by inhibitor concentration: a perspective from microscopic adsorption behaviorInd. Eng. Chem. Res.5320141678516792
- C.VermaE.E.EbensoL.O.OlasunkanmiM.A.QuraishiI.B.ObotAdsorption behavior of glucosamine based pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studiesJ. Phys. Chem. C1202120161159811611