Abstract
In this study, a novel, selective and highly sensitive spectrophotometric method has been developed for the determination of copper(II) by using a new chromogenic reagent N,N′-bissalicylidene-2,3-diaminopyridine (H2IF). The absorbance was measured at λmax = 414 nm. Experimental conditions were optimized, and Beer’s law was observed in the concentration range of 6.35–318 μg L−1 of copper. The molar absorptivity of the complex was calculated and found to be 1.46 × 105 L mol−1 cm−1. The detection and quantification limits were calculated and found to be 6.38 μg L−1 and 21.27 μg L−1, respectively. The calculated relative standard deviation (n = 5) was 0.62% for a standard solution including 63.5 μg L−1 copper(II). The interference effects of the common ions have been studied and the statistical assessment of the obtained results is presented. The developed method was applied for the determination of copper in water samples. The reliability and accuracy of the developed method was proven by the analysis of a waste water standard.
1 Introduction
Currently, the selective and sensitive monitoring of trace metal ions from different sources, especially in water, have become more important due to their toxic effects on environmental and biological systems. Copper and its salts are used in industries, laboratories, medicines, foods and beverages. Therefore, it is of significant importance to develop uncomplicated, rapid and effective methods for quantitative analysis of copper in food, industrial and environmental samples.
For copper(II) determination, several analytical techniques have been used such as atomic absorption spectrometry [Citation1–Citation3], voltammetry [Citation4,Citation5], potentiometry [Citation6–Citation10], inductive coupled plasma-emission spectrometry [Citation11] and inductive coupled plasma-mass spectrometry [Citation12]. Spectrophotometry is the most widely used analytical technique for analysis because it is simple, economic, and easily available to most laboratories. Many spectrophotometric reagents have been used for determination of Cu(II); however, most of these reagents have various limitations such as requiring more time for colour development, heating, a narrow Beer’s range, or interferences from many ions [Citation13–Citation20].
H2IF is among the foremost salophen ligands that have many fruitful applications and was first proposed by Bosner and co-worker [Citation21]. The neutral Cu(II) complex ([CuL]) of H2IF has been successfully crystallized by Atakol et al. [Citation22] and characterized by Asadi et al. [Citation23]. Recently, the potential DNA binding features and photo physical behaviours have been investigated by Kocak et al. [Citation24]. In one of its recent applications among numerous studies throughout the past few decades, the evaluation of potentiometric cation recognition characteristics of the ligand has been proposed by our group [Citation25,Citation26]. In our up-to-date studies, addition of Cu(II) to the solution of H2IF resulted in a rapid colour change from yellow to light brown with an accompanying new band appearing at 414 nm in the absorption profile. However, the compound has not been previously applied for the spectrophotometric determination of Cu(II).
Herein, we have proposed a novel, highly sensitive, simple and feasible method for the spectrophotometric monitoring of Cu(II) ion with a H2IF in a methanol–water medium. Various experimental conditions, e.g., the amount of chromogenic agent, the effect of coexistence ions, the effect of pH, the ranges of applicability of Beer’s law and limit of detection on the spectrophotometric determination of copper have been considered. The method was successfully performed for the analysis of copper(II) in water samples and reference materials C12X3500 and SPS-WW1 (waste water).
2 Experimental
2.1 Reagents, solutions and samples
Throughout the study, analytical-reagent grade chemicals were used.
The solutions of metal salts were prepared with the relevant cations. For each cation, a 10−1 M stock solution was prepared and then the dilute solutions (1 × 10−2 to 1 × 10−9 M) were prepared by serial dilution of the stock solutions.
Acetate buffer solutions were prepared in the pH range of 2.20–5.60 by mixing appropriate proportions of sodium acetate solution (0.1 M) and acetic acid (0.2 M).
The river and sea water samples collected from various resources were filtered over a 0.45 m filter from Millipore (Milford MA).
Certified materials C12X3500 and SPS-WW1 were supplied from MBH Analytical Ltd. and LGC Group (UK).
2.2 Characterization of H2IF
H2IF was synthesized according to the method carried out in our prior studies using the same ratio of reactants [Citation25,Citation26]. Yield: 85%. Anal.Calc. C, 71.91; H, 4.76; N, 13.24. Found: C, 71.92; H, 4.82; N 13.67. IR (KBr, cm−1): 3476 νas(OH); 3043 νar(CH); 1615-1606 ν(C=N); 1553 ν(C=C). 1H-NMR (400 MHz, ppm, CDCl3) 9.54 (s, 1H, –OH) 8.67 (s, 1H, imine) 8.45 (s, 1H, imine-pyridine nitrogen side) 7.28-7.75 (m, 4H, benzene) 6.89-7.15 (m, 3H, pyridine). ESI-MS (m/z, positive mode): 318 (H2IF2+, 64%); 301 (H2IF-OH2+, 100%). An optimized structure of the ligand based on density functional theory calculations is given in (see Ref. [Citation25] for computational details).
A 1 × 10−3 M solution of the H2IF was prepared by dissolving a proper weight of reagent in 10.0 mL methanol and then diluted with methanol to 50 mL in a calibrated flask.
2.3 Apparatus
A Thermo Array Evolution UV–Vis spectrophotometer (USA) was used for the observation of the absorption spectra.
A glass pH electrode (Schott) connected to a Jenway 3040 model Ion Analyzer was used for pH measurements of the buffer solutions.
2.4 Calibration procedure
A 1 mL of 5 × 10−4 M H2IF and 1 mL of acetate buffer (pH 4.8) were added into a 4-mL glass tube. A 1 mL aliquot containing between 0.0 and 635 μg L−1 Cu(II) was transferred into tube and mixed well. The absorbance was measured at 410 nm against a blank solution. The blank solution was prepared in a similar way, but without containing any copper. All absorbance measurements were implemented at room temperature. The amount of Cu(II) in the sample was determined from the calibration graph prepared in the same way.
3 Results and discussion
3.1 Spectral properties of ligand and complex
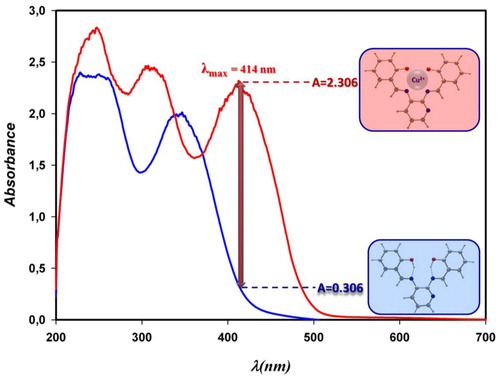
The H2IF ligand reacts with a Cu(II) ion to form a stable square-planar neutral complex of 1:1 adduct, which is highly soluble in a methanol-water mixture. The electroneutral nature of the complex is derived from deprotonation of H2IF induced upon complexation with Cu(II). The obtained absorption spectra of the free ligand and the Cu(II) complex is given in . The spectra were recorded in semi-aqueous media of 5 × 10−4 M H2IF and Cu(II)–H2IF complex in the range of 200–600 nm. The absorption of H2IF occurs within the 200–400 nm range of the spectrum, whereas the Cu(II) complex further shows a distinct band along the 400–450 nm range with a 414-nm transition maximum. It is apparent from that the 414-nm maximum makes spectrophotometric determination of the metal very feasible. Investigations were carried out to define the most appropriate conditions. The influence of each of the following variables such as the effect of pH and time, and the amount of chromogenic reagent on the reaction was tested. Using a univariate optimization method, the selected parameter was changed while the other variables were kept constant to obtain the optimum value for each of the variables.
3.2 Investigation of the appropriate experimental conditions
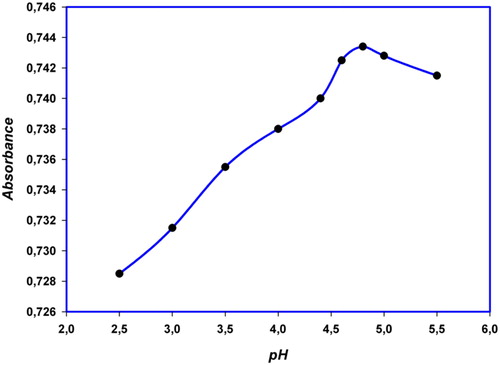
The workable pH range for the determination of Cu(II) was studied and ascertained. Several types of buffers such as universal, acetate and phosphate were prepared and tried. An acetate buffer was used to preserve the optimum buffer solution. This buffer gave the highest absorbance value. Keeping the other conditions constant, the pH values were varied in the range 2.20–5.60, as shown in . The inherent features of the spectral curves persisted nearly constant at pH 4.60–5.00. A pH of 4.80 was arbitrarily chosen for complete colour development. Therefore, all following studies were carried out at pH 4.80. It was observed that, under each condition, the absorbance persisted constant with the addition of 1 mL buffer for 1 mL sample.
The concentration of H2IF was another important variable that could affect the absorbance intensity of the complex in the solution. The influence of the H2IF concentration was studied in the range 1 × 10−5 to 1 × 10−4 M. Increasing the chromogenic reagent concentration resulted in an increment in the absorbance of the complex. It was observed that the concentration of the reagent should be in the range of 5.0 × 10−5 to 5.2 × 10−5 M. The maximum absorbance of the coloured complex incorporated with minimum blank reading was found to correspond to 5 × 10−5 M H2IF. In the subsequent studies, the volume of 5 × 10−5 M H2IF was fixed at 1.0 mL.
The effect of time on the complex formation reaction between Cu(II) and H2IF was studied. The solution colour changed immediately from light yellow to light brown upon addition of Cu(II) to the solution. In only one minute, the absorbance of the system at 414 nm reached the maximum with the addition of Cu(II) to the solution and did not increase more with a longer time duration. This result depicts a rapid interaction of H2IF with Cu(II) in a one-minute period under experimental conditions. This method could be applied for real-time detection of Cu(II) with no long interaction time required to obtain the whole absorbance before measurement of the signal.
In our previous potentiometric studies, interferences from other coexisting ions were computationally demonstrated through the comparisons of the calculated host-guest interaction energies in the framework of conceptual DFT and only the interference of Cr3+ was found to be noteworthy [Citation25].
In the study herein, the effect of common interfering ions on the analysis of Cu(II) was also studied in detail. The tolerance limit was taken as the amount that caused a ±5% absorbance error in the determination of 63.5 μg L−1 Cu(II). Great amounts of Na+, K+, NH4+, Ca2+, ClO4−, SO42−, CO32−, NO3−, 10000-fold excess of Cd2+, Ba2+, F−, Br−, 5000-fold excess of Pb2+, Mn2+, 2000-fold excess of Zn2+ and Mg2+, and 1000-fold excess of Cr3+, Co2+ and Ni2+ did not interfere in the analysis of copper(II).
3.3 Applicability of Beer’s Law and sensitivity
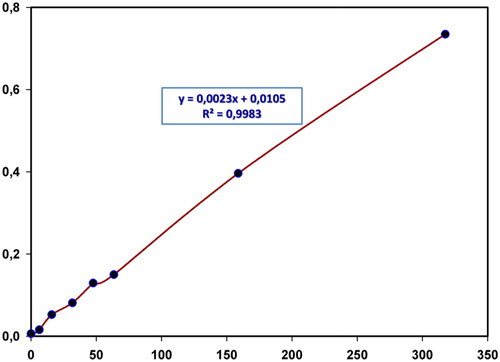
Under the optimum conditions, the calibration curve for the determination of copper(II) was obtained and is depicted in . The Beer’s law was observed in the range of 6.35–318 μg L−1 copper(II) concentration. The obtained linear regression equation was: A = 0.002 + 0.010 CCu (μg L−1). The correlation coefficient was 0.997. The apparent molar absorption coefficient (ε) was 1.46 × 105 L mol−1 cm−1, calculated from the slope of calibration curve. The detection (LOD = 3Sa/b) and quantification (LOQ = 10Sa/b) limits were calculated and found to be 6.38 μg L−1 and 21.27 μg L−1, respectively. (where S a is the standard deviation of the response and b is the slope of the calibration curve). The calculated relative standard deviation (n = 5) was 0.62% for a standard solution containing 63.5 μg L−1 Cu(II).
3.4 Application for real samples
The analytical applicability of the developed method was evaluated by performing it for the determination of copper in sea and river water samples. The samples collected from various resources were filtered, spiked with 25 or 50 μg L−1 Cu and reanalysed. The obtained results of these spike/recovery experiments are given in . Good recoveries (98.5–102.96%) were obtained for Cu(II) in these samples, demonstrating the accuracy of the developed method. As another validation protocol, reference standards C12X3500 and SPS-WW1 were tested. The results were found to be in good agreement with the certificated copper concentrations ().
Table 1 Analytical results of water samples and the recovery of spiked analyte, and standard reference materials.
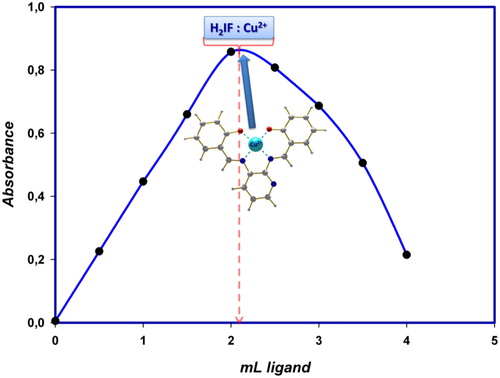
3.5 Determination of the stoichiometry of the complex
Job method was used for the determination of the composition of the Cu(II)–H2IF complex. Equimolar (1 × 10−4 mol L−1) solutions of Cu(II) and H2IF were prepared. The metal(II) and reagent solutions were mixed in constantly ranging proportions, keeping the total volume of metal and ligand constant at 4.0 mL. The pH was kept at a constant value of 4.8 by the addition of acetate buffer. The absorbance originating from the complex which develops a light brown colour was measured at 414 nm. The obtained values are shown in . The maximum absorbance value was read at approximately 2 mL ligand volume and was assigned to a molar [Cu(II)]:[H2IF] ratio of 1:1 adduct suggesting a Cu(II)–H2IF stoichiometry.
3.6 Comparison with the current literature
In summary, a relevant benchmark of spectrophotometric methods from the attached literature [Citation27–Citation32] and from the present study is compiled and given in . All parameters in are nearly comparable within a reasonable data range and seem not superior to each other in general trend. Nonetheless, the shortness of reaction time which is favourable for practical application of spectrophotometric methods was found to be shortest one for the present method among existing values in .
Table 2 Comparison of the present spectrophotometric data to others in the determination of copper(II).
4 Conclusion
In this work, a novel, highly selective and feasible method was developed for the spectrophotometric monitoring of Cu(II) by using N,N′-disalicylidene-2,3-diaminopyridine (H2IF) as a new chromogenic reagent. The ligand has been found to be a highly sensitive and selective spectrophotometric reagent for Cu(II) ions. In the presence of Cu(II), H2IF displays an obvious colour change from light yellow to light brown in a very short period. H2IF also exhibits a good selectivity and sensitivity towards Cu(II) over other common competitive alkali, alkaline earth and transition metal ions. Experimental findings have indicated that the reagent can be readily suggested among one of selective spectrophotometric reagents and has a potential application for rapid, selective and sensitive detection of Cu(II) in aqueous media. Moreover, the spectrophotometric efficiency of H2IF would pave the way for flow-injection spectrophotometric applications of the reagent.
Acknowledgement
Equipment support from the Ondokuz Mayıs University is gratefully acknowledged.
Notes
Peer review under responsibility of Taibah University.
References
- R.J.CassellaO.I.B.MagalhaesM.T.CoutoE.L.S.LimaM.A.F.S.NevesF.M.B.CoutinhoSynthesis and application of a functionalized resin for flow injection/F AAS copper determination in watersTalanta672005121128
- M.H.MashhadizadehM.PestehM.TalakeshI.SheikhshoaieM.Mazloum-ArdakaniM.A.KarimiSolid phase extraction of copper(II) by sorption on octadecyl silica membrane disk modified with a new Schiff base and determination with atomic absorption spectrometrySpectrochim. Acta B632008885888
- C.A.SahinI.TokgozA novel solidified floating organic drop microextraction method for preconcentration and determination of copper ions by flow injection flame atomic absorption spectrometryAnal. Chim. Acta66720108387
- A.MohadesiM.A.TaherVoltammetric determination of Cu(II) in natural waters and human hair at a meso-2,3-dimercaptosuccinic acid self-assembled gold electrodeTalanta72200795100
- H.BagheriA.ShirzadmehrM.RezaeiDetermination of copper ions in foodstuff products with a newly modified potentiometric carbon paste electrode based on a novel nano-sensing layerIonics2016112
- M.AndacF.ColdurS.BilirA.BirinciS.DemirH.UzunSolid-contact polyvinyl chloride membrane electrode based on the bis[(2-(hydroxyethylimino)phenolato]copper(II) complex for trace level determination of copper ions in wastewaterCan. J. Chem.922014324328
- M.Ghanei-MotlaghM.A.TaherV.SahebM.FayaziI.SheikhshoaieTheoretical and practical investigations of copper ion selective electrode with polymeric membrane based on N,N′-(2,2-dimethylpropane-1,3-diyl)-bis(dihydroxyacetophenone)Electrochim. Acta56201153765385
- A.IslamnezhadN.MahmoodiPotentiometric Cu(II)-selective electrode with subnanomolar detection limitDesalination2712011157162
- Y.M.IssaH.IbrahimO.R.ShehabNew copper(II)-selective chemically modified carbon paste electrode based on etioporphyrin I dihydrobromideJ. Electroanal. Chem.66620121118
- A.ShokrollahiA.AbbaspourM.GhaediA.N.HaghighiA.H.KianfarM.RanjbarConstruction of a new Cu(II) coated wire ion selective electrode based on 2-((2-(2-(2-(2-hydroxy-5-methoxybenzylidene amino)phenyl)disufanyl)phenylimino)methyl)-4-methoxyphenol Schiff baseTalanta8420113441
- T.KamiuraK.FunasakaY.TajimaT.KawarayaK.KurodaPretreatment by yeast for determination of nickel and vanadium in bitumen-in-water emulsion by inductively coupled plasma atomic emission spectrometryAnal. Chim. Acta32719966164
- A.Martin-CameanA.JosM.PuertoA.CallejaA.Iglesias-LinaresE.SolanoA.M.CameanIn vivo determination of aluminum, cobalt, chromium, copper, nickel, titanium and vanadium in oral mucosa cells from orthodontic patients with mini-implants by Inductively coupled plasma-mass spectrometry (ICP-MS)J. Trace Elem. Med. Biol.3220151320
- F.J.HuoC.X.YinY.T.YangJ.SuJ.B.ChaoD.S.LiuUltraviolet-visible light (UV-Vis)-reversible but fluorescence-irreversible chemosensor for copper in water and its application in living cellsAnal. Chem.84201222192223
- F.DayouY.DongSpectrophotometric determination of trace copper in water samples with thiomichlersketoneSpectrochim. Acta A662007434437
- J.Y.KwonY.J.JangY.J.LeeK.M.KimM.S.SeoW.NamJ.YoonA highly selective fluorescent chemosensor for Pb2+J. Am. Chem. Soc.12720051010710111
- Q.LinP.ChenJ.LiuY.P.FuY.M.ZhangT.B.WeiColorimetric chemosensor and test kit for detection copper(II) cations in aqueous solution with specific selectivity and high sensitivityDyes Pigments982013100105
- J.H.SohK.M.K.SwamyS.K.KimS.KimS.H.LeeJ.YoonRhodamine urea derivatives as fluorescent chemosensors for Hg2+Tetrahedron Lett.48200759665969
- H.YangZ.G.ZhouK.W.HuangM.X.YuF.Y.LiT.YiC.H.HuangMultisignaling optical-electrochemical sensor for Hg2+ based on a rhodamine derivative with a ferrocene unitOrg. Lett.9200747294732
- Z.Y.YaoB.H.HuangX.P.HuL.ZhangD.P.LiM.GuoX.H.ZhangH.YuanH.C.WuColorimetric detection of copper ions based on a supramolecular complex of water-soluble polythiophene and ATPAnalyst138201316491652
- L.ZengE.W.MillerA.PralleE.Y.IsacoffC.J.ChangA selective turn-on fluorescent sensor for imaging copper in living cellsJ. Am. Chem. Soc.12820061011
- Z.CimermanN.GalesicB.BosnerStructure and spectroscopic characteristics of Schiff-bases of salicylaldehyde with 2,3-diaminopyridineJ. Mol. Struct.2741992131144
- O.AtakolH.NazirM.N.TahirD.UlkuCrystal structure of (N,N′-disalicylidene-2,3-diaminopyridine)copper(II) complexAnal. Sci.131997519520
- M.AsadiZ.AsadiS.TorabiN.LotfiSynthesis, characterization and thermodynamics of complex formation of some new Schiff base ligands with some transition metal ions and the adduct formation of zinc Schiff base complexes with some organotin chloridesSpectrochim. Acta A942012372377
- A.kocakH.YilmazÖ.FaizO.AndacExperimental and theoretical studies on Cu(II) complex of N,N′-disalicylidene-2,3-diaminopyridine ligand reveal indirect evidence for DNA intercalationPolyhedron1042016106115
- S.DemirH.YilmazM.DilimulatiM.AndacAn efficient ab initio DFT and PCM assessment of the potentiometric selectivity of a salophen type Schiff baseJ. Mol. Model.202014
- S.DemirH.YilmazM.DilimulatiM.AndacSpectral and thermal characterization of salophen type Schiff base and its implementation as solid contact electrode for quantitative monitoring of copper(II) ionSpectrochim. Acta A1502015523532
- J.G.ZhangL.ZhangY.L.WeiJ.MaS.M.ShuangZ.W.CaiC.DongA selectively fluorescein-based colorimetric probe for detecting copper(II) ionSpectrochim. Acta A1222014731736
- R.A.NalawadeA.M.NalawadeG.S.KambleM.A.AnuseRapid synergistic extractive spectrophotometric determination of copper(II) by using sensitive chromogenic reagent N00,N000-bis[(E)-(4-fluorophenyl) methylidene]thiocarbonohydrazideSpectrochim. Acta A1462015297306
- A.A.GoudaA.S.AminCloud-point extraction preconcentration and spectrophotometric determination of trace quantities of copper in food, water and biological samplesSpectrochim. Acta A12020148896
- G.S.KambleS.S.KolekarM.A.AnuseSynergistic extraction and spectrophotometric determination of copper(II) using 1-(2,4-dinitro aminophenyl) 4,4,6-trimethyl-1,4 dihydropyrimidine-2-thiol: analysis of alloys, pharmaceuticals and biological samplesSpectrochim. Acta A78201114551466
- G.RamanjaneyuluP.R.ReddyV.K.ReddyT.S.ReddyDirect and derivative spectrophotometric determination of copper(II) with 5-bromosalicylaldehyde thiosemicarbazoneOpen Anal. Chem. J.220087882
- H.A.PanahiM.KarimiE.MoniriH.SoudiDevelopment of a sensitive spectrophotometeric method for determination of copperAfr. J. Pure Appl. Chem.21020089699