?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Captopril is an angiotensin-converting enzyme (ACE) inhibitor that is used for the treatment of hypertension and congestive heart failure. This article addresses the accurate measurements of densities and refractive indices of solutions containing captopril in pure solvents such as water, methanol, ethanol and 1-propanol and aqueous mixtures of methanol, ethanol and propan-1-ol of 30%, 50% and 70% by volume in a wide range of drug concentration at 26 °C. This article also includes the evaluation of apparent molar volume, partial molar volume at infinite dilution and transfer volumes. The concentration dependence of the refractive indices studied and respective fitting parameters have been reported. Different properties are interpreted in terms of intermolecular interactions, effect of drug on structure of solvent/solvent mixture and overall structural fittings in solutions.
1 Introduction
Water-co-solvent mixtures are widely used in the pharmaceutical sciences to enhance the solubility of drugs. These mixtures are highly non-ideal due to the existence of intermolecular interactions. The drug solutions in these water-co-solvent systems have applications in drug solubility and the design of homogeneous pharmaceutical dosage forms such as syrups and elixirs. Therefore, the physicochemical behaviour of the drug captopril (CPT) in water-co-solvent mixtures is highly significant in view of its pharmaceutical applications.
Table 1 Density, ρ (g cm−3) and refractive index data for CPT in pure solvents at 26 °C.
Table 2 Density, ρ (g cm−3) and refractive index data for CPT in aqueous-alcoholic mixtures at 26 °C.
The study of molecular interactions in solutions containing alcohols is of great interest, as alcohols are –OH group-containing highly polar organic molecules and self-associate through hydrogen bonding [Citation1–Citation3]. They are polar compounds in which hydrogen bonding or dipole-dipole Van der Waals forces are present. The hydrophobic character of alcohols increases with chain length; therefore, hydrophobic effects are studied using alcohol solvents.
Studies on physicochemical properties of drugs in aqueous-alcoholic mixtures are of interest due to peculiar results in different solvent systems. Volumetric data from drug studies can provide clues to the interactions occurring in cellular fluids. A survey of the literature reveals that studies on volumetric properties of drugs are of increasing interest by a number of workers in this field of research.
Drugs are the organic molecules containing hydrophobic and hydrophilic groups. Pharmacological properties of drug molecules are highly dependent on their solution behaviour [Citation4,Citation5]. Drugs may break or preserve the structure of the solvent or solvent mixture through interactions due to the presence of hydrophobic and hydrophilic groups. These interactions are specific and electrostatic interactions. Drug transport, anaesthesia and protein binding are important processes where drug and biomacromolecules interact, which affects their physicochemical behaviour.
Volumetric properties are dependent on different interactions such as solute-solute, solute-solvent and solvent-solvent interactions and structural effects due to volume difference among different components of solution [Citation6]. Systems containing hydrogen bonds are of great interest due to the vital role of these bonds in chemical, physical and biological processes [Citation7]. Pure and mixed (with water) alcohols are widely used in fields such as the pharmaceutical industry, ecology, cosmetics, and energy source [Citation7,Citation8]. Density, refractive index and viscosity and derived properties are useful in collecting analytical information required for industrial purposes [Citation9]. The study of refractive indices of different systems containing various solutes has been carried out by many researchers [Citation10–Citation15]. Knowing the densities and refractive indices of drug solutions is helpful for understanding molecular interactions and structural fittings in solution. Molecular interactions in solution are understood from studies based on measurements of refractive indices of solutions [Citation16–Citation19].
Therefore, in continuation of our earlier work in understanding the solution behaviour of different systems [Citation20–Citation24], the systematic study of volumetric and refractometric properties of CPT drug in water, alcohols and in alcohol-water mixtures of different volume ratios is carried out in the present work.
2 Experimental
Captopril (CPT) was received as a gift from Wockhardt Ltd. Aurangabad (MS) India, and it was used as is. Deionized distilled water (HPLC grade, pH = 6.91) obtained from a Millipore prefiltration kit (Direct-Q™ system series) was used. Methanol (MeOH, Merck, 99.0%), ethanol (EtOH, SD fine, 99.9%) and 1-propanol (1-PrOH, SD fine, 99.0%) were used. Solutions of drugs having different concentrations were prepared in pure solvents and solvent mixtures of different volume ratios. Density of solutions was measured using a single capillary calibrated pycnometer. The pycnometer was calibrated by ethanol at experimental temperature. Weight was measured on a single pan electronic balance (±0.001 g). The refractive index of solutions was measured using a thermostatically controlled Cyber LAB-Cyber Abbe Refractometer (Amkette Analytics, ±0.0002, 1.3000 to 1.7000). Averages of three readings of density and refractive index are reported.
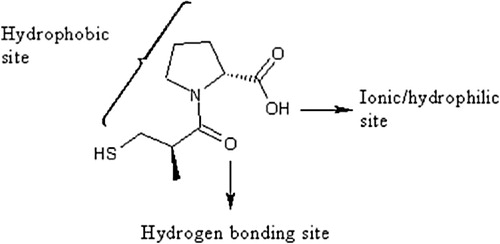
CPT has different interaction sites as shown in . It has hydrophilic and hydrophobic parts (functional groups) along with a hydrogen bonding site. The amide (–CONH) group can undergo hydrogen bond interactions with polar molecules, and the acid (–COOH) group is polar and ionic; therefore, CPT shows interesting interactions with polar solvent molecules.
3 Results and discussion
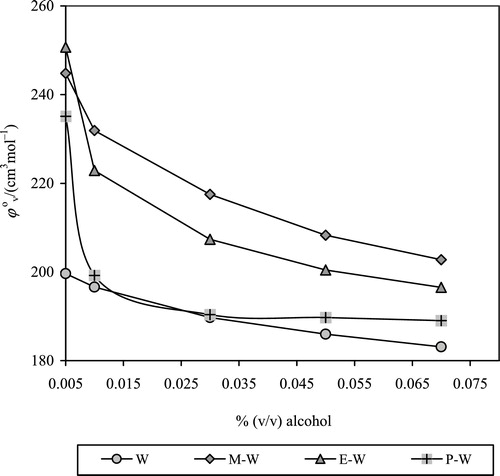
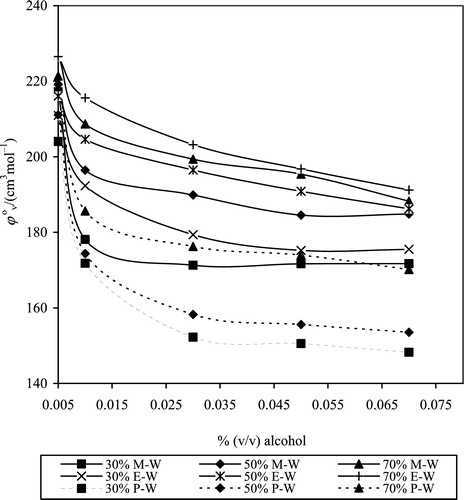
Experimental densities of drug in pure solvent and solvent mixtures are reported in and . It is seen that the density increased with drug concentration in any given solvent system, and density largely decreased with increase in volume ratios of MeOH, EtOH and PrOH for a given drug concentration, which is consistent with the densities of these alcohols. Behaviour density with drug concentration indicates a change in volume, strengthening of drug-solvent interactions and modification of structural orientation in solution.
The apparent molar volume (φv, AMV) of drug in different solvent systems is calculated from accurately measured densities of solution [Citation25] using Eq. Equation(1)(1)
(1) as follows:
(1)
(1) where c is molar concentration, Μ2 = molecular weight of solute, ρ0 = density of solvent and ρ = density of solution. AMV values of drug in pure solvents and aqueous-alcoholic mixtures with different composition are presented in and .
It is seen that AMV decreases with drug concentration in each solvent/solvent mixture and increases with increase in volume ratio of alcohols in the respective systems. A decrease in AMV with increasing drug concentration is attributed to solvophobic hydration between the polar groups of CPT and the solvent and compression in volume due to modified mean distance [Citation26]. Increase in AMV with increased volume ratio of alcohols is due to relative weakening of drug-solvent interactions and reduction in electrostriction. The AMVs of drug in pure MeOH, EtOH and PrOH are greater than in water due to the presence of additional alkyl groups (–CH3, –CH2CH3 and –CH2CH2CH3 respectively) in these alcohols. The AMV is the sum of the geometric volume of solute molecules, and changes in volume occurred due to the interaction of solute with solvent [Citation27]. A survey of the literature on model compounds revealed that the volumes of the water-soluble compounds are generally smaller in aqueous medium compared to non-aqueous medium.
AMV data is fitted to Masson’s relation [Citation28,Citation29] Eq. Equation(2)(2)
(2) :
(2)
(2)
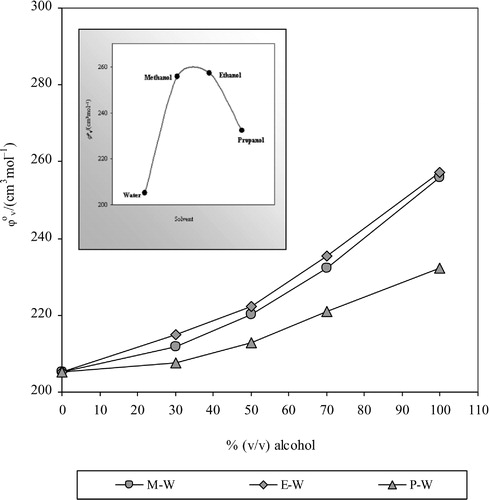
The φov (partial molar volume, PMV) and Sv are determined as the intercept and slope of the linear plots for drug + pure alcohols and drug + mixed solvents. Values of φov and Sv are obtained from the graph by linear fitting and are reported in .
Table 3 The φov (cm3 mol−1), Sv (cm3 dm3/2 mol−3/2) and transfer volumes, Δtrφ0v (cm3 mol−1) of CPT in pure solvents and aqueous-alcoholic mixtures.
The φov is independent of solute-solute interactions in solution; its values for CPT are positive and large in all the solvent systems due to strong drug-solvent interactions because of reduction in the electrostriction, change in the volume and solvation behaviour of drug The φov values in MeOH-H2O and EtOH-H2O are nearly the same but greatly differ from the values in 1-propanol-water. Positive φov values also suggest no restriction on the molecular motion in solution [Citation30]. The trend of φov values with solvent composition is: MeOH-H2O ≈ EtOH-H2O > 1-PrOH-H2O as shown in . This observation suggests that relatively more of the structure is disrupted in aqueous solutions of 1-PrOH due to the hydrophobic chain of alcohols [Citation25]. The φov increased with alcohol content and is attributed to the solvation behaviour of drug and change in volume due to reduction in the electrostriction and migration of solvent from the second layer around the drug to bulk solvent.
Sv values are negative (negative slopes) for all the solvent systems, which indicates the presence of weak ion-ion or solute-solute interactions and insufficient counter ion binding of the drug molecule due to its hydrophobic nature [Citation26]. At infinite dilution, the drug molecules are far away from each other and cannot interact strongly, which results in the negative slopes and weak drug-drug interactions and strong drug-solvent interactions. A negative Sv value also suggests that the drug molecule tries to occupy the empty space of the solvents. There is no appreciable change in the Sv with solvent composition, even from water to 1-propanol.
Partial molar volume of transfer (standard transfer volume of drug, Δtrφ0v) from aqueous solution to aqueous-alcoholic solutions is calculated [Citation31] from the following Eq. Equation(3)(3)
(3) and is reported in .
(3)
(3) In all cases, the Δtrφ0v values are positive. Δtrφ0v increased with increase in alcohol content. These values are large and positive in pure solvents such as MeOH, EtOH and 1-PrOH. Δtrφ0v values are smaller in 1-PrOH-H2O solutions compared to MeOH-H2O and EtOH-H2O. The trend of Δtrφ0v values with solvent composition is: MeOH-H2O ≈ EtOH-H2O < 1-PrOH-H2O.
The hydration number (the number of water molecules hydrating drug) of the drug decreases in the presence of alcohol in aqueous drug solution and further decreases with increase in the alcohol content in solution due to the existence of drug-alcohol polar-hydrophilic interactions and intermolecular interactions (intermolecular hydrogen bonding) between water and alcohol [Citation32]. Positive Δtrφ0v values are attributed to decrease in volume of shrinkage because of interactions between drug and alcohols, the existence of hydrophilic-hydrophilic and ion-hydrophilic interactions, and the reduction in the electrostriction (less hydration of drug in presence of alcohol) due to intermolecular hydrogen bonding between water and alcohol. These effects increase with increase in the alcohol content of the solution. This result also suggests the presence of dominating hydrophilic-hydrophilic interactions in solution. The increase in the values of φ0v and positive Δtrφ0v values are due to strong-drug-solvent interactions and decrease in the shrinkage volume of water by the drug in presence of alcohols, i.e., alcohols have a dehydrating effect on the hydrated drug [Citation33]. Apart from the interactions of -OH groups with polar/hydrophilic/ionic groups of the drug, additional interactions between hydrophobic alkyl groups (–CH3, –CH2CH3 and –CH2CH2CH3) of MeOH, EtOH and PrOH and the hydrophobic part of the drug molecule occur in aqueous-alcoholic solutions.
The experimental refractive indices of drug in pure and mixed solvents are reported in and . The refractive index increases with drug concentration in each solvent system. It also increases with volume ration of MeOH, EtOH and 1-PrOH for a given drug concentration. In pure MeOH, the refractive index values are small compared to H2O and aqueous-MeOH mixtures. However, in the case of aqueous-EtOH and aqueous-1-PrOH, the refractive index values increased with an increase in alcohol content for a given drug concentration.
In general, the refractive index data in different aqueous-alcoholic mixtures follows the following trend: Aqueous-1-PrOH > Aqueous-EtOH > Aqueous-MeOH. In pure solvents, the refractive index data follows the following trend: 1-PrOH > EtOH > H2O > MeOH. Refractive index trends in various systems are in accordance with the refractive indices of the respective solvents and solvent composition. The relationship between refractive index and concentration of solute depends on the nature of the solute, solvent, temperature and wavelength. The dependence on temperature and wavelength is eliminated by using a thermostat and monochromatic light, respectively. The linear relationship between refractive index and concentration of drug is checked [Citation34] using the following Eq. Equation(4)(4)
(4) and graphical parameters, namely, refractive index at infinite dilution, no and constant, K which depends on nature of solute are determined graphically and are reported in along with r2 values of the respective plots:
(4)
(4) where n = refractive index of solution, n0 = infinite dilution refractive index and K = constant.
Table 4 Graphical parameters of concentration dependence of n, {n = K (dm3 mol−1) × c (mol dm−3) + n0} for CPT in pure solvents and aqueous-alcoholic mixtures.
The refractive index at infinite dilution increases with the volume ratio of MeOH, EtOH and 1-PrOH for a given drug concentration except in the case of pure MeOH. The r2 values of various plots indicate that the relationship between refractive index and concentration of drug is linear in most cases [Citation35]. Further, the linear relationship between refractive index and density of solutions is checked. From the r2 values of various plots, the relationship between refractive index and density of solutions seems to be linear.
4 Conclusions
From the volumetric and refractometric properties of CPT in pure solvents and solvent mixtures of different compositions, it is concluded that strong drug-solvent interactions are present in each solution. The large positive values of φov confirm the reduction in electrostriction, change in volume and solvation behaviour of CPT. Relatively more structure disruption is observed in aqueous solutions of 1-PrOH due to the hydrophobic chain. Drug-drug interactions are weak in all aqueous-alcoholic systems and are not greatly affected by the nature of alcohol. A standard transfer volume, Δtrφ0v, indicates reduction in hydration number of the drug with increase in alcohol content. Hydrogen bonding interactions and ionic/hydrophilic-hydrophilic interactions between drug (–C=O, amide group and –COOH, acid group) and solvent/co-solvent (–OH) exists in solution. Refractive index data suggests that the packing of drug molecules becomes tighter with increases in concentration of drug in each system due to strengthening of drug-solvent interactions and structural cause of change in density and existence and modification of molecular interactions. The present work is helpful for prediction of absorption and permeability of CPT through membranes, which has applications in the field of medicinal and pharmaceutical chemistry.
Acknowledgements
The authors are thankful to Wockhardt Ltd. Aurangabad Ltd. Aurangabad (MS) India for the generous gift of the drug sample.
Notes
Peer review under responsibility of Taibah University.
References
- S.ElangovanS.MullainathanDielectric relaxation studies of ethyl formate with primary alcohols using time domain reflectometryMater. Sci. Res. Ind.920128183
- B.K.SarkarA.ChoudhuryB.SinhaExcess molar volumes excess viscosities and ultrasonic speeds of sound of binary mixtures of 1,2-dimethoxyethane with some aromatic liquids at 298.15 KJ. Solut. Chem.4120125374
- G.MahendranL.PalaniappanMolecular interactions of aniline in toluene + iso-butanol systemIndian J. Pure Appl. Phys.492011803808
- P.S.NikamT.R.MahaleM.HasanDensity and viscosity of binary mixtures of ethyl acetate with methanol, ethanol, propan-1-ol, propan-2-ol, butan-1-ol, 2-methylpropan-1-ol, and 2-methylpropan-2-ol at (298.15, 303.15, and 308.15) KJ. Chem. Eng. Data41199610551058
- V.K.SayalS.ChavanP.SharmaConductance measurements of narcotic-analgesic drugs in ethanol + water mixtures at 25 °CJ. Indian Chem. Soc.822005602607
- R.B.TôrresA.C.M.MarchioreP.L.O.VolpeVolumetric properties of binary mixtures of (water + organic solvents) at temperatures between T = 288.15 K and T = 303.15 K at p = 0.1 MPaJ. Chem. Thermodyn.382006526541
- Y.MarcusEffect of ions on the structure of waterPure Appl. Chem.82201018891899
- F.KoohyarF.KianiS.SharifiM.SharifiradS.H.RahmanpourStudy on the change of refractive index on mixing, excess molar volume and viscosity deviation for aqueous solution of methanol, ethanol, ethylene glycol, 1-propanol and 1,2,3-propantriol at T = 292.15 K and atmospheric pressureRes. J. Appl. Sci. Eng. Technol.4201230953101
- R.BeldaJ.V.HerraezO.DiezRheological study and thermodynamic analysis of the binary system (water/ethanol): influence of concentrationPhys. Chem. Liq.422004467479
- M.V.RathnamS.MohiteM.S.KumarVolumetric, viscometric and optical study of molecular interactions in binary mixtures of diethyl malonate with ketones at 303.15, 308.15 and 313.15 KJ. Serb. Chem. Soc.772012507521
- S.C.BhatiaR.RaniJ.SangwanR.BhatiaDensities, viscosities, speeds of sound, and refractive indices of binary mixtures of 1-decanol with isomeric chlorotoluenesInt. J. Thermophys.32201111631174
- A.FucaloroA.ZanellaS.WidjajaJ.WidjajaPartial molar volumes and refractions of cobalt(III) complexes, part 1: homologous series of hexaaminecobalt(III) complexesJ. Solut. Chem.34200513571370
- D.Rudan-TasicC.KlofutarApparent specific volume and apparent specific refraction of some poly(oxyethylene) glycols in 1,4-dioxane and benzene solutions at 298.15 KMonatsh. Chem.135200412091224
- D.Rudan-TasicC.KlofutarApparent molar volume and apparent molar refraction of mono-, di-, tri-, and tetra(oxyethylene) glycol in aqueous, 1,4-dioxane, and benzene solutions at 298.15 KMonatsh. Chem.134200311851193
- A.ArceE.RodiiA.SotoMolar volume, molar refraction, and Isentropic compressibility changes of mixing at 25 °C for the system ethanol + methanol + dibutyl etherJ. Solut. Chem.271998911923
- M.J.IqbalM.A.ChaudhryThermodynamic study of three pharmacologically significant drugs: density, viscosity, and refractive index measurements at different temperaturesJ. Chem. Thermodyn.412009221226
- I.BanikM.N.RoyStudy of solute-solvent interaction of some bio-active solutes prevailing in aqueous ascorbic acid solutionJ. Mol. Liq.1692012814
- J.V.HerraezR.BeldaRefractive indices, densities and excess molar volumes of monoalcohols + waterJ. Solut. Chem.35200613151328
- R.BeldaJ.V.HerraezO.DiezA study of the refractive index and surface tension synergy of the binary water/ethanol: influence of concentrationPhys. Chem. Liq.43200591101
- S.D.DeosarkarU.B.ShaikhPhysico-chemical properties and components interaction in the solutions of para-substituted benzoic acids in aqueous ethanolRuss. J. Gen. Chem.83201323922394
- S.D.DeosarkarT.M.KalyankarStructural properties of aqueous metoprolol succinate solutions. Density, viscosity, and refractive index at 311 KRuss. J. Phy. Chem. A87201310601062
- S.D.DeosarkarA.L.PuyadP.S.KattekarT.M.KalyankarThe density and viscosity of aqueous solutions of sodium 2-({[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methyl}sulfinyl)benzimidazol-1-ide and solute-solvent molecular interactions studyRuss. J. Phy. Chem. A872013524527
- S.D.DeosarkarR.T.SawaleA.R.BanA.L.PuyadDensities, refractive indices and apparent molar volumes of potassium hexacyanoferrate(II) trihydrate in acidic media at 35 °CJ. Chem. Pharm. Chem.62014390394
- S.D.DeosarkarS.M.DeorayeT.M.KalyankarTemperature and concentration dependences of density and refraction of aqueous duloxetine solutionsRuss. J. Phy. Chem. A.88201411291132
- P.SharmaS.ChauhanV.K.SyalM.S.ChauhanStudies of partial molar volumes of some narcotic-analgesic drugs in aqueous-alcoholic mixtures at 25 °CInt. J. Thermophys.292008643655
- A.Q.MunirM.A.AsianStudy of solvent and temperature effects on volumetric, viscometric and conductometric studies of amlodipine besylateAsian J. Biomed. Pharm. Sci.43520142229
- S.K.SikarwarV.R.ChoureyA.A.AnsariApparent molar volume and viscometric study of alcohols in aqueous solutionInt. J. Chem. Phys. Sci.42015115120
- A.AliShahjahanN.H.AnsariDensity and viscosity of α-amino acids in aqueous solutions of cetyltrimethylammonium bromideRuss. Chem. Bull.59201019992004
- D.O.MassonSolute molecular volumes in relation to solvation and ionizationPhilos. Mag.81929218235
- J.D.PandeyK.MishraA.ShuklaR.D.RaiUltrasonic and thermodynamic studies of tetracyclines in solutionsCan. J. Chem.651987303306
- B.SinhaP.K.RoyM.N.RoyApparent molar volumes and viscosity B-coefficients of glycine in aqueous silver sulphate solutions at T = (298.15 308.15, 318.15) KActa Chim. Slov.572010651659
- V.P.KorolevA.L.SerebryakovaHydration numbers of glycine in an aqueous urea solutionJ. Struct. Chem.52201111061110
- M.N.RoyR.S.SahP.P.PradhanP.K.RoyIon-solvent and ion-ion interactions of phosphomolybdic acid in aqueous solution of catechol at 298.15 308.15, and 318.15 KRuss. J. Phy. Chem. A.83200918871895
- F.KoohyarA.A.RostamiM.J.ChaichiF.KianiStudy on thermodynamic properties for binary systems of water + l-cysteine hydrochloride monohydrate glycerol, and d-sorbitol at various temperaturesJ. Chem.20132013110
- A.KumarEstimates of internal pressure and molar refraction of imidazolium based ionic liquids as a function of temperatureJ. Solut. Chem.372008203214