Abstract
Syntheses of bioactive annulated pyrimidine derivatives are the most significant tasks in N-heterocyclic chemistry because these compounds have proved to be very attractive and useful for the design of new molecular frameworks of potential drugs with varying pharmacological activities. This review paper summarizes the one-pot multicomponent synthesis of annulated nitrogen- and oxygen-containing heterocycles, such as pyrano[2,3-d]pyrimidines, pyrido[2,3-d]pyrimidines and pyrido[2,3-d;5-6-d]dipyrimidines. The synthetic procedure is based on the chemistry of the domino Knoevenagel-Michael addition mechanism.
1 Introduction
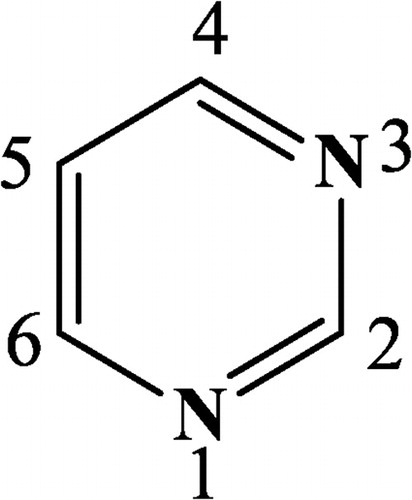
Genetic materials are made up of heterocycles such as adenine, guanine, cytosine, thymine and uracil. Nitrogen-containing six-member heterocycles and their fused derivatives, such as pyrano/pyridopyrimidines, are reported to show a wide range of biological activities, including antibacterial [Citation1], antiallergic [Citation2], antitumour [Citation3], antifolate [Citation4], tyrosine kinase [Citation5], antimicrobial [Citation6] calcium channel antagonistic [Citation7], anti-inflammatory, analgesic [Citation8], antihypertensive [Citation9], antileishmanial [Citation10], tuberculostatic [Citation11], anticonvulsant [Citation12], diuretic potassium-sparing [Citation13] and antiaggressive [Citation14] activities. Therefore, the synthesis of fused pyrimidines is of significant interest for the fusion of biodynamic heterosystems. This has proved to be very attractive and useful for the design of new molecular frameworks of potential drugs with varying pharmacological activities. Pyrimidine-containing molecules are a continuously expanding area of research in heteroaromatic chemistry because of their unique structures that provide several applications in different areas of pharmaceutical and agrochemical research or, more recently, in material sciences [Citation15]. Pyrimidine () is the parent ring system of a variety of substances that play vital roles in biological processes. This structural design also has a subset of a large number of pharmaceutical agents and naturally occurring substances such as vitamins, coenzymes, purines, pterins, nucleotides and nucleic acids. The properties of pyrimidines are governed to a large extent by the electron-attracting properties of the two nitrogen atoms; each of these reinforces the electronic effect of the other in the 2-, 4- and 6-positions.
Therefore, electrophilic attack will be strongly hindered at these positions, and nucleophilic attack is strongly facilitated. The 5-position is affected only by the inductive effect of the two nitrogen atoms, thereby making this position susceptible to electrophilic attack.
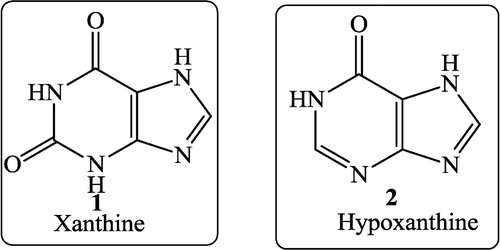
The pyrimidine nucleus has a wide occurrence in nature viz. in nucleic acids and nucleotides as well as in alkaloids obtained from tea, coffee, and cocoa and in uric acid. Most of the natural pyrimidines are hydroxyl and amino derivatives. Xanthine (1), which was first discovered by Marget in 1817 in bladder stones, also occurs in tea and in animal tissues along with hypoxanthine (2).
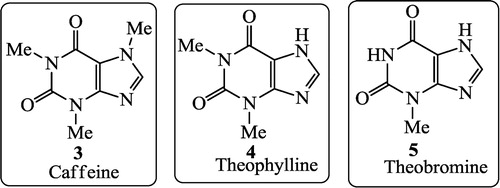
Coffee beans contain approximately 1.5% caffeine, tea leaves contain approximately 1.5% caffeine (3) and some theophylline (4), and cocoa beans contains approximately 1.3% theobromine (5). These methylated derivatives of xanthines have a stimulating effect on the central nervous system.
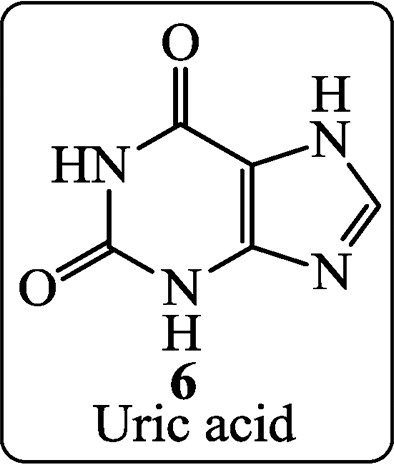
Uric acid (6), discovered by Swedish chemist Scheele in 1776, is a constituent of human urinary calculi (pebbles) and is a metabolite of purine nucleosides.
Pyrimidines (cytosine, thymine and uracil) are key components of deoxyribonucleic acid (DNA) molecules and participate directly in the encoding of genetic information. They also pass information to related ribonucleic acid (RNA) molecules that control protein synthesis and the sequence of amino acids [Citation16]. Vitamins in the B group, such as thiamine, folic acid, riboflavin, and cyanocobalamine, are nitrogen-containing heterocycles and function as either coenzymes or their precursors. Other vitamins, such as ascorbic acid (vitamin C) [Citation17] and α-tocopherol (vitamin E), are oxygen-containing heterocycles [Citation18]. Thus, the majority of commercially available pharmacologically active molecules are nitrogen- and oxygen-containing heterocycles. These molecules have attracted considerable attention from chemists as well biologists as a consequence of their exciting biological properties. Nitrogen- and oxygen-containing annulated heterocycles such as pyrano[2,3-d]pyrimidines, pyrido[2,3-d]pyrimidines and pyrido[2,3-d;5-6-d]dipyrimidines serve as both biomimetic and reactive pharmacophores owing to their varied therapeutic properties and thus play an essential role in natural and synthetic organic chemistry.
A major challenge of modern synthetic chemistry is to design highly efficient chemical reaction sequences that provide molecules containing maximum complexity and structural diversity with interesting bioactivities in the minimum number of synthetic steps. The use of organocatalysts has increased dramatically in the last few years both as a result of the novelty of this concept and, more importantly, because the efficiency and the selectivity of many organocatalyst reactions meet the standards of established organic reactions under reflux conditions. In light of these observations, intense efforts have been focused on reviewing the synthetic development of annulated pyrimidine derivatives via different pathways.
2 Multicomponent reactions (MCRs) in organic synthesis
In the past two decades, it has been extensively verified that multicomponent reaction (MCRs) have become standard in organic synthesis for creating molecular diversity and complexity in one step and have found a broad spectrum of applications in the discovery and development of pharmaceutical intermediates. Moreover, the combination of these reactions with suitable post-reaction modifications leads to scaffold multiplicity, which is one of the most important features of diversity-oriented synthesis. The development of multi-component reactions (MCRs) to produce biologically active compounds (annulated pyrimidines) has become an important area of research in organic, combinatorial, and medicinal chemistry [Citation19]. MCRs leading to attractive heterocyclic scaffolds are particularly useful for the creation of diverse chemical libraries of “drug-like” molecules, because the combination of three or more small-molecular-weight building blocks in a single operation leads to high combinatorial efficacy in creating diversity [Citation20]. MCRs can be considered a subclass of domino processes, as they are usually performed by placing all substrates in one pot under similar reaction conditions, where the compounds undergo the transformations in a time-resolved mode, meaning “after each other”. Since several substrates are placed together, it is not only the molecular complexity that is built up very rapidly but also the possibility of generating multiple analogues [Citation21]. One-pot multi-component reaction strategies offer significant advantages over conventional linear-type syntheses by virtue of their convergence, atom-economy, environmental concerns, productivity, facile execution and high yields [Citation22].
In modern heterocyclic synthesis, research efforts have been made to pursue ‘efficiency’ as a key goal. In the development of new synthetic methodologies, the development of new reactions with high yields and high selectivity is believed to generate ‘efficiency’, and research efforts have been made to achieve these goal. The review literature reported the one-pot multicomponent synthesis of targeted pyrano/pyridopyrimidine derivatives proceeded via domino Knoevenagel–condensation of active methylene compounds (malononitrile, methylcyanoacetate, ethylcyanoacetate, etc.) with different aromatic aldehydes through the loss of a water molecule followed by Michael addition of barbituric acid/thio-barbituric acid/6-amino uracil on the β-position on the electron-deficient C-atom and an intramolecular heterocyclization.
3 Fused heterocycles containing pyrano/pyridopyrimidines
Several methods have been reported in the literature for the synthesis of annulated pyrimidine derivatives based on different pathways using one-pot multi components. The significant reported work related to the synthesis of fused pyrano/pyridopyrimidines is described below.
3.1 Pyrano[2,3-d]pyrimidines
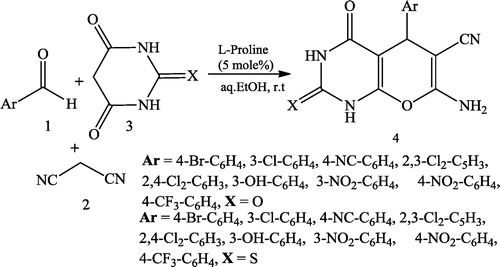
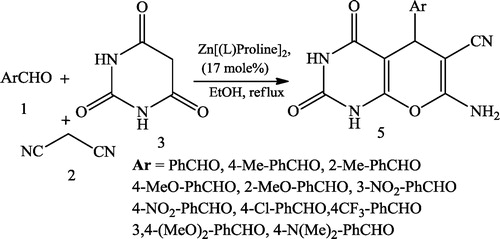
Bararjanian et al. [Citation23] reported a one-pot synthesis of pyrano[2,3-d]pyrimidinone derivatives 4 using a mixture of aromatic aldehydes 1, malononitrile 2 and barbituric acid/thiobarbituric acid 3 catalysed by L-proline in aqueous media (). The same type of product 5 was reported by Majid et al. [Citation24] using Zn[(L)proline]2 as an efficient catalyst and aromatic aldehydes 1, malononitrile 2, and barbituric acid as starting multicomponent reactants ().
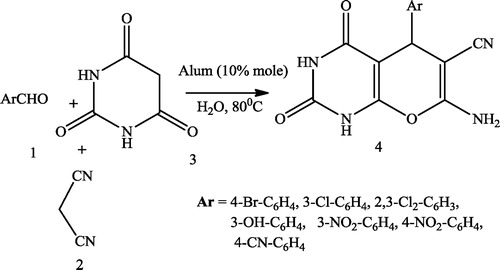
Akbar et al. [Citation25] reported an environmentally friendly and efficient synthesis of pyrano[2,3-d] pyrimidinone derivatives 4 using barbituric acid 1, aromatic aldehyde 2, malononitrile 3 and alum (10% mol) as catalysts in water at 80 °C ().
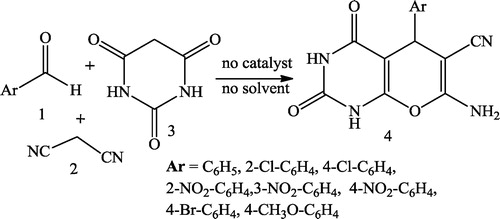
Sara et al. [Citation26] reported a novel one-pot synthesis of pyrano [2,3-d] pyrimidine-2,4 (1H,3H)-diones 4 in excellent yield from a mixture of aldehydes 1, malononitrile 2 and barbituric acid 3 without using any catalyst or solvent ().
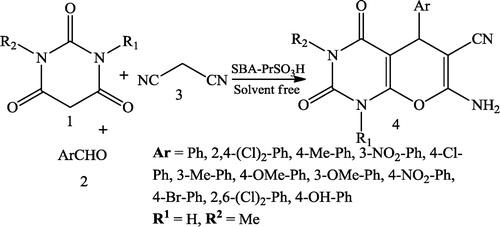
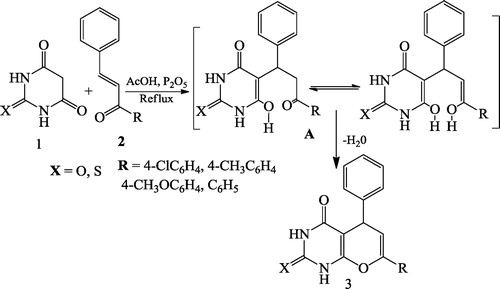
Ghodsi et al. [Citation27] reported a three-component synthesis of pyrano[2,3-d]pyrimidine dione derivatives 4 using a reaction mixture of barbituric acid 1, aldehyde 2, malonitrile 3 and nanoporous silica (SBA-Pr-SO3H) as the catalyst (). Giasuddin et al. [Citation28] further reported a one-step synthesis of pyrano[2,3-d]pyrimidine derivatives 3 using a mixture of barbituric acid or thiobarbituric acid 1 and arylideneacetophenone 2 in the presence of acetic acid/P2O5 ().
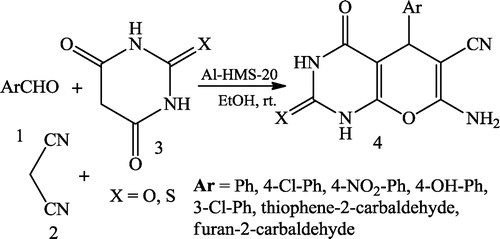
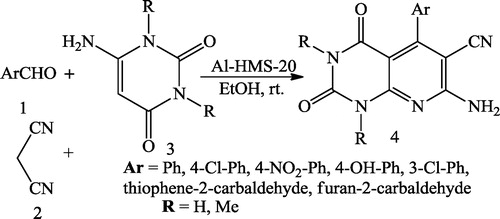
Behrouz et al. [Citation29] reported the Al-HMS-20-catalysed synthesis of pyrano[2,3-d]pyrimidines 4 via a three-component reaction of aromatic benzaldehyde 1 malononitrile 2 and barbituric acid 3 using EtOH as the solvent ().
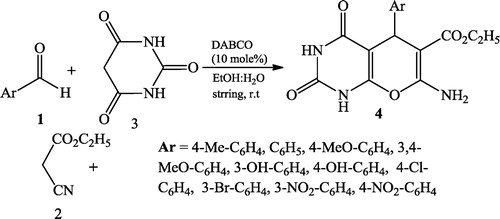
Bhat et al. [Citation30] reported the synthesis of pyrano[2,3-d]pyrimidinones 4 using a multicomponent solution of aromatic aldehydes 1, active methylene compound 2, barbituric acid 3 (2 mmol each) and 10 mol% 1,4-diazabicyclo[2,2,2]octane (DABCO) in 15 mL of an ethanol: water (1:1 ratio) mixture with stirring for 30–40 min at room temperature ().
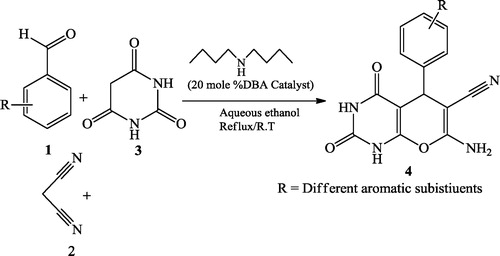
Bhat et al. [Citation31] reported pyrano[2,3-d]pyrimidinones 4 using equimolar amounts of aromatic aldehydes 1, malononitrile 2, barbituric acid 3 and 20 mol % dibutylamine (DBA) organocatalyst in an RB flask with 16 mL of aqueous media (1:1 ratio). The reaction was stirred for 43–129 min at room temperature until the product was formed ().
3.2 Pyrido[2,3-d]pyrimidines
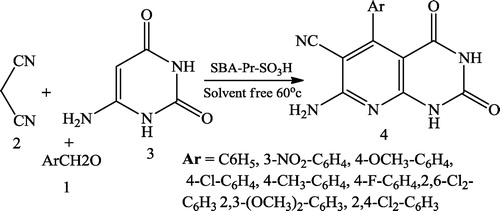
Ghodsi et al. [Citation32] reported the one-pot synthesis of pyrido[2,3-d]pyrimidine derivatives 4 using equimolar amounts of aromatic aldehyde 1, malononitrile 2 and 6-amino uracil 3 in the presence of SBA-Pr-SO3H as a highly active heterogeneous acid catalyst. The resulting mixture was heated under solvent-free conditions at 60 °C ().
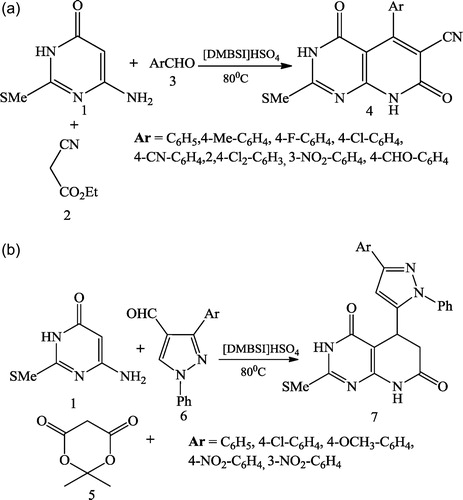
Roghayeh et al. [Citation33] reported the one-pot multicomponent synthesis of pyrido[2,3-d]pyrimidine (4 & 7) derivatives using a Brønsted-acidic ionic liquid as catalyst. A mixture of equimolar amounts of 6-amino-2-(methylthio) pyrimidin-4(3H)-one 1, ethylcyanoacetate 2 or meldrum 5 and aldehyde (3 or 6) was added to a vial containing a magnetic stirring bar with [DMBSI]HSO4 as an ionic liquid and heated to 80 °C ().
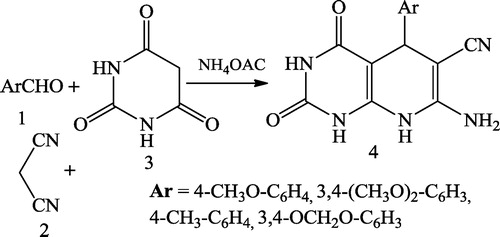
Shallu et al. [Citation34] reported the synthetic studies of some varied structural systems of biologically potent polynitrogen heteropolycyclics using equimolar amounts of aromatic aldehydes 1, malononitrile 2 and barbituric acid 3 along with excess ammonium acetate in methanol as the solvent under reflux in a water bath ().
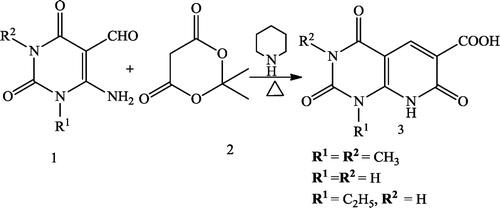
Mohit et al. [Citation35] reported the synthesis of novel classes of pyrido[2,3-d]-pyrimidines 3 using equimolar amounts of 1,3-dimethyl-6-amino-5-formyl uracil 1 and Meldrum’s acid 2 in the presence of piperidine with refluxing in a mixture of methanol and chloroform ().
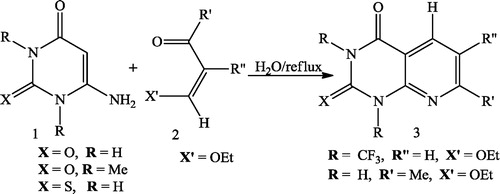
Robabeh et al. [Citation36] reported that a series of pyrido[2,3-d]pyrimidines 3 were easily constructed by cyclocondensation reactions of 6-aminouracils or 6-aminothiouracil 1 with α,β-unsaturated carbonyl compounds (aldehyde, ketone and ester) 2 possessing a leaving group on the β-position and using H2O as the solvent under reflux conditions ().
Shahrzad et al. [Citation37] reported a facile one-pot synthesis of pyrido[2,3-d]pyrimidinones 4 using a mixture of aromatic aldehyde 1, methylcyanoacetate 2, 4(6)-aminouracil 3 and ZrO2 NPs catalyst at 70 °C for 1 h. The organic solution was then poured into cold water (20 mL), filtered and washed with aqueous ethanol to give the pure product (). Similar pyrido[2,3-d]pyrimidine derivatives 5 were reported by Shan et al. [Citation38] in a 100-mL dry flask containing arylaldehyde 1, malononitrile 2, 4-amino-2,6-dihydroxy pyrimidine 3 using KF/Al2O3 as the catalyst and EtOH as the solvent ().
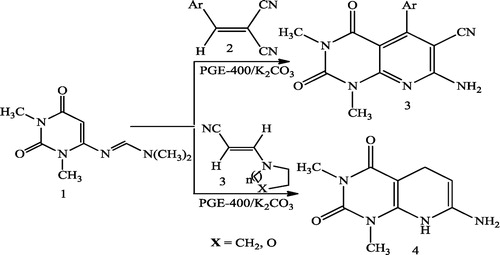
Rupam et al. [Citation39] reported unexpected deviation of uracil amidine from diene behaviour toward the synthesis of some pyrido[2,3-d]pyrimidine derivatives. The products 3 and 4 were obtained by mixing uracil amidine 1 and ylidine malononitrile 2 or 2-amino-substituted ylidine nitrile 3 in 2 mL of PEG-400 and K2CO3 and then stirring at room temperature for an appropriate time ().
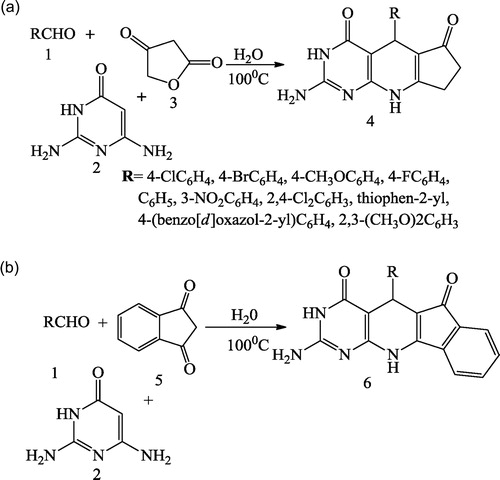
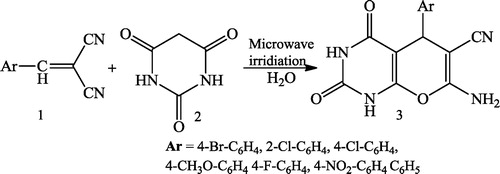
Jiang et al. [Citation40] reported a simple synthesis of pyrido[2,3-d]pyrimidine derivatives (4 & 6) using an aldehyde 1, 2,6-diaminopyrimidine-4(3H)-one 2, and either tetronic acid 3 or indane-1,3-dione 5 in water under microwave irradiation and traditional heating conditions without the use of any catalyst. The synthesis of the compounds was achieved in an aqueous medium ().
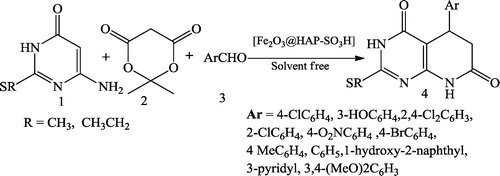
Moona et al. [Citation41] reported the one-pot multicomponent synthesis of novel pyrido[2,3-d]pyrimidines 4 using HAp-encapsulated-γ-Fe2O3-supported sulfonic acid-nanocatalysed condensation of 6-amino-2-(methylthio or ethylthio)pyrimidin-4(3H)-one 1, Meldrum’s acid 2 and aryl aldehydes 3 under solvent-free conditions ().
Behrouz et al. [Citation42] reported the Al-HMS-20-catalysed synthesis of pyrido[2,3-d]pyrimidines 4 via the three-component reaction of aromatic benzaldehyde 1 malononitrile 2 and 6-amino-uracils 3 using EtOH as the solvent ().
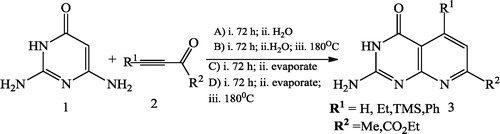
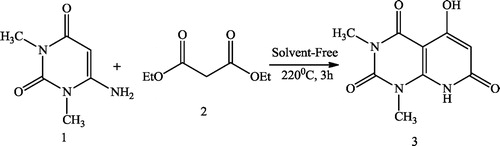
Mark et al. [Citation43] reported a new and highly expedient synthesis of pyrido[2,3-d]pyrimidines 3 using pyrimidinone 1 and alkynone 2 in different solvents (). Somayeh et al. [Citation44] used domino Knoevenagel condensation–Michael addition–cyclization for the synthesis of pyrido[2,3-d]pyrimidine-trione 3 as a new heterocyclic active methylene compound via the reaction of 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione 1 and diethyl malonate 2 at 220 °C under solvent-free conditions ().
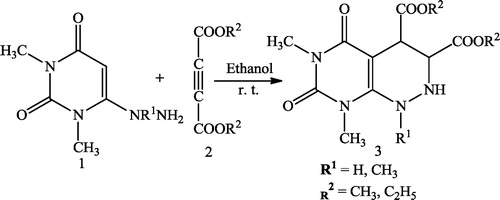
Pulak et al. [Citation45] reported a simple and efficient method for the synthesis of novel pyrimido[4,5-c]pyridazines 3 using 6-hydrazino uracils 1 and acetylenedicarboxylates 2 at room temperature with ethanol as the solvent ().
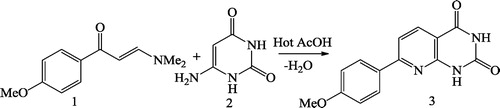
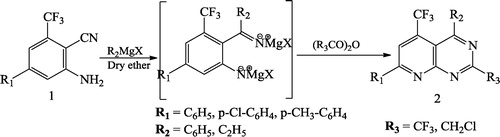
Gwydion et al. [Citation46] reported improved synthesis of substituted pyrido[2,3-d]pyrimidinediones 3 using enaminone 1 and 6-aminouracil 2 (). Ravikanth et al. [Citation47] synthesized novel 5-trifluoromethyl-2,4,7-trisubstituted pyrido[2,3-d]pyrimidines 2 by reacting 2-amino-3-cyano-4-trifluoromethyl-6-substituted pyridines 1 with the Grignard reagent (R2Mgx), followed by the condensation with anhydride/chloroacetylchloride ().
Rahmati and Khalesi [Citation48] reported the one-pot multicomponent synthesis of pyrido[2,3-d]pyrimidines 4 using 3-oxo-3-phenylpropanenitrile 1, aromatic aldehydes 2 and 6-amino-1,3-dimethyl pyrimidine-2,4(1H,3H)-dione 3 in water (5 mL) at 90 °C ().
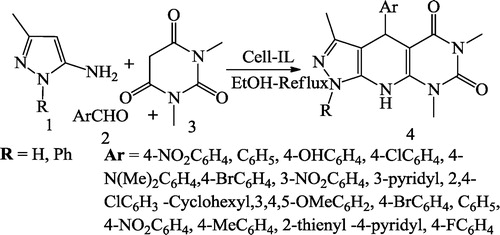
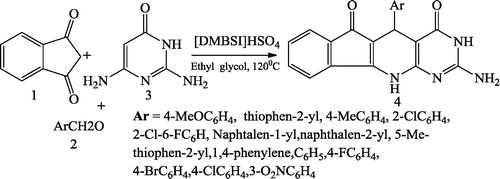
Satasia et al. [Citation49] reported pyrido[2,3-d]pyrimidine-diones 4 using aminopyrazoles 1, aldehydes 2, 1,3-dimethylbarbituric acid 3 and 5 mL of ethanol heated under reflux in the presence of 30 mg of Cell-IL (). Mamaghani et al. [Citation50] reported fused pyrido[2,3-d]pyrimidines 4 obtained using the equimolar mixture of 1,3-indanedione 1, arylaldehyde 2 and 2,6-diamino-4-hydroxy pyrimidine 3 in ethylene glycol containing [DMBSI]HSO4 under reflux at 120 °C ().
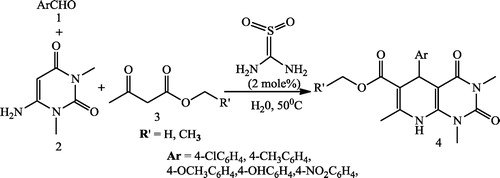
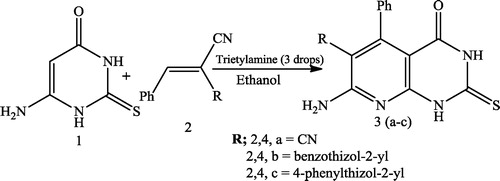
Verma et al. [Citation51] reported dihydropyrido[2,3-d]pyrimidine-2,4-diones 4 using a reaction mixture of aromatic aldehyde 1, 6-amino-1,3-dimethyl uracil 2, and 1,3-dicarbonyl compound 3 in the presence of a catalytic amount of thiourea dioxide in water at 50 °C (). Aziem et al. [Citation52] reported the synthesis of pyrido[2,3-d]pyrimidines (3a-c) using equimolecular amounts of 6-amino-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one 1 and 2-benzylidenemalononitrile (2a), 2-(benzo[d]thiazol-2-yl)3-phenylacrylonitrile (2b) and 3-phenyl-2-(4-phenylthiazol-2-yl) acrylonitrile (2c) in absolute ethanol (10 mL) containing triethylamine (3 drops), which was heated under reflux for 3 h ().
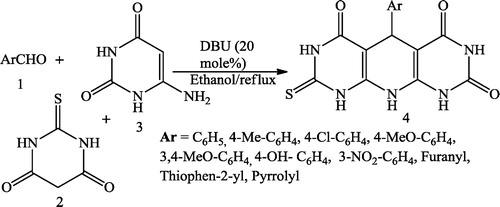
Bhat et al. [Citation53] synthesized pyrido[2,3-d:6,5-d]dipyrimidine derivatives 4 using equimolar mixture of aromatic aldehyde 1, thio-barbituric acid 2, 6-amino-uracil 3 in aqueous ethanol using organocatalyst 1,8-diazabicyclo [5.4.0]undec-7-ene (DBU) ().
4 Microwave-assisted Pyranopyrimidines
Green chemistry has now become a subject of significant research in modern organic synthesis [Citation54]. The concept of “green chemistry” emerged in the early 1990s [Citation55] and is now widely used to meet the fundamental scientific challenges involved in protecting the human health and the environment while simultaneously achieving commercial feasibility [Citation56]. Nonclassical methods following the principles of green chemistry [Citation57] reduce or even eliminate the generation of hazardous substances [Citation58] and increase product yield. Microwave (MW)-assisted organic synthesis is one of the important green chemistry techniques used during the recent years [Citation59]. The ability of MW-assisted organic synthesis to rapidly synthesize organic compounds is of significant benefit for library generation. Moreover, MW-assisted organic synthesis allows for modifications of selectivity (chemo-, regio-, and stereo-selectivity) and the use of solvent, catalyst-free conditions [Citation60]. In the past few decades, the synthesis of heterocyclic compounds has played a major role in medicinal chemistry research, and many significant advances in the practical aspects of organic chemistry have included novel synthetic strategies and methods as well as the advent of a vast array of analytical techniques. Hence, present-day chemists are no longer confined to only using thermal energy for driving chemical reactions. With the increasing complexity of the problems and the availability of newer methods for activation of chemical reactions, chemists have resorted to using microwave techniques for rapid and efficient synthesis of a variety of compounds, owing to the selective absorption of microwave energy by polar molecules. The application of microwave irradiation has been shown to provide enhanced reaction rate and improved product yield in chemical synthesis and also enabled the formation of a variety of carbon-heteroatom bonds.
Microwaves couple directly with the molecules of the entire reaction mixture, leading to a rapid rise in the temperature. Since this process is not limited by the thermal conductivity of the vessel, the result is an instantaneous localized superheating of any substance that will respond to either dipole rotation or ionic conductivity. Only the reaction vessel contents are heated, not the vessel itself; better homogeneity and selective heating of polar molecules may be achieved.
A plethora of very recent synthetic applications describes a variety of new chemistries that can be obtained with microwave irradiation, but a wide range of microwave-assisted applications is still waiting. There have been tremendous successes in the synthesis of numerous heterocyclic compounds and in the development of new processes under clean, environmentally benign methodologies that are sustainable for the long term. Below, we describe studies of microwave-assisted synthesis of N and O-type heterocyclic compounds reported in the literature.
Ipsita et al. [Citation61] reported a novel three-component one-pot synthesis of pyrano [2,3-d] pyrimidines 4 and pyrido[2,3-d]pyrimidines 5 under microwave irradiation at 60% power and 80 °C for 4–5 min in the solid state. The reaction occurs by reacting equimolar amounts of N,N-dimethyl barbituric acid 1, benzaldehyde 2, and malononitrile 3 without using a catalyst ().
Yuan et al. [Citation62] achieved effectives synthesis of 7-amino-6-cyano-5-aryl-5H-pyrano[2,3-d]pyrimidine-2,4(1H,3H)-diones 3 under microwave irradiation. A dry flask (25 mL) was charged with the arylidenemalononitrile 1, barbituric acid 2 and water (3 mL). The flask was then connected with refluxing equipment under microwave irradiation at power 285 W for 3–5 min ().
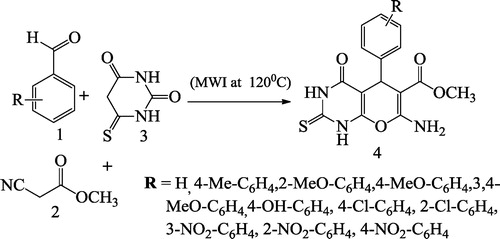
Bhat et al. [Citation63] reported pyrano[2,3-d]pyrimidinone derivatives 4 via a multicomponent solution of substituted aromatic aldehydes 1, methyl cyanoacetate 2 and thio-barbituric acid 3 using water as the solvent under microwave (MW) irradiation at 250 W and 120 °C (). Shahrzad et al. [Citation64] reported an efficient synthesis of pyrido[2,3-d]pyrimidine derivatives 4 via one-pot three-component reaction of aromatic aldehyde 1, malononitrile 2, and 4(6)-aminouracil 3 under microwave irradiation in aqueous media ().
Jiang et al. [Citation65] also reported a simple synthesis of pyrido[2,3-d]pyrimidine derivatives 4 using 3-(4-chlorobenzylidene)furan-2,4(3H,5H)-dione 1, 2,6-diaminopyrimidine-4(3H)-one 2 using water under microwave irradiation at 150 W at 100 °C for a given time (). Mont et al. [Citation66] synthesized functionalized pyrido[2,3-d]pyrimidines 4 via cyclocondensation α,β-unsaturated esters 1, active methylene compounds 2 (malononitrile, or methyl cyanoacetate) and guanidinium carbonate 3 under microwave irradiation at 140 °C ().
Kamlesh et al. [Citation67] synthesized microwave and conventional novel pyrido[2,3-d]pyrimidine derivatives 2 using 6-amino-1-(4-fluorophenyl)-2-oxo-4-phenyl-1,2-dihydro pyridine-3,5 dicarbonitrile derivatives 1 in acetic acid used as a self-solvent. A catalytic amount of conc. sulfuric acid was added to promote the reaction. The reaction mixture was heated in an oil bath at the reflux temperature, and the same reaction mixture was also monitored under microwave irradiation at 180 MW (). Shujiang et al. [Citation68] also reported microwave-assisted one-pot synthesis of dihydropyrido[2,3-d]pyrimidine derivatives 4 using a mixture of 2,6-diaminopyrimidin-4-one 1, aldehyde 2, 1,3-dicarbonyl compound 3 in glycol without a catalyst under microwave irradiation at 300 W power and 198 °C for 4–7 min ().
Kamlesh et al. [Citation69] reported the microwave assisted synthesis of novel pyrido[2,3-d]pyrimidines using dihydropyridines. The reaction mixture was subjected to MW at 180 W power ().
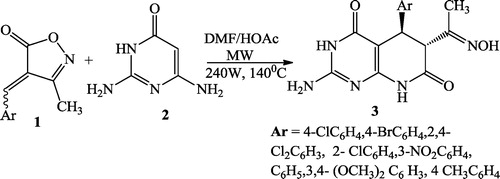
Shujiang et al. [Citation70] synthesized an efficient route of a new class of pyrido[2,3-d]pyrimidine derivatives 3 using a reaction mixture of 4-arylidene-3-methylisoxazol-5(4H)- one 1, 2,6-diamino-pyrimidin-4(3H)-one 2, DMF and HOAc at 240 W power and 140 °C temperature ().
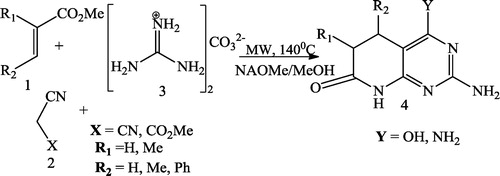
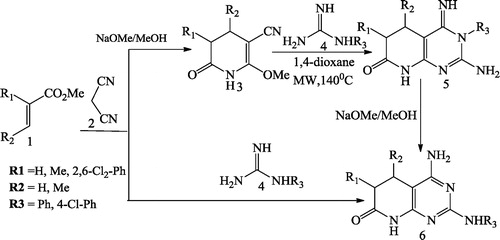
Galve et al. [Citation71] reported the synthesis of 2-arylamino substituted 4-amino-5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-ones 6 from treatment of pyridones 3 (synthesized from α,β-unsaturated esters 1 and malononitrile 2) with the aryl guanidines 4 to form 3-aryl substituted pyridopyrimidines 5 undergoing the Dimroth rearrangement by NaOMe/MeOH. The overall yields of such a 3-step protocol are higher than those of the multicomponent reaction between α,β-unsaturated ester 1, malononitrile 2, and aryl guanidine 4 ().
5 Ultrasound-irradiated Pyridopyrimidines
Ultrasound is extensively used in organic chemistry, offering a versatile and facile pathway for a large variety of heterocyclic syntheses. The study of ultrasound is concerned with understanding the effect of sound waves and wave properties on chemical systems. Ultrasound frequencies range from approximately 20 kHz to 10 MHz and can be roughly subdivided into three main regions: low-frequency high-power ultrasound (20–100 kHz), high-frequency medium-power ultrasound (100 kHz–1 MHz), and high-frequency low-power ultrasound (1– 10 MHz). It is transmitted through any substance such as solid, liquid or gas that possesses elastic properties. The movement of the sound source is transmitted to the particles of the medium, which oscillate in the direction of the wave and produce longitudinal as well as transverse waves. As the molecules of the medium vibrate, the average distance between the molecules decreases in the compression cycle and increases during the expansion. When the average distance between the molecules exceeds the critical molecular distance necessary to hold the liquid intact, the liquid breaks down; cavities (cavitation)' and bubbles are formed. This process, known as cavitation, refers to the formation and the subsequent dynamic life of bubbles in liquids. Therefore, cavitation is the formation, growth, and implosive collapse of bubbles irradiated with sound, which is the impetus for sonochemistry. The bubbles can be filled with gas or vapour and occur in water, organic solvents, biological fluids, liquid helium, molten metals or other fluids. Bubble collapse results in high temperature (as much as 4700 °C) and pressure changes (10 Pa). The solvent/reagent vapour suffers fragmentation generating reactive species such as free radicals or carbenes. These high-energy species are concentrated at the interface and lead to intermolecular reactions. However, if there are non-volatile solutes, these would also collect at the interface and react with the high-energy species. Furthermore, the shock wave produced by a bubble collapse can influence the reactivity by altering the solvation of the reactive species.
A number of common reactions used in synthetic heterocyclic chemistry have been performed more efficiently using ultrasound techniques. The use of ultrasound has several advantages. Generally, the yield increases, and the percentage of by-products decreases. Reactions occur faster, so lower temperatures can be used. Ultrasound provides alternative pathways for reactions, owing to the formation of high-energy intermediates. Thus, ultrasound is an alternative method for rapid and facile synthesis of a variety of heterocycles and fused heterocycles. Ultrasonic-assisted organic synthesis (UAOS) is a powerful and green approach that is increasingly being used to accelerate the synthesis of organic compounds. Furthermore, it has significant applications in various fields such as chemical synthesis, pharmaceutical industry, food and polymer industry, electroplating, and decontamination. Compared to the conventional methods, this method is more convenient showing high yields, short reaction times, mild conditions and easily control [Citation72]. Below, several specific applications of ultrasound-assisted synthesis are described.
Anshu et al. [Citation73] used cerric ammonium nitrate (CAN) as an efficient catalyst for the synthesis of pyrano[2,3-d]pyrimidine-2,4,7-triones 4 in an aqueous medium under sonication via a one-pot three-component reaction of benzaldehyde 1, barbituric acid 2 and Meldrum’s acid 3 at 50% power of the processor and in a 4 s pulse mode until the separation of the solid product. Completion of the reaction was monitored by TLC using n-hexane: ethyl acetate (7:3) as an eluant. All reactions were invariably completed in 25–40 min ().
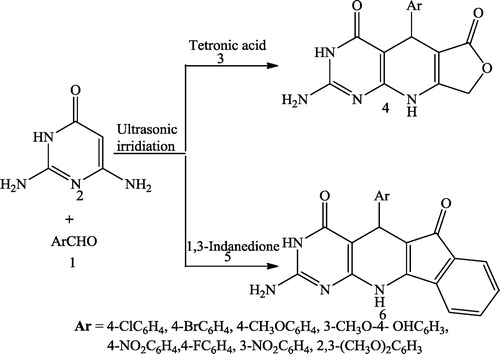
Shujiang et al. [Citation74] reported a series of pyrido[2,3-d]pyrimidine derivatives (4 & 6) and related compounds via the condensation reaction of aromatic aldehyde 1, 2,6-diaminopyrimidine-4(3H)-one 2 and either tetronic acid 3 or 1,3-indanedione 5 under ultrasonic irradiation in an open vessel at 65 °C until the disappearance of the starting material for 20–45 min without a catalyst. This protocol has the advantages of higher yields, lower cost and more convenient procedure ().
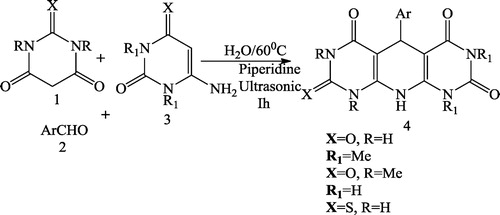
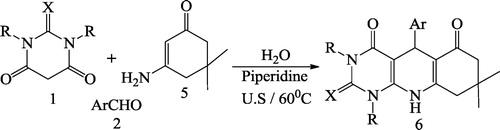
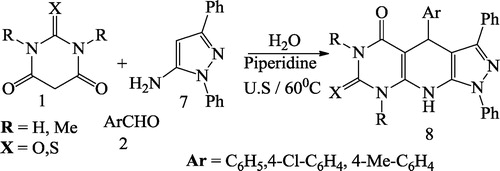
Mosslemin et al. [Citation75] reported a one-pot, three-component condensation reaction of barbituric acid 1, aromatic aldehyde 2, and enamine 3 in the presence of piperidine and water under ultrasonic irradiation at 60 °C to give the desired pyridodipyrimidines 4 in good yields (). The same authors also synthesized pyridopyrimidines 6 with the same effectiveness under ultrasonic irradiation at 60 °C by reacting barbituric acid 1, aromatic aldehyde 2 and aminocyclohex-2-enone 5 (). The reaction was further carried out using barbituric acid 1, aromatic aldehyde 2 and pyrazole 7 to give pyrazolo pyridopyrimidines 8 under ultrasonic irradiation in good yields ()
6 Conclusion
In conclusion, this review summarizes and highlights the recent major advances for the synthesis of annulated pyrimidine derivatives such as pyrano/pyridopyrimidines via a straightforward domino Knoevenagel–Michael addition procedure. The one-pot syntheses of targeted annulated pyrimidine utilize multicomponent solutions of active methylene compounds and aromatic aldehydes with or without catalyst and solvents under various conditions. The reports in the literature also showed the synthesis of targeted annulated pyrimidines using both conventional and non-conventional techniques. The non-conventional (microwave and ultrasound irradiations) pathways showed advantages such as an environmentally friendly and safe procedure, spectacular accelerations, higher yields under milder reaction conditions, short reaction times and simple work up relative to the conventional techniques.
Acknowledgements
The authors are thankful to Prof. J. S. Meshram and Prof. H. D. Juneja Head, Department of Chemistry, R.T.M. Nagpur University, Nagpur, India-44003.
Notes
Peer review under responsibility of Taibah University.
References
- A.R.BhatR.S.DongreR.S.SelokarPotent in vitro antibacterial and antifungal activities of pyrano[2,3-d]pyrimidine derivatives with quantitative yieldInt. J. Pharma and Bio. Sci.52014422430
- N.KitamuraA.OhnishiPyrimidopyrimidinedione derivatives and their use antiallergic agentEur. Pat. 163599; Chem. Abstr..1041984186439
- E.M.GrivskyS.C.LeeW.SigelD.S.DuchC.A.NicholSynthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidineJ. Med. Chem.231980327329 G.L.AndersonJ.L.ShimA.D.Broompyrido[2,3-d]pyrimidines. IV. Synthetic studies leading to various oxopyrido[2,3-d]pyrimidinesJ. Org. Chem.41197610951099
- A.RosowskyC.E.MotaS.F.QueenerSynthesis and Antifolate Activity of 2,4-Diamino-5,6,7,8-tetrahydro- pyrido[4,3-d]pyrimidine Analogues of Trimetrexate and PiritreximJ. Heterocyclic Chem.321995335340
- A.M.ThompsonA.J.BridgesD.W.FryA.J.KrakerW.A.DennyTyrosine Kinase Inhibitors.7.7-amino-4-(phenylamino)-and7-amino-4-[(phenylmethyl)amino] pyrido [4,3-d]pyrimidines: A New Class of Inhibitors of the Tyrosine Kinase Activity of the Epidermal Growth Factor ReceptorJ. Med. Chem.38199537803788
- I.O.DonkorC.L.KleinL.LiangN.ZhuE.BradleyA.M.ClarkSynthesis and Antimicrobial Activity of Some 6,7-Annulated Pyrido[2,3-d]pyrimidineJ. Pharmaceutical Sci.841995 661–644
- A.PastorR.AlajarinJ.J.VaqueroJ.Alvarez-BuillaM.F.Casa-JuanaC.SunkelJ.G.PriegoI.FonsecaJ.S.AparicioSynthesis and Structure of New Pyrido[2,3-d]pyrimidine Derivatives with Calcium Channel Antagonist ActivityTetrahedron50199480858098
- V.E.KollaA.B.DeyanovF.Y.NazmetdinovZ.N.KashinaL.P.DrovosekovaInvestigation of the Anti-Inflammatory and Analgesic Activity of 2-Substituted 1-Aryl-6-carboxy-(carbethoxy)-7-methyl-4-oxo-1,4- dihydropyrido[2,3-d]pyrimidineJ. Pharm. Chem.2719939
- J.W.EllingboeN.J.PrincetonSubstituted Pyridopyrimidines and AntihypertensivesChem. Abstr.1996124 Article ID: 176134q S.FurujaT.OhtakiPyridopyrimidine derivatives, their production and useChem. Abstr.1211994205395
- A.AgarwalR.AshutoshN.GoyalP.M.S.ChauhanS.GuptaDihydropyrido [2,3-d]pyrimidines as a New Class of Antileishmanial AgentsJ. Bio-organic & Med. Chem.1324200566786684
- I.D.BystryakovaO.A.BurovaG.M.ChelyshevaS.V.ZhilinkovaN.M.SmirnovaT.S.SafonovaSynthesis and Biological Activity of Pyrido[2,3-d]pyrimidineJ. Pharmaceutical Chem.25121991874876
- A.B.DeyanovR.K.NiyazovF.Y.NazmetdivovB.Y.SyropyatovV.E.KollaM.E.KonshinSynthesis and Biological Activity of Amides and Nitriles of 2- Arylamino-5-carboxy(carbethoxy)-6-methylnicotinic ac-ids and 1-aryl-6-carbethoxy-7-methyl-4-oxo-1,4-dihydro- pyrido[2,3-d]pyrimidineJ. Pharmaceutical Chem.251991248250
- A.MongeV.Martinez-MerinoC.SanmartinF.J.FernandezM.C.OchoaC.BerllverP.ArtigasE.F-Alvarez2-Arylamino-4-oxo-3,4-dihydropyrido-[2,3-d]pyrimidines: Synthesis and Diuretic ActivityEur. J. Med. Chem.24198924
- H.SaladowskaA.Bartoszko-MalikT.ZawiszaSynthesis and Properties of New Derivatives of Ethyl 7-Methyl-2,4-dioxo-1,2,3,4-tetrahydropyrido [2,3-d]pyrimidine-5-carboxylateFarmaco451990101110
- A.A.FayedH.M.HosniE.M.FlefelA.E.AmrSynthesis and pharmacological activities of some new thieno [2,3-d] pyrimidine and pyrimidino pyrazolo thieno pyrimidine derivativesW. J. Chem.420095865
- R.DahmHuman Genetics1222008565581 J.D.WatsonF.H.CrickNature1711953737738
- M.B.DaviesJ.AustinD.A.PartridgeVitamin C: Its chemistry and biochemistryR.S.C. Cambridge19913047
- H.M.EvansO.H.EmersonG.A.EmersonThe isolation from wheat germ oil of an alcohol, α-tocopherol, having the properties of vitamin E, EJ. Biological Chem.1131936319332
- R.V.A. Orru, M. De Greef, Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds, Synthesis 10 (200) 1471.A.DomlingI.UgiMulticomponent Reactions with IsocyanidesAngew. Chem.39200031683210
- L.WeberMulti-component reactions and evolutionary chemistryDrug Discovery Today72002143147 R.W.ArmstrongA.P.CombaP.A.TempstS.BrownT.A.KeatingMultiple-component condensation strategies for combinatorial library synthesisAcc. Chem.291996123131
- G.H.PosnerMulticomponent one-pot annulations forming 3 to 6 bondsChem. Rev.861986831844
- L.WeberK.IllegelM.AlmstetterDiscovery of new multi component reactions with combinatorial methodsSynlett1999366377 A.DomlingRecent advances in isocyanide-based multi component chemistryCurr. Opin. Chem. Biol.62002306313
- M.BararjanianS.BalalaieB.MovassaghA.M.AmaniOne-Pot Synthesis of Pyrano[2,3-d]pyrimidinone Derivatives Catalyzed by L-Proline in Aqueous MediaJ. Iran. Chem. Soc.62009436442
- M.MajidA.G.HeraviB.KhadijehD.FatemehZn[(L)proline]2: An Efficient Catalyst for the Synthesis of Biologically Active Pyrano[2,3-d]pyrimidine DerivativesSynth. Communs.40201019271931
- M.AkbarF.NaserA.B.F.MohammadEcofriendly and Efficient Synthesis of Pyrano[2,3-d] pyrimidinone and Tetrahydrobenzo[b]pyran Derivatives in WaterSynth. & React. Inorg. Metal-Org & Nano-Metal Chem.402010179185
- M.SaraM.R.Naimi-JamalMechanochemical Solvent-Free and Catalyst-Free One-Pot Synthesis of Pyrano[2,3-d]Pyrimidine-2,4(1H,3H)-Diones with Quantitative YieldsMolecules142009474479
- M.Z.GhodsiF.SakinehA.ShimaB.AlirezaB.RoyaA.MassoudThree-component synthesis of pyrano[2,3-d]pyrimidine dione derivatives facilitated by sulfonic acid nanoporous silica (SBA-Pr-SO3H) and their docking and urease inhibitory activityDARU J. Pharmaceu. Sci.320133
- M.A.GiasuddinU.K.R.RommanA.KawsariM.E.HalimM.M.RahmanS.M.AhmadA one-step synthesis of 5,7-diaryl-1,5-dihydro (or 1,2,3,5-tetrahydro)-pyrano [2,3-d]pyrimidin-2,4-diones (or 2-thioxo-4-ones)Indian J. Chem.2011946948
- S.BehrouzH.P.MohammahH.MahdiAl-HMS-20 catalyzed synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines via three-component reactionChem. Intermed.201310.1007/s11164-013-1277
- A.R.BhatA.H.ShallaR.S.DongreSynthesis of new annulated pyrano[2,3-d]pyrimidine derivatives using organo catalyst (DABCO) in aqueous mediaJ. Saudi Chem. Soc.201410.1016/j.jscs.2014.03.008
- A.R.BhatA.H.ShallaR.S.DongreDibutylamine (DBA): A highly efficient catalyst for the synthesis of pyrano[2,3-d]pyrimidine derivatives in aqueous mediaJ. Taibah Univ. for Sci.201510.1016/j.jtusci.2015.03.004
- M.Z.GhodsiH.N.NargesR.MahshidA.S.AliOne-pot synthesis of pyrido[2,3-d]pyrimidine derivatives using sulfonic acid functionalized SBA-15 and the study on their antimicrobial activitiesJ. Saudi Chem. Soci.201410.1016/j.jscs.2014.06.007
- H.N.RoghayehM.ManouchehrT.KhalilS.FarhadR.MehdiA Rapid One-pot Synthesis of Pyrido[2,3-d]pyrimidine Derivatives Using Brønsted-acidic Ionic Liquid as CatalystActa Chim. Slov.602013889895
- G.ShalluG.PoonamS.AnandL.S.RattanSynthetic studies of some varied structural systems of biologically potent polynitrogen heteropolycyclicsIndian J. Chem.49201012431256
- L.D.MohitJ.B.PulakSynthesis of Novel Classes of Pyrido[2,3-d]-pyrimidines, Pyrano[2, 3-d]pyrimidines and PteridinesSynth. Communs.36200630853090
- B.RobabehA.RaziehA clean and efficient cyclocondensation to pyrido[2,3-d]pyrimidine derivatives in aqueous mediaChinese Chem. Letts.22201111831186
- A.ShahrzadA.MaryamFacile one-pot synthesis of pyrido[2,3-d]pyrimidine derivatives over ZrO2 nanoparticles catalystChinese Chem. Letts.232012257260
- W.X-ShanZ.Z-SenS.D-QingW.X-YongZ.Z-MinKF-Alumina Catalyzed One-Pot Synthesis of Pyrido[2,3-d]Pyrimidine DerivativesSynth. Communs.34200443314338
- S.RupamJ.B.KalyanD.YuvarajP.DipakUnexpected deviation from diene behaviour of uracil amidine: towards synthesis of some pyrido[2,3-d]pyrimidine derivativesMol. Divers152011697705
- T.Shu-JiangY.ZhangH.JiangB.JiangZ.Jun-YongJ.Run-HongF.ShiA Simple Synthesis of Furo[3,4:5,6]pyrido[2,3-d]pyrimidine Derivatives through Multicomponent Reactions in WaterEur. J. Org. Chem.200715221528
- M.MoonaM.ManouchehrS.FarhadS.MehdiA.M.FatemehOne-pot synthesis of novel pyrido[2,3-d]pyrimidines using HAp-encapsulated-γ-Fe2O3 supported sulfonic acid nanocatalyst under solvent-free conditionsChinese Chem. Letts.24201413871391
- S.BehrouzH.P.MohammahH.MahdiAl-HMS-20 catalyzed synthesis of pyrano [2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines via three-component reactionRes. Chem. Intermed.201310.1007/s11164-013-1277
- C.MarkD.D.BagleyR.L.HughesE.C.VickiA new and highly expedient synthesis of pyrido[2,3-d]pyrimidinesTetrahedron Letts.42200165856588
- A.SomayehK.TelmaA.MahsaR.K.HamidB.AyoobDomino Knoevenagel condensation–Michael addition–cyclization for the diastereoselective synthesis of dihydrofuropyrido[2,3-d]pyrimidines via pyridinium ylides in waterR.S.C. Adv.4201472967300
- P.J.BhuyanK.C.LekhokJ.S.SandhuStudies on Uracils: A Simple and Efficient Method for the Synthesis of Novel Pyrimido[4,5-c]pyridazinesTetrahedron Letts.40199917931794
- G.H.ChurchillS.A.RawL.PowellImproved synthesis of substituted pyrido[2,3-d]pyrimidinedionesTetrahedron Letts.52201136573661
- S.RavikanthG.V.ReddyD.MaitraieV.V.V.N.S.Rama RaoP.S.RaoB.NarsaiahSynthesis of Novel 5-Trifluoromethyl-2,4,7- Trisubstituted Pyrido[2,3-d]PyrimidinesSynth. Communs.34200444634469
- A.RahmatiZ.KhalesiCatalyst free synthesis of fused pyrido [2,3- d] pyrimidines and pyrazolo [3,4- b] pyridines in waterChinese Chem. Lett.23201211491152
- S.P.SatasiaP.N.KalariaD.K.RavalCatalytic regioselective synthesis of pyrazole based pyrido[2,3-d]pyrimidine-diones and their biological evaluationOrg. Biomol. Chem.12201417511758
- M.MamaghaniF.ShiriniE.BasserehR.H.Nia1,2-Dimethyl-N-butanesulfonic acid imidazolium hydrogen sulfate as Efficient Ionic Liquid Catalyst in the Synthesis of Indeno Fused Pyrido[2,3-d]pyrimidinesJ. of Saudi Chem. Soc.201410.1016/j.jscs.2014.12.003
- S.VermaS.L.JainThiourea dioxide in water as a recyclable catalyst for the synthesis of structurally diverse dihydropyrido [2,3-d] pyrimidine-2, 4-dionesTetrahedron Lett.53201225952600
- A.A.AziemM.S.El-GendyA.O.AbdelhamidSynthesis and antimicrobial activities of pyrido[2,3-d]pyrimidine, pyridotriazolopyrimidine, triazolopyrimidine, and pyrido[2,3-d:6,5-d']dipyrimidine derivativesEur. J. Chem.32012455460
- A.R.BhatR.S.DongreOne-pot synthesis of annulated pyrido[2,3-d:6,5d]dipyrimidine derivatives using nitrogen based DBU catalyst in aqueous ethanol mediumJ. Taiwan Inst. Chem. Eng.201510.1016/j.jtice.2015.04.020
- V.SantagadaF.FrecenteseE.PerissuttiF.FiorinoB.SeverinoG.CaliendoThe application of microwave irradiation as new convenient synthetic procedure in drug discoveryMini Rev. Med. Chem.92009340358 M.PhukanK.J.BorahR.BorahHenry reaction in environmentally benign methods using imidazole as catalystGreen Chem. Lett. Rev.22009249253
- P.T.AnastasaE.S.BeachaGreen chemistry: the emergence of a transformative frameworkGreen Chem. Lett. Rev.12007924
- A.R.KatritzkyC.W.ReesThe structure, reactions, synthesis, and uses of heterocyclic compounds1984Pergamon PressOxford18 V.PolshettiwarR.S.VarmaMicrowave assisted organic synthesis and transformations using Benign Reaction MediaAcc. Chem. Res.412008629639
- V.SantagadaE.PerissuttiG.CaliendoThe application of microwave irradiation as new convenient synthetic procedures in drug discoveryCurr. Med. Chem.9200212511283
- J.L.TuckerGreen chemistry, a pharmaceuticalOrg. Process Res. Dev.102006315319
- A.DastanA.KulkarniB.TorokEnvironmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approachesGreen Chem.1420121737
- A.De-HozA.Díaz-OrtizA.MorenoA.S-MigallónP.PrietoJ.R.CarrilloE.VázquezM.V.GómezM.A.HerreroMicrowave-assisted reactions in heterocyclic compounds with applications in medicinal and supramolecular chemistryComb. Chem. High Throughput Screening102007877902
- D.IpsitaB.S.D.KumarJ.B.PulakA novel three-component one-pot synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines using microwave heating in the solid stateTetrahedron Lett.44200383078310
- G.YuanT.ShujangL.TuanjieZ.XiaojingZ.SongleiF.FangS.DaqingEffective Synthesis of 7-Amino-6-cyano-5-aryl-5H-pyrano[2,3-d]pyrimidine-2,4(1H,3H)-diones Under Microwave IrradiationSynth. Commun.34200412951299
- A.R.BhatA.H.ShallaR.S.DongreMicrowave assisted one-pot catalyst free green synthesis of new methyl-7-amino-4-oxo-5-phenyl-2-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carboxylates as potent in vitro antibacterial and antifungal activityJ. Adv. Res.201410.1016/j.jare.2014.10.007
- A.ShahrzadB.SaeedIntern. An Efficient Synthesis of Pyrido[2,3-d]pyrimidine Derivatives via One-Pot Three-Component Reaction in Aqueous MediaJ. Org. Chem.22012714
- T.Shu-JiangY.ZhangH.JiangB.JiangZ.Jun-YongJ.Run-HongF.ShiA Simple Synthesis of Furo[3,4:5,6]pyrido[2,3-d]pyrimidine Derivatives through Multicomponent Reactions in WaterEur. J. Org. Chem.200715221528
- N.MontJ.TeixidJ.I.BorrellC.O.KappeA three-component synthesis of pyrido[2,3-d]pyrimidinesTetrahedron Lett.44200353855387 N.MontJ.TeixidC.O.KappeJ.I.BorrellA one-pot microwave assisted synthesis of pyrido[2,3-d]pyrimidinesMol. Divers72003153159
- M.K.KamleshM.G.RavindraT.K.TaslimahemadK.P.PrafulMicrowave and conventional synthesis of novel pyrido[2,3-d]pyrimidine scaffold as an antimicrobial agentChem. & Bio. Interface42014119130
- T.ShujiangZ.JunyongX.ZouF.FangL.TuanjieMicrowave-assisted one-pot synthesis of 2-amino-6,7-disubstituted-5-methyl-5,8-dihydropyrido[2,3-d]pyrimidin-4(3H)-one without catalystArkivoc20057681
- K.KamleshK.TaslimR.VijayP.PrafulMicrowave assisted synthesis and biological investigations of novel derivatives of pyrido[2,3-d]pyrimidinesChemistry & Biology Interface32013192200
- T.ShujiangZ.JunyongJ.RunhongB.JiangY.Z.HongAn efficient route for the synthesis of a new class of pyrido[2,3-d]pyrimidine derivativesJ. Org. Biomol. Chem.520071451453
- I.GalveR.P.D-BellacasaD.Anchez-GarcX.BatlloriJ.T.BorrellSynthesis of 2-arylamino substituted 5,6-dihydropyrido[2,3-d]pyrimidine-7(8H)-ones from arylguanidinesJ. Mol. Divers162012639649
- H.M.MohammadR.N.MohammadRapid and efficient synthesis of fused heterocyclic pyrimidines under ultrasonic irradiationUltrason. Soochem.172010162167 K.JadidiR.GharemanzadehM.MehrdadH.R.DarabiH.R.KhavasiD.AsgariA facile synthesis of novel pyrrolizidinee under classical and ultrasonic conditionsUltrason. Sonochem.152008124128
- D.AnshuL.ShyamS.B.GuptaCerric ammonium nitrate (CAN) catalysed synthesis of pyrano[2,3-d]pyrimidine-2,4,7-Triones in aqueous medium under sonication via one-pot three component reactionEur. Chem. Bull.22013836841
- T.ShujiangC.LongjiZ.YanS.QingqingZ.DianxiangL.ChunmeiAn efficient synthesis of pyrido[2,3-d]pyrimidine derivatives and related compounds under ultrasound irradiation without catalystUltrason. Sonochem.152008217221
- M.H.MossleminM.R.NateghiRapid and efficient synthesis of fused heterocyclic pyrimidines under ultrasonic irradiationUltra Sonochem.172010162167