Abstract
A hydroponic experiment was carried out under controlled conditions to investigate the impact of different levels of nickel (Ni) in the nutrient solution on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings, including membrane lipid peroxidation (LPO), proline accumulation and superoxide dismutase (SOD). The Ni levels investigated were 0, 25 and 50 μg per litre. A statistically significant increase in LPO was recorded in the seedlings grown at concentrations of 25 or 50 μg Ni per litre, but SOD (1.15.1.11) activity was not significantly affected. Plant height and chlorophyll content were affected significantly in a dose dependent manner. Proline content increased considerably in response to Ni concentration. The results indicate that the increase in the activities of the antioxidant enzymes were not sufficient to protect cell membrane against Ni toxicity.
Abbreviations:
Keywords:
1 Introduction
Nickel (Ni), a heavy metal, is an essential micronutrient for plant growth and development [Citation1]. However, it becomes toxic at high concentrations. Excess Ni destroys photosynthesis and membrane functions, inhibits seed germination, plant growth and development, and markedly decreases the yields of plants [2–5Citation[2]Citation[3]Citation[4]Citation[5]]. Soil and water contamination by heavy metals was originally restricted to metalliferous soils but has become a general problem caused by anthropogenic sources including smelting of ore, electroplating and municipal sludge [Citation6,Citation7]. Heavy metals tend to bind sulphydryl groups of enzymes and hence suppress the functioning of essential biological components [Citation8]. They can also lead to ionic imbalances as a result of altered translocation of ions such as Fe2+, Mn2+, Cu2+, Zn2+ and/or metal substitution.
In the last decades, there has been increasing demand for assessing the ecological risks of soil contamination, using phyto-toxicity tests as important tools for risk assessment and environmental monitoring of heavy metal-polluted soils [Citation9]. Phytotoxicity tests generally use toxicological endpoints such as seedling growth and biomass production [Citation10]. There is increasing interest in using other parameters based on physiological and biochemical biomarkers, such as photosynthesis, chlorophyll fluorescence and enzymatic activities in plant tissues [10–12Citation[10]Citation[11]Citation[12]].
Phytotoxicity from heavy metals is closely related to the production of reactive oxygen species (ROS) in plants [Citation13]. It has been demonstrated that excess Ni and cadmium lead to a significant increases in the content of hydrogen peroxide (H2O2) [Citation2] and the membrane lipid peroxidation in a few plant species [Citation14].
In 1996, the US Environmental Protection Agency recommended several plants that have an economic and ecological importance as biomarkers for toxicity assessment in terrestrial and aquatic ecosystems. So in this study we investigated the effects of Ni toxicity on growth parameters, membrane LPO, proline accumulations and some antioxidative enzyme activities (SOD) in tissues of wheat seedlings.
2 Materials and methods
2.1 Plant growth, sample analysis and metal estimation
Seeds of the wheat cultivar Gerek used in this study were obtained from the Central Agricultural Research Institute, Ankara (Turkey). The seeds were surface-sterilized with 10% sodium hypochlorite solution for 10 min, washed and imbibed in distilled water for 1 day. Ten to 15 imbibed seeds were then planted onto plastic cups covered with cheesecloth and containing Hoagland's nutrient solution. The cups were kept for 7 days in a growth chamber at 25 °C and an 8 h dark 16 h light cycle, using a light intensity of 40 mmol m−2 s−1. The treatment was started on the 8th day and continued for three days, by replacing the original solution by Hoagland's solutions containing 25 or 50 μg Ni per litre, using Ni(NO3)2. These Ni concentrations had been earlier established in preliminary experiments. The nutrient solutions were changed every 24 h. After 15 days, the leaves were harvested and either used directly for analysis or frozen in liquid nitrogen followed by storage at −20 °C until further use.
Dried wheat samples were digested with 10 mL of concentrated HNO3, using a CEM microwave digestion system. After digestion, the volume of each sample was adjusted to 25 mL using double deionized water [Citation15]. Determinations of the Ni concentrations in all samples were carried out using Inductively Coupled Plasma Optical Emission Spectrometry (Varian). The samples were analysed in triplicate.
2.2 Chlorophyll
The amount of chlorophyll in the leaves was determined according to the method described by Knudson et al. [Citation16].
2.3 Lipid peroxidation
Lipid peroxidation (LPO) was determined using the method of Teresa et al. [Citation17] by measuring the amount of TBARS. The leaf tissues (approximately 0.3 g) of control and treated plants were homogenized in 3 mL of 5% trichloroacetic acid (TCA) solution using a cold mortar and pestle. The homogenates were then transferred to fresh tubes and centrifuged at 12,000 × g for 15 min at room temperature. Four mL of 0.5% thiobarbituric acid (TBA) in 20% TCA solution (freshly prepared) were then added to 1 mL of the supernatant and incubated at 96 °C for 30 min. The tubes were cooled by transferring them to an ice bath and then centrifuged at 12,000 × g for 10 min. The absorbance of the supernatant was recorded at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. TBARS content was determined by using its extinction coefficient, i.e., 155 mM−1 cm−1.
2.4 Proline
The proline content was determined according to the method described by Bates et al. [Citation18]. The leaf tissues (about 0.3 g) of control and treated plants were homogenized by adding 5 mL of 3% sulphosalicylic acid solution. The leaves were homogenized using a cold pestle and mortar. The homogenates were centrifuged at 5000 × g for 10 min at 4 °C. For each sample, a glass tube containing 2 mL of acid ninhydrin (0.31 g ninhydrin, 7.5 mL of acetic acid, and 5 mL of 6 M phosphoric acid), 2 mL of 96% acetic acid and 1 mL of 3% sulphosalicylic acid was prepared and supernatant (2 mL) from each homogenate was added to the tubes. The tubes were incubated at 96 °C for 1 h in a hot water bath and after incubation 4 mL of toluene was added to each tube followed by mixing. The absorbance of the pink red upper phase was recorded at 520 nm against a toluene blank. A standard curve for proline in the range of 0.01 μM to 1.5 mM was constructed to determine the proline content of each sample.
2.5 Enzyme and protein
Fresh leaf tissue (1 g) was homogenized in liquid nitrogen using a pestle and mortar and suspended in 3.0 mL of 0.1 M Tris–HCl buffer (pH 7.5) containing 0.5 mM ethylenediaminetetraacetic acid (EDTA) and 1.0% polyvinylpyrrolidone (PVP). The homogenates were centrifuged at 15,000 × g for 20 min. The supernatant was used as an enzyme source in all enzyme analysis. The protein content in the leaf extracts was determined according to the Bradford method using bovine serum albumin as a standard [Citation19].
2.6 Superoxide dismutase (1.15.1.11)
To determine superoxide dismutase (SOD) activity, the assay buffer consisted of 20 mM sodium phosphate (pH 7.5), 0.1 mM EDTA, 10 mM methionin, 0.1 mM p-nitro blue tetrazolium chloride (NBT), 0.005 mM riboflavin and the enzyme extract containing 50 mg protein in a final volume of 3 mL. SOD activity was determined by the inhibition of NBT photo-reduction. A fluorescent lamp was positioned at a distance of 20 cm from the samples for 5 min. One unit of SOD was defined as the amount of enzyme that inhibits NBT photo-reduction by 50% when monitored at 560 nm. A standard curve for SOD in the range of 20–200 ng mL−1 was constructed to determine the SOD content in each sample [Citation20].
2.7 Statistical analysis
Statistical significance of the difference between mean values obtained from at least three independent analyses was determined by one-way analysis of variance (ANOVA) at 95% confidence interval using SPSS 10.0 for Windows [Citation21].
3 Results
3.1 Plant growth Ni accumulation
Increasing the Ni concentration in the nutrient solution affected plant height considerably (). Mean seeding height at 50 μg Ni L−1 was 15.5 cm against 24 cm in the control (no Ni) (). In this study, the seedlings grown on 50 μg Ni L−1 were most affected, but the ones at 25 μg Ni L−1 were also significantly different from the control (p < 0.05).
The mean values of Ni in the seedling tissues are given in . The variation in Ni contents was large: 1.12–17.7 μg kg−1 for roots and 0.3–12.7 μg kg−1 for leaves. The accumulation of Ni in roots was higher than in leaves.
Table 1 Nickel contents (μg g−1 DW) in roots and leaves of wheat (T. aestivum) seedlings grown at different Ni concentrations of the nutrient solution. Means in the same row, followed by a different letter are statistically different (p < 0.05).
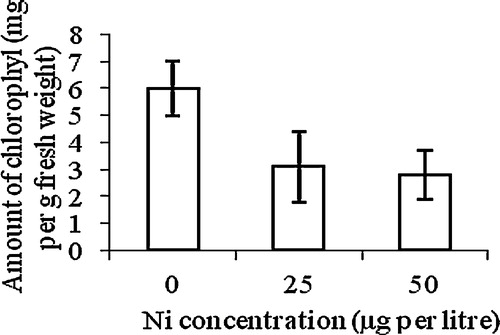
3.2 Chlorophyll content
A statistically significant (p < 0.05) decrease in chlorophyll content was observed at both 25 and 50 μg Ni L−1. The seedlings grown at 50 μg Ni L−1 were affected most: compared with the control their chlorophyll content was reduced by 50% ().
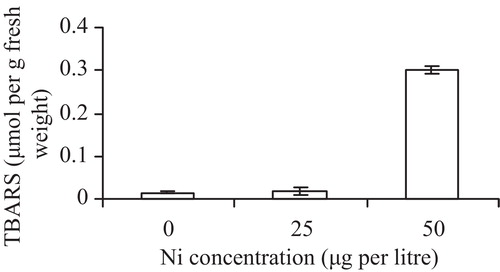
3.3 Lipid peroxidation
The effect of Ni on lipid peroxidation (LPO) was determined by evaluating the tissue TBARS contents. Ni toxicity caused a significant increase in TBARS content (). The difference in accumulation of TBARS between the control and 25 μg Ni L−1 was small but it increased significantly (p < 0.05) between 25 and 50 μg Ni L−1.
3.4 Proline accumulation
The differences in proline content between the control and the Ni treatments were statistically significant (p < 0.05). Mean proline content increased significantly between 25 and 50 μg Ni L−1 (p < 0.05). At 50 μg Ni L−1 it had doubled compared with the control ().
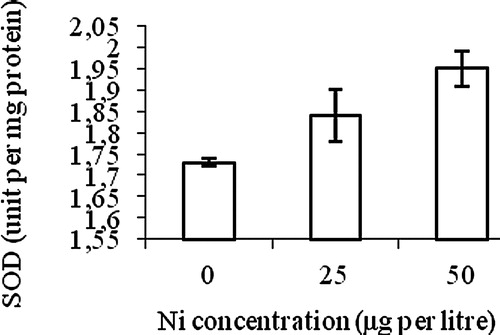
3.5 Superoxide dismutase activity
The differences in superoxide dismutase (SOD) activity between the control and the Ni treatments were statistically significant (p < 0.05). SOD activity differed between 25 and 50 μg Ni L−1 but the difference was not statistically significant ().
4 Discussion
Plants have a remarkable but differential ability to take up minerals from their external environment and accumulate various essential and non-essential elements including heavy metals.
In the present study, wheat seedlings were grown in hydroponic culture in the presence of increasing Ni concentrations to evaluate their Ni uptake and growth and identify possible defence mechanisms. The results presented here indicate that Ni was toxic to wheat seedlings. The greater accumulation of Ni in the roots as found in our study could reflect different cellular mechanisms for the bioconcentration of essential and non-essential trace metals in plants [Citation22,Citation23].
4.1 Plant growth and metal accumulation
The most common response of plants to stress conditions, such as heavy metals, is growth reduction. According to Wang and Zhou [Citation24], tolerances of plant to heavy metals are usually estimated on the basis of the degree of their root or shoot growth inhibition by the metal present in a nutrient solution. In their study, the critical level of Ni above which a significant reduction in dry matter yield occurred was found to be different for different plant species. In the hydroponic cultures of our study, a clear plant growth reduction was observed at a Ni concentration in the nutrient solution of 25 μg L−1 (). Reduced seedling vigour in wheat due to excess Ni could possibly be attributed to the interference of Ni with the metabolic and biochemical processes, such as protein and chlorophyll synthesis.
Results obtained from this study demonstrated that Ni concentrations ranging from 0 to 50 μg L−1 inhibited seedling growth. At increasing Ni concentrations the wheat seedlings exhibited morphological deformations. Ni also caused a reduction in root volume in wheat.
Literature data indicates that tolerance to heavy metals depends on plant species and may even be organ-dependent. Treatment of spinach with equal Cd and Ni doses had similar inhibitory effect on its leaf growth [Citation25]. According to Pandey and Sharma [Citation26], shoots of cabbage exposed to equal Cd and Ni concentrations responded similarly, as in wheat, whereas cabbage roots showed a higher tolerance to Ni.
Our results indicate an increase in Ni accumulation in leaves and roots with increasing Ni concentration in the nutrient solution (). Ni accumulated primarily in the roots, and small amounts were transferred to the leaves. Ni distribution in plant tissues is an indication of a change in chemical form resulting from complexation with plant-produced ligands. Studies on the uptake of heavy metals by plants have shown that heavy metals can also be transported passively from roots to shoots through the xylem vessels.
The results of our study show that little Ni was translocated to the above-ground parts of the wheat seedlings: the Ni being mostly retained by the roots. This difference in distribution of the metals taken up over roots and shoot was probably responsible for differential growth inhibition of these organs. Gajewska and Sklodowska [Citation27] noticed that a decrese in the root/shoot length ratio was related to an increase in the root/shoot ratio of the metal contents in the seedling.
4.2 Chlorophyll content
The chlorophyll content of the leaf tissues were significantly lower when Ni levels in the nutrient solution were above 25 μg L−1. In leaves, the inhibition of chlorophyll accumulation was proportional to the Ni concentration in the nutrient solution. The reduced chlorophyll accumulation in leaves could be explained by the inhibition of chlorophyll biosynthesis. Dhir et al. [Citation28] explained that this decline in chlorophyll levels could be due to (i) a lower Fe content, (ii) reduced efficiency of enzymes involved in chlorophyll biosynthesis and (iii) replacement of central Mg2+ molecules in chlorophylls by the heavy metals.
Several authors report that decreased chlorophyll contents in the leaves of Ni-treated plants [Citation26,Citation29,Citation30]. Such chlorosis could result from both Fe and Mg deficiency and the inhibition of chlorophyll synthesis [Citation30].
4.3 Lipid peroxidation
The level of malondialdehyde (MDA) content has been considered as an indicator of oxidative stress. TBARS contents of leaf tissues were slightly higher at 25 μg Ni L−1 and were more prominent at a Ni concentration of 50 μg L−1 (). The level was twice Ni than that of the control. The effect of Ni on plant lipid peroxidation (LPO) was dependent on element dosage. At the higher concentration, the antioxidative function of Ni seemed to be decreased or reversed and LPO was higher than at the lower Ni concentration. LPO might have been induced due to the production of OH generated in response to the metal. It has been reported that LPO can be induced by heavy metals via free radicals such as OH+, or by lipoxygenase [Citation31,Citation32].
4.4 Proline accumulation
In plants and micro-organisms, proline has been shown to play an important role in the recovery from environmental stresses including heavy metal stress. Drought, salinity, and heavy metal toxicity induce proline accumulation in algae and higher plants [Citation33]. However, the function of proline accumulation in response to metal stress has still not been very clear. We observed a significant accumulation of proline in the leaves of the Ni treated wheat seedlings in a dose dependent manner (). Proline accumulation might be induced as a result of accumulation of reactive oxygen species (ROS). The mechanisms by which proline reduces oxidative damage include physical quenching of singlet oxygen and chemical reaction with hydroxyl radicals [Citation34]. Due to its chelating ability, proline can also be a defence mechanism for survival of stressed plants by binding metal ions. An increase in both proline and TBARS contents with increasing metal ion concentration is indicative of a correlation between free radical generation and proline accumulation. It has been suggested that proline might protect plants from heavy metal toxicity [Citation35]. Nonetheless, many researchers believe that proline accumulation is simply an indicator of various stresses, and is not involved in protecting plants against metal toxicity. Proline accumulation did not occur until plant tissues had been damaged and therefore was not linked to preventing metal toxicity [Citation36]. Schat et al. [Citation37] demonstrated that heavy metal-induced proline accumulation was not realized until the damage had been caused and as a result it obviously did not prevent metal toxicity. Similarly, despite substantial proline accumulation, the membrane damage as exhibited by LPO did not decrease during the present study. These results suggest that proline accumulation is not involved in protection against Ni-induced oxidative damage.
4.5 Superoxide dismutase
Superoxide dismutase (SOD) is a key enzyme in protecting the cell against oxidative stress, which catalyzes the dismutation of O2− to H2O2 and O2. Plants exposed to different metals respond differently as regards enzymatic activities.
Our results demonstrate that the Ni toxicity mediated changes in the activities of antioxidant enzymes are dose dependent. We did not observe any significant change in total SOD activity in leaves of wheat seedlings upon Ni stress. A decrease in the levels of superoxide radicals may reduce the need for their scavenger, i.e., the SOD [Citation38]. Similarly, SOD activity was also not altered when barley seedlings were exposed to toxic boron levels in a study reported by Karabal et al. [Citation39].
In summary, our results strongly suggest that higher Ni levels cause oxidative stress in wheat cells and may cause membrane damage through the production of ROS and interfere with chlorophyll metabolism. Furthermore, although significantly induced, antioxidant enzymes are not sufficient to protect wheat cells from Ni toxicity. Consequently, it is more likely that antioxidant enzyme activities may not be part of mechanism(s) involved in Ni stress tolerance. Although the physiological mechanisms responsible for the effect of Ni on antioxidant enzymes cannot be explained with the results obtained from the present study, it may be worth mentioning that higher Ni concentrations triggered the antioxidant responses in wheat through the activation of oxido-reductases. Nevertheless, Ni-induced oxidative stress occurred at high concentrations exhibiting its role as a pro-oxidant, which is supported by previous reports [40–43Citation[40]Citation[41]Citation[42]Citation[43]]. These results suggest that there must be additional ways in which Ni tolerant plants detoxify the effects of this metal. They also suggest that detection and analysis of induced proteins in stressed Ni tolerant plants might help to increase our understanding of the mechanisms of Ni tolerance in plants.
References
- D.L.EskewR.M.WelchW.A.NorvellNickel an essential micronutrient for legumes and possibly all higher plantsScience2221983621623
- R.BoominathanP.M.DoranNi-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertoloniiNew Phytologist1562002205215
- T.PandolfiniR.GabbrielliC.CompariniNickel toxicity and peroxidase activity in seedlings of Triticum aestivum L.Plant, Cell & Environment151992719725
- J.L.MoyaR.RosI.PicazoInfluence of cadmium and nickel on growth, net photosynthesis and carbohydrate distribution in rice plantsPhotosynthesis Research3619937580
- K.V.Madhava RaoT.V.S.SrestyAntioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stressesPlant Science1572000113128
- I.DéportesJ.L.Benoit-GuyodD.ZmirouHazard to man and the environment posed by the use of urban waste compost: a reviewScience of the Total Environment1721995197222
- V.BarcanE.KovnatskySoil surface geochemical anomaly around the copper–nickel metallurgical smelterWater, Air, & Soil Pollution1031998197218
- F.Van AsscheH.ClifstersEffects of metals on enzyme activity in plantPlant, Cell & Environment131993195206
- D.GongC.A.GrimesO.K.VargheseTitanium oxide nanotube arrays prepared by anodic oxidationJournal of Materials Research16200133313334
- A.AliM.AterI.SunaharaR.P.YvesPhytotoxicity and bioaccumulation of copper and chromium using barley (Hordeum vulgare L.) in spiked artificial and natural forest soilsEcotoxicology and Environmental Safety572004363374
- G.OuzounidouCopper induced changes on growth, metal content and photosynthetic function of Alyssum montanum L. plantsEnvironmental and Experimental Botany341994165172
- J.Hartley-WhitakerG.AinsworthA.A.MehargCopper and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivityPlant, Cell & Environment242001713722
- A.MithoferB.SchulzeW.BolandBiotic and heavy metal stress response in plants: evidence for common signalsFEBS Letters566200415
- S.BaccouchA.ChaouiE.E.FerjaniNickel toxicity induces oxidative damage in Zea mays rootsJournal of Plant Nutrition24200110851097
- D.Demirezen YılmazEffects of salinity on growth and nickel accumulation capacity of Lemna gibba (Lemnaceae)Journal of Hazardous Materials14720077477
- L.L.KnudsonW.T.TibbittsE.G.EdwardsMeasurement of ozone injury by determination of leaf chlorophyll concentrationPlant Physiology601977606608
- M.M.TeresaS.CristinaC.HermanN.FlaviaAntioxidative responses of wheat treated with realistic concentration of cadmiumEnvironmental and Experimental Botany502003265276
- L.S.BatesR.P.WaldreuT.D.TeakRapid determination of free proline for water stress studiesPlant and Soil391973205207
- M.M.BradfordA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnalytical Biochemistry721976248254
- N.MisraA.GuptaEffect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catarantus roseus seedlingsJournal of Plant Physiology16420061118
- SPSSSPSS Version 10.02002SPSS Inc.233 S. Wacker Drive, Chicago, IL
- L.A.RichardsDiagnosis and Improvement of Saline and Alkali Soils1954U.S. Department of Agriculturep.60
- S.ErdeiA.HegedüsHeavy metal induced physiological changes in the antioxidative response systemProceedings of the 7th Hungarian Congress on Plant Physiology2002
- M.WangQ.ZhouSingle and joint toxicity of chlorimuron-ethyl, cadmium, and copper acting on wheat Triticum aestivumEcotoxicology and Environmental Safety602005169175
- S.MishraS.B.AgrawalInteractive effects between supplemental ultraviolet-B radiation and heavy metals on the growth and biochemical characteristics of Spinacia oleracea LBrazilian Journal of Plant Physiology18200618
- N.PandeyC.P.SharmaEffect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbagePlant Science1632002753758
- E.GajewskaM.SklodowskaDifferential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlingsEcotoxicology and Environmental Safety7320109961003
- B.DhirP.SharmilaP.P.SaradhiS.A.NasimPhysiological and antioxidant responses of Salvinia natans exposed to chromium-rich wastewaterEcotoxicology and Environmental Safety72200917901797
- E.GajewskaM.SklodowskaM.SlabaJ.MazurEffect of nickel on antioxidant enzyme activities, proline and chlorophyll contents in wheat shootsBiologia Plantarum502006653659
- I.V.SereginA.D.KozhevnikovaPhysiological role of nickel and its toxic effects on higher plantsRussian Journal of Plant Physiology532006257277
- A.MishraM.A.ChoudhuriEffect of salicylic acid on heavy metal induced membrane deterioration mediated by lipooxygenases in ricePlant Biology421999409415
- S.ChoudhuryS.K.PandaToxic effect, oxidative stress and ultrastructural changes in moss Taxitheelium nepalense (Schwaegr.) Broth. under lead and chromium toxicityWater, Air, & Soil Pollution16720057390
- T.C.StadtmanSe biochemistryAnnual Review Biochemistry591990111127
- M.P.AliaJ.MatysikEffect of proline on the production of singlet oxygenAmino Acids212001195200
- M.E.FaragoW.A.MullenPlants which accumulate metals. Part IV. A possible copper–proline complex from the roots of Armeria maritimeInorganica Chimica Acta3219799394
- S.LuttsJ.M.KinetJ.BouharmontEffects of various salts and of mannitol on ion and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) callus culturesJournal of Plant Physiology1491996186195
- H.SchatS.S.SharmR.VooijsHeavy metal-induced accumulation of free proline in metal-tolerant and a nontolerant ecotype of Silene vulgarisPlant Physiology1011997477482
- T.F.ChenW.J.ZhengY.S.WongF.YangSe-induced changes in activities of antioxidant enzymes and content of photosynthetic pigments in Spirulina platensisJournal of Integrative Plant Biology5020084048
- E.KarabalM.YucelH.A.OktemAntioxidant responses of tolerant and sensitive barley cultivars to boron toxicityPlant Science1642003925933
- P.SinhaB.K.DubeC.ChatterjeeManganese stress alters phytotoxic effects of chromium in green gram physiology (Vigna radiata L.) cv. PU 19Environmental and Experimental Botany572006131138
- P.CartesL.GianfredaM.L.MoraUptake of Se and its antioxidant activity in ryegrass when applied as selenate and selenite formsPlant and Soil2762005359367
- H.HartikainenT.XueV.PiironenSe as an anti-oxidant and pro-oxidant in ryegrassPlant and Soil2252000193200
- J.NowakK.KaklewskiM.LigockiInfluence of Se on oxidoreductive enzymes activity in soil and plantsSoil Biology and Biochemistry36200415531558