Abstract
An important part of the study of the policy response of government in the area of a novel technology, such as genomics, lies in identifying the technological trajectory followed in the sector and how it intersects or impacts upon existing policy, regulatory and innovation regimes. Part of the challenge in studying the impacts and outcomes of such trajectories, therefore, is their multilayered nature. This has led to the proliferation of different models or frameworks for the analysis of many sectors, each one tackling a specific level and obscuring the linkages between levels and units of analysis. Research into innovations, however, benefits from an understanding of the overall policy and regulatory regimes present in a sector while an understanding of regulatory behaviour is in turn linked to the overall policy framework set up to govern a sector. As such, analyses of both regulation and innovation in a sector such as genomics can profit from an integrated, multi-level approach grounded in the overall nature of the policy regime present in the sphere of activity under examination. We offer such an approach by synthesizing four existing models of policy, regulatory and innovation behaviour that fit the three levels of analysis – the policy regime, regulatory regime and the innovation regime – in the sphere of biotechnology.
1 Introduction
An important part of the study of the policy response of government in the area of a novel technology, such as genomics, lies in identifying the technological trajectory followed in the sector and how it intersects or impacts upon existing policy, regulatory and innovation regimes. Part of the challenge in studying the impacts and outcomes of such trajectories, therefore, is their multilayered nature (CitationHowlett, 2009). This has led to the proliferation of different models or frameworks for analysis of the sector, each one tackling a specific level and obscuring the linkages between levels and units of analysis.
Research into innovations, however, benefits from an understanding of the overall policy and regulatory regimes present in a sector while an understanding of regulatory behaviour is in turn linked to the overall policy framework set up to govern a sector. As such, analyses of both regulation and innovation in a sector such as genomics can profit from an integrated, multi-level approach grounded in the overall nature of the policy regime present in the sphere of biotechnology. We aim to offer such an approach by synthesizing four existing models of policy, regulatory and innovation behaviour that fit the three levels of analysis: the policy regime, regulatory regime and the innovation regime.
At the most general level of the biotechnology policy regime, a very useful model is CitationPaarlberg's (2000) comparative analysis in which government activity in issue areas germane to biotechnology innovations are scored to provide a measure of the precautionary or permissive nature of country or sector policy as a whole. We combine this model with the discussion of CitationIsaac (2001) concerning the nature of the origin of risk assessments in either the scientific of social communities in order to generate a simple two-dimensional model of possible overall biotechnology policy regime types.
Within this broad framework, a third model developed by CitationHaga and Willard (2006) of genomics regulatory behaviour, focusing on the manner in which specific sectors of activity – biomedical, agri-food and others – can be placed on a continuum of legal vs consultative regulatory approaches, allows us to more precisely place countries (and sectors) on a quadrant according to their specific approach to genomics regulation.
Finally, at the level of innovation cluster/actor activity, a fourth model developed by CitationPickernell, Rowe, Christie, and Brooksbank (2007) is used to allow us to identify eight ideal-type innovations systems based on their level of formality and verticality of interactions between network members. This provides us with a third framework allowing the classification of innovation activity in specific industries and industrial groupings within the more general regulatory and policy regime-level classifications set out by Paarlberg and Haga and Willard.
Taken together these three frameworks help identify (1) the overall policy approach taken by governments involved in the application of genomics technologies generated from the biotechnology sector as a whole; (2) the regulatory aspects of, and approaches taken to, such applications; and (3) the nature of the innovation systems involved in the development and utilization of such novel technologies and the role government plays within them.
Each framework identifies the key issues and factors that contribute to policy-making in each activity and sets out some general expectations for the nature and direction of policy developments in each area derived from comparative research into similar applications in other countries and jurisdictions. The utility of using such frameworks to arrive at a basic understanding of the nature and direction of genomics, and more generally, biotechnology development is illustrated through their application to the activities of Canadian governments in this area since the prospects and potential of genetic manipulations first came to the attention of Canadian government regulators and industrial actors in the late 1970s and early 1980s.
2 Three frameworks for analyzing biotechnology policy, genomics regulation, and innovation processes
The study of biotechnology policy has generated a large number of models and elaborating and modifying these frameworks is necessary in order to better map the nature of technological trajectories and their impact on public policy-making in this sector. By linking and merging several existing models, we are able to offer an integrated framework for the study of regulation and innovation that provides researchers with a parsimonious set of variables to investigate in order to describe the overall nature of biotechnology policy, and genomics regulation and innovation activity occurring in that regime. Taken together the three frameworks set out below provide a set of tools for the comparative analysis of both industry and government behaviour as it relates to innovation and regulation within biotechnology-driven policy processes.
2.1 The Paarlberg/Isaac model of overall policy regime-level biotechnology policy orientations
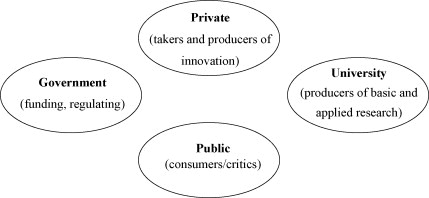
In general, within a science-based technological sector such as genomics, four sets of policy actors exist: the private sector, governments, the public and the university research community. These actors take on different tasks according to their level of engagement in the sector and their role in either promoting or monitoring and controlling innovation. Private companies, for example, may actively develop innovation or may be open to adopt innovations developed elsewhere. Universities tend to provide basic research, but in the current climate are actively seeking ways to spin off their research in the private sector in the form of applied research. Governments can provide funding for university and private sector activity as well as establish and sustain the legal and regulatory framework in which innovations occur. The public, including the media and interest groups and other forms of non-governmental organizations can take on a variety of roles from uneducated consumer of science-based products to sophisticated critics of private, university and government behaviour (see Fig. 1).
Governments tend to have a bifurcated approach to innovation recognizing on the one hand the importance of innovative products and processes in fostering economic development and, on the other, the need to regulate innovation in order to allay public concerns and promote product safety. CitationPaarlberg (2000:6) developed a high-level framework for classifying the general tendency or trend of governments in terms of their orientation towards either promotion or precaution in dealing with biotechnological innovations. His work dealt with the attitudes of governments in developing countries in dealing with the introduction of genetically modified organisms (GMOs) and this same classificatory model has been successfully utilized by CitationTalukder and Kuzma (2008) in their work analyzing the Indian government policy approach towards Bt cotton. However, the model not only provides a description of the policy space occupied more generally by food-related biotechnology applications but also with slight modification can address the specific concerns and issues found in the biomedical sector.Footnote1
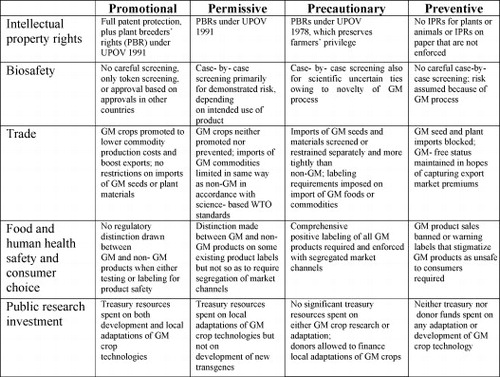
Paarlberg's framework delineates the key elements of the sector at the level of a national policy regime. He uses a matrix identifying the general set of issues faced by policy-makers and other actors in the sector, ultimately classifying typical responses to each issue along a scale running from “preventive” to “promotional” (on the use of this terminology see also CitationSheingate, 2006); classifying policies on the basis of whether they would “tend to promote use of the new technology [genetic modification] or prevent its use”. He found national policies to cluster into four different distinct policy regimes – promotional, permissive, precautionary and preventive. Policies that accelerate the spread of GM crop and food technologies within the borders of a nation can be termed “promotional.” Policies that are neutral towards the new technology, in tending neither to speed nor to slow its spread, can be termed “permissive.” Policies intended to slow the spread of GM crops and foods for various reasons are called “precautionary.” Finally, policies that tend to block or ban entirely the spread of this new technology are “preventive” (CitationPaarlberg, 2000:4). Paarlberg argued these regimes link together and synthesize five distinct policy issue areas – intellectual property rights, biosafety, trade, food safety and public research investment. For example, a promotional policy in the food safety area would provide no regulatory distinction between GM and non-GM foods, while a preventive one would ban GM food sales. This model can be extended with relatively minor changes to medical biotechnology by adding a human health policy issue area to Paarlberg's food safety criterion (see Fig. 2).
Paarlberg's framework can be improved by adding in a second dimension, however, that of whether or not risk assessments and orientations are driven by the scientific community or by social actors. As CitationIsaac (2002) suggested, country-level regimes also differ significantly in this second area (see Fig. 3).
Fig. 3 Scientific vs social rationality in biotechnology regulation. Source: CitationIsaac (2001:2).
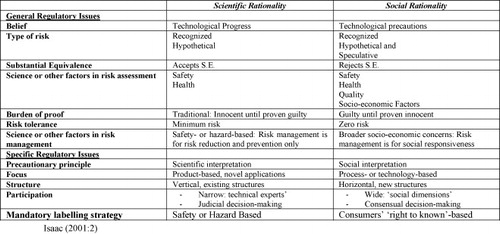
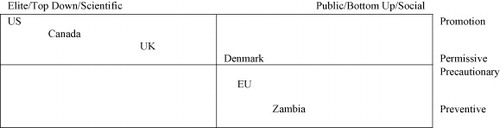
This insight allows us to organize countries and/or sectors within a two-dimensional policy space by taking into account both Paarlberg's insight into the promotional vs precautionary nature of national and sectoral regimes and also Isaac's observation about whether or not this orientation is driven by bottom-up “social” concerns or by top-down “scientific” ones. US approaches to food safety, for example, a key element in the area of genomics research applied to commercial crops, are premised on the individual freedom of the consumer to choose a particular product but are supported by relatively elite/top-down policy models. In the European Union, the leading principle in food safety policy has been a precautionary one (CitationLindner, 2008:142) mixed with several examples of increased bottom-up assessment activities (CitationSeifert, 2006). Canada has followed an intermediate path. In the biotechnology field, regulation in the 1980s tended to be connected to the development of a GMO industry that was often centered on agri-business (canola, for example) and was relatively permissive. However, genetically modified organisms faced strong resistance from the public both in Canada and abroad. After 1990, the Canadian biotechnology sector stabilized and shifted its focus from GMOs to genomics and the policy approach coalesced in a permissive-promotional direction supported by both funding and organizational frameworks like Genome Canada and its provincial offshoots. Thus, using the combined Paarlberg/Isaac model, the US and Canada, for example, can be seen to apply a scientific rationality model to a more or less promotional approach to biotechnology while the EU follows a social rationality/precautionary approach. Denmark, for example, follows a tradition of organizing consensus conferences in which the public is involved in commenting on biotechnology (CitationSeifert, 2006) but while this country has rather strict guidelines regarding cloning, the genomics aspect is more open to research. On the other hand, in 2001/2002, Zambia refused a shipment of what might have possibly been GMO corn after a rather interesting process of public debate revealing a preventive, bottom-up policy strategy (CitationMwale, 2006) (see Fig. 4).
These general policy regimes are difficult to change once established, since passing from one quadrant to another in the above matrix has attendant costs for both policy-makers and other actors. For example, a move from the elite-promotional Canadian model to a public-permissive such as the Danish one would reduce the ‘freedom of movement’ of the scientific community. Therefore, identifying the location of a particular country or sector within a specific quadrant is a key step in identifying and understanding subsequent activities which take place within a regime, such as regulatory and innovation activity.
2.2 Regulatory regimes: The Haga and Willard framework
Within the policy regime, one area of government activity is at the regulatory level – broadly conceived as including the provision of both constraints and incentives for specific industry actor and consumer behaviour. Activity at this level can be illuminated by applying Paarlberg and Isaac's categories of key overall biotechnology policy orientations to a second framework focusing specifically on regulatory activity. This second framework helps identify the general nature of the issues with which regulators and the industry grapple and the manner in which different countries and sectors have developed different ‘styles’ of regulation vis-à-vis genomics issues.
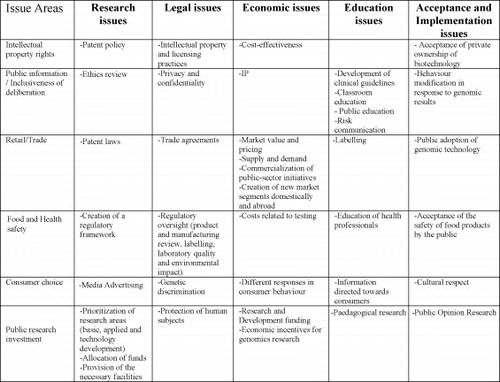
The Paarlberg/Isaac's model of “permissive-promotional” and ‘elite-public’ policy regimes can be adapted to deal with the components of regulatory regimes as CitationHaga and Willard (2006) identified by in their discussion of GMO regulatory behaviour. These authors argued, like Paarlberg, that five types of regulatory issues exist in the genomics or biotechnology sector.Footnote2 And, like Isaac, they highlighted the centrality to risk management and regulatory oversight of the degree of inclusiveness of social actors in the policy deliberation process (see Fig. 5).Footnote3
Fig. 5 Haga and Willard list of Biotechnology regulatory issue areas. Source: CitationHaga and Willard (2006:967).
Based on their application of this framework to their own study of regulatory behaviour in several jurisdictions, Haga and Willard argued that in each issue area governments choose a regulatory approach based on a spectrum between legal and consultative approaches to a subject. That is, that a government would adopt one or more of five basic approaches to dealing with these issues: a legislative approach in which laws are passed banning specific types of activity; a regulatory approach in which various kinds of standards and tests are established to govern genomics research activities; a guidelines approach, in which best practices are identified and encouraged; a voluntary approach in which users are encouraged to develop their own guidelines for good conduct; and a public consultation approach in which government or arms-length agencies undertake public hearings or meetings designed to solicit public opinion and input into future government activities (see Fig. 6).
Fig. 6 Haga and Willard Biotechnology regulatory spectrum. Source: CitationHaga and Willard (2006:968)

This framework suggests that regulatory policy-making in the area of biotechnologies is basically about designing and adopting a mix of policies that fit the circumstances of the particular sector in question within the generally established elite-social/permissive-precautionary regime logic. This can be seen in the peculiar differences distinguishing US and Canadian GMO policies, for example (CitationMontpetit, 2005), and in the differences between the regulations that the US have imposed on agricultural and medical GMOs uses (CitationSheingate, 2006). Generally speaking, the differences between the European Union and the United States and Canada extend to the broader area of food health and safety, where variations in values result in different regulative environments (CitationLindner, 2008). This difference was highlighted by CitationIsaac (2002) who argued that the different approaches that the Canadian and European Union (EU) followed in regulating agricultural biotechnology originated in different perspectives on the interpretation of the precautionary principle found in each jurisdiction.
2.3 Application to innovation studies: the Pickernell et al. framework
A second important area of government policy-making in the genomics sphere is at the level of support provided for innovation where governments can facilitate or constrain the development of national, regional or micro-level innovation systems in the biotechnology area.
In analyzing such innovation systems, researchers often use a network approach to map the industry, academic and government actors and their interactions in the sector, accompanied by a documentary analysis of key statements by network actors pertaining to their goals and intentions. This method helps clarify the relations and actors found in biotechnology innovation. The nature of the connections between the scientific community and producers is of particular interest, but interactions between the public, the private sector, universities and governments all take place. A policy network approach, in general, based primarily on the methods used successfully in the past by authors such as CitationKnoke and Laumann (1982) and CitationLaumann and Knoke (1987) and others in the social network analysis (SNA) mode is a useful one to adopt in mapping these interactions.
A fundamental pre-requisite for such an approach is the careful delimitation of the boundaries separating networks clearly, delineating relationships in one sector from those existing in others (CitationLaumann, Marsden, & Prensky, 1983). One method of establishing such limits involves categorizing the types of issues found in legislative acts and other official statements that government policy-makers themselves use to frame their activity in the sector. As long as policy-makers are responsive to consumer and producer interests, such issue lists will typically represent both governmental and non-governmental actor perceptions of the key issues facing the industry and government and the aggregate list of such issues can thus be taken as representing the overall policy space occupied by the sector.
The use of the lists of issues constructed by both Paarlberg and Haga and Willard is useful in this regard and CitationPickernell et al. (2007) have generated a model of innovation activity in the biotechnology sector which can be seen as being ‘nested’ within the larger regime and regulatory frameworks set out above. The authors organize the broad literature on the subject of innovation systems to identify a set of variables that are typically related to innovation systems, networks or clusters, and on this basis, outline several basic innovation network types which are helpful for understanding genomics innovation system behaviour (CitationNewell, 2008). These variables include the structure and purpose of networks and clusters, the focus and mode of the firms involved the network mode, the management focus, and the learning processes that are in place in a sector. Each specific network/cluster type reflects the kinds of costs associated with the sector, the different goals and behaviours of the actors, and the ways in which management choices and knowledge flow through the structure.
This particular framework (see Fig. 7) allows us to map the existing and any emerging structures in any biotechnology industry and helps to delineate the preferences of the various actors for a specific cluster or network model type. Ultimately Pickernell et al. argue that the eight basic types they identify can be arranged in a two-dimensional space based on the degree of formality found in key relationships and the vertical or horizontal (cross-industrial) nature of network links (see Fig. 8). This framework again allows for the comparative treatment of innovation systems in various biotechnology policy regimes, such as those in genomics as countries and sectors can be placed into this innovation policy space much as they were in the case of the Haga and Willard model of regulatory space set out above regulatory activity.
Fig. 7 General clusters and networks frameworks. Source: CitationPickernell et al. (2007:343).
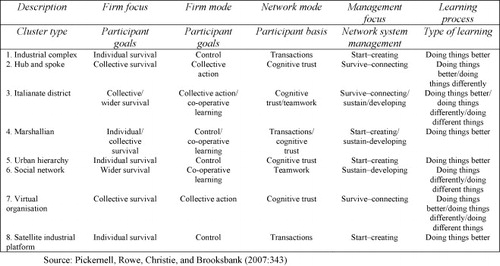
Fig. 8 Cluster/network structural typology. Source: CitationPickernell et al. (2007:344).
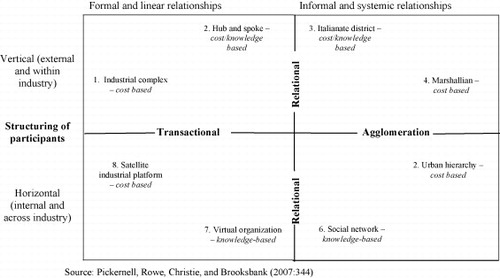
Since the nature of permissive and precautionary regulatory activity and the tendency towards elite or participatory risk assessment is a determinant of aspects of innovation activity – such as the structuring of participants within or across industrial sectors – and of the nature of formal or informal relations in a sector, this third framework can be seen to be nested within the other two, much as the regulatory framework itself is nested inside the overall policy regime one.
3 Application to the Canadian genomics case
The utility of these three frameworks to understanding trajectories in regulatory and innovation behaviour in specific countries and sectors can be seen in their application to a case such as that of Canadian regulatory behaviour in the genomics sector.
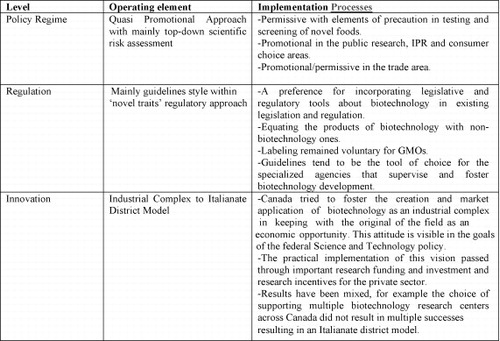
At a ‘meta-level’, Canadian policy-makers recognized early on the economic potential of life science applications and tried to develop a biotechnology policy regime that fostered private investment in the areas such as GMOs for agriculture. While the focus of the federal Science and Technology policy has since shifted to genomics, a strongly promotional attitude remains in place.
And Canada has also always operated on the principle of novel traits (CitationMontpetit, 2005), grounding the system in scientific rationality (CitationIsaac, 2002). As genomics took center stage during the last decade, the policy orientation remained grounded in novelty and therefore assumes that no specific ‘genomic policy’ is required. In the end biosafety (both human safety in foods and drugs and environmental safety) is left to legislative and regulatory devices, but these are mostly pre-existing tools to which ad hoc sections have been added dealing with biotechnology's potential risks. Thus we can say that the Canadian regulative framework both reflects and reproduces the notion that biotechnology is a ‘business as usual’ sector that only requires an adaptation of existing rules.
In the regulatory sphere, important yardsticks are embedded in legislative tools like the 1988 Canadian Environmental Protection Act, which recognized the potential danger inherent in this technology or the Assisted Human Reproduction Act that sets limits regarding cloning and the creation of human embryos for research. As mentioned above, the trigger for risk oversight is based on the novelty of the product, a broader trigger than in the United States, but not as broad as the European ones (CitationMontpetit, 2005; Kurzer & Cooper, 2007). In 1999, Health Canada also introduced mandatory notification of all genetically modified foods. However a promotional approach is found in the food safety and consumer choice area, where no policy provision mandates labeling for GMO foods but leaves the same to a voluntary regime. The labeling of non-food products is under the supervision of the Competition Bureau (Industry Canada). But the Consumer Packaging and Labelling Act (1999) does not expressly cover the use of biotechnology. This generates an interesting opposition between a relatively strict novelty approach combined with the regulatory compulsory notification of all GMO foods, and a voluntary labeling system for GMO foods. This means that even relatively unproblematic modifications like the addition of Vitamin C to a vegetable must follow the screening and approval process and yet the final product is not discernible from any other one sold by Canadian retailers. The result has been a ‘negative’ labeling stressing the lack of GMOs in organic and natural foods. In general, medical biotechnology is covered under the Food and Drug Regulations Footnote4 and the Medical Devices Regulations. These extend to biotechnology the existing regulations for drugs (Section C – Division 03 and 04). The system protects the rights of companies that invest in biotechnology as the decision to support Monsanto against Saskatchewan farmer Percy Schmeiser in 2004 showed (Monsanto Canada Inc. v. Schmeiser, 2004 1 SCR 902, SCC 34).
At the level of research, guidelines, especially within the context of specialized agencies overseeing and/or participating in ongoing scientific research, as is the case for medical genomics, can be a much more reactive and efficient tool than legislation to deal with sudden breakthroughs or dangers. In terms of public consultation, most government agencies involved in the supervision and development of biotechnology have (and have had in the past) public contact and public consultation processes in place. However, risk assessment remains very much a top-down, science-driven process combining several elements of the ideal-type approaches identified by Haga and Willard.Footnote5
At the innovation level, the Canadian policy regime is organized around policies that are meant to generally promote genomics research and use. From the point of view again of public research, the Canadian public sector is an important player investing important sums in the sector (CitationVan Beuzekom & Arundel, 2006:17–19). At the trade and economic development levels, Canada had counted on genomics research to behave as a transmission belt for its economy. It has also favored the sector in its Science and Technology policy, which consistently advocates an increased role for genomics in the Canadian economy. During the early phase of biotechnology development, GMOs especially in the agricultural field (for example, canola) were heavily supported by the Canadian federal authorities (CitationMoore, 2007). Exports, imports and consumption of these products were expected to be a boost for the Canadian economy. Non-invasive genomic tools like Marker-Assisted Selection (MAS) are heavily promoted at the research level in Canada and are protected through strong Intellectual Property Rights (IPR) legislation, but are not otherwise regulated as they do not present any novel traits, being mostly diagnostic tools. In terms of IPR, Canada protects the work of scientists developing GMOs and genomic tools through the Canadian Intellectual Property Office (CIPO) in the same way in which it protects other intellectual property. For example, plants are protected by patents and by a Plant Breeder's Rights (PBR) system. However, the overall system has proven less promotional than that of the USA since in December 2002, the Supreme Court of Canada ruled that higher life forms are not patentable (Harvard College v. Canada (Commissioner of Patents), 2002 SCC 76). At the policy level, this meant that while growing GMO crops was promoted, their import was regulated in the same way as non-GMO ones, placing this particular policy between promotional and permissive areas.
The innovation side of biotechnology in Canada is thus more complex and broader than the regulatory one as there was an initial phase when various biotechnology research centers were set up (CitationCanada, 1998) in the hope that they would function as core elements for the development of a industrial complex in this area. The creation of regional research centres and of the Centres of Excellence Program in 1986, and of Genome Canada in 2000 all added to a strong innovation-oriented and supportive institutional and regulatory structure. However, achieving successful economic returns and large-scale innovation adoption has been more elusive (CitationPhillips, 2005), and the leadership of the Science and Technology policy and of its administrative structures have been questioned (CitationCanada, 2005; Phillips, 2007). Only three of the hubs subsequently emerged as key players: Toronto, Montreal, and Vancouver (CitationHolbrook et al., 2004). The others progressively slipped behind (CitationNiosi & Banik, 2005) and the research centres that the National Research Council and the federal government created in places such as Ottawa and Saskatoon played at best a secondary role in the development of biotechnology clusters (CitationNiosi & Bas, 2001). The Canadian Biotechnology Strategy is said to have not provided the unifying top-down approach it was meant to create (CitationCanada, 2005:10).Footnote6 The contribution of the Genome Canada centres in creating regional systems of innovation has also been questioned (CitationNiosi, 2005) and also in question is its capacity to bridge the gap between academia and commercial application “…it is far from clear whether [Genome Canada research] will result…in the transformation of inventions into socially-valued products or services” (CitationPhillips, 2005:29). The shortcomings are often seen as policy failure (CitationPaquet, 2005; Stanley, 2007) and connected to the overly optimistic notion that RISs would naturally emerge after the creation of a national innovation strategy. In Pickernell et al's terms, this has led to the development of at best an ‘Italianate District’ in the 4–5 centres scattered across the country (CitationGertler & Levitte, 2005).
Overall, utilizing the three frameworks set out in this article, the following comparative profile of the Canadian genomics sector can be discerned (see Fig. 9).
4 Conclusion
The utility of utilizing the Paarlberg/Isaac, Haga and Willard and Pickernell et al. frameworks to help understand the current status and trajectory of policy-making in emerging biotechnology sectors such as genomics can be seen through their application to a case such as Canada. The application of these models shows that the life sciences in Canada, overtime, have developed two quasi-promotional regimes in the areas of innovation and regulation, which both should be understood as dynamic and evolving. Such an evolution is linked not only to the technological developments they center upon, but also on their economic viability, on the response of the public to the new products they offer, and on existing political attitudes and policies at the national and international levels.
In Canada, as elsewhere, the situation is complex as the actors involved in the process of developing, regulating and applying genomic tools have different agendas and approaches to the use of genomics techniques. Governments are sometimes very interested in fostering genomic applications and technologies in the sector, and some, as in Canada, have adopted a relatively permissive set of regulations to accommodate research and testing of genetically modified organisms (CitationCanada, 1998, 2003; Moore, 1999; Phillips & Wolfe, 2001). On the other hand, some actors are much more wary of applying genetic and genomic modifications and technologies especially when it is unclear if consumers can tell the difference between unpopular genomic techniques such as GMOs and lesser known but benign ones such as Marker-Assisted Selection in terms of their very different levels and types of genetic modifications (CitationDurant and Legge, 2006; Leger Marketing, 2001; Western Opinion Research, 2005).
Adopting a three-tiered approach to policy analysis in the area of genomics (policy regimes, regulatory regimes, and networks/clusters) has two major benefits. Firstly it allows the generation of a comprehensive model of national development using already existing frameworks, increasing our explanatory capacities without adding additional models to an already cluttered field. Secondly, it helps in describing the roles that government may need to take on in the area in the future as the technology continues to develop and intensify.
Notes
1 Policy regime strategies for genomics sciences should be understood as emerging at the national level, but as having strong correlations and links with the international level in terms of their connection to regulatory frameworks like the Cartagena Protocol on Biosafety (CitationNewell, 2008), or to the Codex Alimentarius in terms of food safety (CitationLindner, 2008). In Canada most biotechnology firms are now concentrated in the health sector (65%) with agri-food (28%) and industrial environmental applications (8%) following far behind (CitationVan Beuzekom and Arundel, 2006:27–29).
2 Because their analysis is heavily informed by the medical genetics area, not all of Haga and Willard's categories are of immediate use to the analysis of agricultural biotechnology, but they can serve as initial orientation points in setting out the basic policy tools that governments have at their disposal when regulating genomics technologies.
3 The issue of participation in the policy process by the public and stakeholder groups in the area of the regulation of new technologies is one which has received a great deal of attention in recent years (CitationFischhoff & Fischhoff, 2001; Haddow, Laurie, Cunningham-Burley, & Hunter, 2007; Sharp, Yudell, & Wilson, 2004; Talukder & Kuzma, 2008:131; Tutton, 2007).
4 The Food and Drug Regulations contain a definition of novel foods (which involve genetic manipulation) under division 28. However, no comparable section exists for drugs.
5 It should be noted that Canadians hold a nuanced view of GMOs (CitationHornig Priest, 2006) and are quite interested in organic foods, which have quickly become a large market (CitationMacey, 2006).
6 The 2005 report of the Auditor General of Canada finds that “Overall, the Canadian Biotechnology Strategy has not functioned as planned. It was designed for leadership from the top, which was not provided; however, management and working-levels did provide some coordination” (CitationCanada, 2005:10).
References
- Canada . The 1998 Canadian biotechnology strategy: An ongoing renewal process. 1998; Industry Canada: Ottawa
- Canada . Biotechnology transforming society: Creating an innovative economy and a higher quality of life-creating an innovative economy and a higher quality of life – Report on biotechnology (1998–2003). 2003; Communications Canada: Ottawa
- Canada . Report of the Auditor General of Canada to the House of Commons. Chapter 4. Managing horizontal initiatives. 2005; Office of the Auditor General: Ottawa
- R.F. Durant , J.S. Legge . Wicked problems. Public policy, and administrative theory. Lessons from the GM food regulatory arena. Administration and Society. 38 2006; 309–334.
- B. Fischhoff , I. Fischhoff . Publics’ opinions about biotechnologies. Journal of Agrobiotechnology Management and Economics. 4 2001; 155–162.
- M.S. Gertler , Y.M. Levitte . Local nodes in global networks: The geography of knowledge flows in biotechnology innovation. Industry and Innovation. 12 2005; 487–507.
- G. Haddow , G. Laurie , S. Cunningham-Burley , K.G. Hunter . Tackling community concerns about commercialisation and genetic research: A modest interdisciplinary proposal. Social Science and Medicine. 64 2007; 272–282.
- S.B. Haga , H.F. Willard . Defining the spectrum of genome policy. Nature Reviews Genetics. 7 2006; 966–972.
- J. Holbrook , M. Adam , N. Salazar , S. Crowden , K. Reibling , N. Warfield . The biotechnology cluster in Vancouver. D.A. Wolfe , M. Lucas . Clusters in a cold climate: Innovation dynamics in a diverse economy. 2004; McGill-Queen's University Press: Montreal/Kingston
- S. Hornig Priest . The public opinion climate for gene technologies in Canada and the United States: Competing voices, contrasting frames. Public Understanding of Science. 15 2006; 55–71.
- M. Howlett . Governance modes, policy regimes and operational plans: A multi-level nested model of policy instrument choice and policy design. Policy Sciences. 42 2009; 73–89.
- G.E. Isaac . Transatlantic regulatory regionalism. AgBiotech Bulletin. 9 2001; 1–4.
- G.E. Isaac . Agricultural biotechnology and transatlantic trade: Regulatory barriers to GM crops. 2002; Oxford University Press: Oxford
- D. Knoke , E.O. Laumann . The social organization of national policy domains: An exploration of some structural hypotheses. P. Marsden , N. Lin . Social structure and network analysis. 1982; Sage: Beverly Hills 255–270.
- P. Kurzer , A. Cooper . What's for dinner? European farming and food traditions confront American biotechnology. Comparative Political Studies. 40 2007; 1035–1058.
- E.O. Laumann , D. Knoke . The organizational state: Social choice in national policy domains. 1987; The University of Wisconsin Press: Madison
- E.O. Laumann , P.V. Marsden , D. Prensky . The boundary specification problem in network analysis. R.S. Burt , M.J. Minor . Applied network analysis: A methodological introduction. 1983; Sage: Beverly Hills 18–34.
- Leger Marketing . How Canadians perceive genetically modified organisms. Vol. 8 2001; Leger Marketing: Montreal
- L.F. Lindner . Regulating food safety: The power of alignment and drive towards convergence. Innovation: The European Journal of Social Science Research. 21 2008; 133–143.
- A. Macey . Certified organic production in Canada 2005. 2006; Certified Organic Growers: Ottawa
- M.D. Mehta . From biotechnology to nanotechnology: What can we learn from earlier technologies?. Bulletin of Science, Technology & Society. 24 2004; 34–39.
- É. Montpetit . A policy network explanation of biotechnology policy differences between the United States and Canada. Journal of Public Policy. 25 2005; 339–366.
- E.A. Moore . Science, internationalization and policy networks: Regulating genetically-engineered food crops in Canada and the United States, 1973–98. 1999; University of Toronto Press.
- E.A. Moore . The new agriculture: Genetically-engineered food in Canada. Policy and Society. 26 2007; 31–48.
- P.N. Mwale . Societal deliberation on genetically modified maize in Southern Africa: The debateness and publicness of the Zambian national consultation on genetically modified maize food aid in 2002. Public Understanding of Science. 15 2006; 89–102.
- P. Newell . Lost in translation? Domesticating global policy on genetically modified organisms: Comparing India and China. Global Society. 22 2008; 115–136.
- J. Niosi . Canada's regional innovation system. The Science-based industries. 2005; McGill-Queen's University Press: Montreal
- J. Niosi , M. Banik . The evolution and performance of biotechnology regional systems of innovation. Cambridge Journal of Economics. 29(3): 2005; 343–357.
- J. Niosi , T. Bas . The competencies of regions: Canada's clusters in biotechnology. Small Business Economics. 17 2001; 31–42.
- R.L. Paarlberg . Governing the GM crop revolution. Policy choices for developing countries. 2000; International Food Policy Research Institute: Washington, DC
- G. Paquet . Productivity and innovation in Canada: A case of governance failure. Policy Options. 2005, March–April; 38–42.
- P.W.B. Phillips , R. Wolfe . Governing food: Science, safety and trade. 2001; Queen's University School of Policy Studies: Kingston
- P.W.B. Phillips . The challenge of creating. Protecting and exploiting networked knowledge. E. Einsiedel . Crossing over: Genomics in the public arena. 2005; University of Calgary Press: Calgary
- P.W.B. Phillips . Governing transformative technological innovation who's in charge?. 2007; Edward Elgar: London
- D. Pickernell , P.A. Rowe , M.J. Christie , D. Brooksbank . Developing a framework for network and cluster identification for use in economic development policy-making. Entrepreneurship and Regional Development. 19 2007; 339–358.
- F. Seifert . Local steps in an international career: A Danish-style consensus conference in Austria. Public Understanding of Science. 15 2006; 73–88.
- R.R. Sharp , M.A. Yudell , S.H. Wilson . Shaping science policy in the age of genomics. Nature Reviews Genetics. 5 2004; 1–6.
- A.D. Sheingate . Promotion versus precaution: The evolution of biotechnology policy in the United States. British Journal of Political Science. 36 2006; 243–268.
- G. Stanley . Upgrading Canada's national innovation system: More than money required. Policy Options. July/August 2007; 68–74.
- K. Talukder , J. Kuzma . Evaluating technology oversight through multiple frameworks: A case study of genetically engineered cotton in India. Science and Public Policy. 35 2008; 121–138.
- R. Tutton . Constructing participation in genetic databases: Citizenship, governance, and ambivalence. Science, Technology and Human Values. 32 2007; 172–195.
- B. Van Beuzekom , A. Arundel . OECD biotechnology statistics – 2006. 2006; OECD: Paris
- Western Opinion Research, Inc. . Exploration of consumer understanding and attitudes toward genetic engineering-related food product label statements. Vol. 91 2005; Agriculture and Agri-Food Canada: Winnipeg and Vancouver