?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this work is to explore the possibility of using mixed culture of mesophilic sulfate-reducing bacteria (SRB) for retrieval of toxic and carcinogenic Cr (VI) from synthetic solution. In order to treat Cr (VI) containing wastewater effectively, SRB culture was adapted to 50 mg/L Cr (VI) and maintained through repeated sub-culturing to enhance the growth and activity of SRB. Batch biosorption experiments were carried out in glass serum vials by cultured SRB, accomplishing the removal of 82.1% Cr (VI), 76.9% sulfate, 85.7% COD under the following optimized conditions: pH 7, hydraulic retention time (HRT) 7 days, temperature 37 °C and initial Cr (VI) concentration of 50 mg/L. Further sorption experiments were conducted on synthetic wastewater under optimal operational conditions and resulted in 89.2% Cr (VI), 81.9% COD and 95.3% sulfate reduction from simulated wastewater. The results of this work contributed to a better understanding of metal uptake by biogenic sulfides and would be beneficial in the development of potential biosorbents that possess high capacities for Cr (VI) uptake from aqueous environments.
1 Introduction
In natural environment, chromium can exist in several oxidation states ranging from Cr (II) to (VI) among which trivalent Cr (III) and hexavalent Cr (VI) are the most dominant chromium species in the environment (CitationDogan et al., 2011; CitationVaiopoulou and Gikas, 2012; CitationCirik et al., 2013). The Cr (VI) compounds are used in metallurgical and chrome plating industries for chrome alloy and chromium metal production. It is used as an oxidizing agent and in the production of other chromium compounds in chemical industry. About 80–90% of leather tanning process utilizes chromium chemicals; out of which 40% of chromium used is discharged in the effluent as Cr (VI) and Cr (III) (CitationSaha and Orvig, 2010). Cr (III) salts are used less widely, yet are being employed in photography, textile dyeing, ceramics and glass industry. Cr (III) is required in trace amount for living organisms as it decreases body fat, cholesterol and triglyceride levels, activating enzyme reactions, and increasing the muscle mass (CitationDhal et al., 2013). Cr (VI) is not only highly toxic to all forms of living organisms but also mutagenic and carcinogenic in humans and animals (CitationLosi et al., 1994). The U.S. Environmental Protection Agency (EPA) has classified Cr (VI) as a Group ‘A’ human carcinogen. So due to its high toxicity, stringent regulations are applied on the discharge of Cr (VI) to surface water. The EPA has set a limit of 100 μg Cr(III) and 50 μg Cr (VI)/L for drinking water and a limit of ≤0.05 mg/L (CitationBaral and Engelken, 2002) for its discharge into surface water, while total chromium, including Cr (III), Cr (VI) and its other forms, should be lower than 2 mg/L (CitationZayed and Terry, 2003). So treatment of wastewater is one of the growing concerns in environmental cleaning. Conventional methods for treatment of metal contaminated wastewater are restricted, because of technical or economical constrains (CitationKiran and Kaushik, 2012; CitationDhal et al., 2013). Therefore, recent studies have concentrated on the development of low cost processes. The use of microorganisms including bacteria, fungi, algae and yeast has gained much more attention in recent years since they carry a wide range of binding sites for heavy metal ions (CitationMona et al., 2011; CitationKiran and Thanasekaran, 2012; CitationSingh et al., 2013). Cr (VI) reduction by biogenic sulfides is one of the major approaches used for detoxification of Cr (VI) containing wastewater. Cr (VI) can be reduced in biological treatment processes to Cr (III), which is almost insoluble and less-toxic chromium form (CitationAgrawal et al., 2006). The best way to reduce the mobility of heavy metals is to transform them into insoluble compounds such as sulfides, which are more stable forms. It has been reported that certain heavy metals such as Cr (VI) may be reduced under sulfate reducing conditions (CitationSingh et al., 2011; CitationSahinkaya et al., 2013; CitationCirik et al., 2013). Sulfate-reducing bacteria (SRB) under anaerobic conditions oxidize simple organic compounds (such as acetic acid and lactic acid) by utilizing sulfate as an electron acceptor and generate hydrogen sulfide. Hydrogen sulfide reacts with heavy metal ions to form insoluble metal sulfides that can be easily separated from solution (CitationSteed et al., 2000; CitationLuptakova and Kusnierova, 2002; CitationLloyd, 2003). The potential advantages of metal precipitation by biogenic sulfides include the production of lower sludge volumes and products with lower solubility as compared to hydroxide precipitation (CitationGadd, 2004). In addition, valuable metals can be recovered from metal sulfide sludges (CitationBoonstra et al., 1999). Several studies have reported process optimization, organic carbon (electron donor) consumption coupled with sulfate reduction, hydrogen production and the efficiency of metal precipitation in the biological treatment with biogenic sulfide (CitationBertolino et al., 2012; CitationZhou et al., 2013; CitationMartins and Pereira, 2013; CitationBai et al., 2013). In the present work, the effect of varying pH, temperature, metal dose and incubation time were optimized so as to attain high removal efficiency of Cr (VI) by SRB. Thus this effort provides optimum conditions for efficient removal of Cr (VI) using consortium of sulfate-reducing bacteria. These optimized parameters were further applied to investigate efficiency of biogenic sulfides produced from sulfate-reducing bacterial consortia in sequestration of chromium (VI), COD and sulfate to treat simulated wastewater in a small scale bioreactor.
2 Materials and methods
2.1 Growth media and conditions
A modified Postgate growth medium was used in all experiments. The composition of the Postgate growth medium used was as (g/L): KH2PO4 0.5; Na2SO4 1.0; NH4Cl 2.0; CaCl2 0.06; FeSO4 0.005; sodium citrate 0.3; yeast extract 0.1; sodium lactate 15 mL; Resazurin 0.1%. All incubations were done at 37 °C in the dark. All chemicals were of analytical grade, and solutions were prepared with sterile deionized water.
2.2 Source of inoculums
The mixed culture of SRB used in the present study was obtained from the sludge sample collected from anaerobic digester sludge of sewage treatment plant. One gram of the screened sludge was inoculated in small scale bioreactor (1000 mL) containing 800 mL CitationPostgate (1984) isolation selective medium. The composition of the Postgate isolation media was as (g/L): Na2SO4 1.0; KH2PO4 0.5; NH4Cl 1.0; CaCl2 0.7; FeSO4 0.1; yeast extract 1.0; agar 15.0; mercaptoacetic acid 0.1; ascorbic acid 0.1; ethanol (absolute) 4.5 mL; and Resazurin 0.1%. Reactor was kept in anaerobic conditions for the growth of anaerobic bacteria for a period of 7 days at 37 °C till the color of media changed to blackish gray. After 7 days of anaerobic incubation, the 200 mL inoculum of isolates was further transferred into 800 mL sterilized Postgate growth medium in another 1000 mL reactor. The Postgate medium is partially selective for SRB, with sodium lactate as electron donor and carbon source. The H2S generation and blackening of the medium were observed as positive indication for the presence of sulfate-reducing bacteria.
2.3 Batch experiments
Experiments were conducted in batch system under anaerobic conditions. Glass serum vials (120 mL) were used for all the experiments. 80 mL of modified Postgate medium with pH 7 was added in the vials for batch tests. After inoculation, the bottles were sealed with butyl rubber stoppers and aluminum crimp seals, and incubated at temperature 37 °C under static condition. Oxygen was replaced from the gaseous phase with nitrogen. Bacteria used in batch experiments were cultivated in closed serum vials using standard procedures for SRB (CitationPostgate, 1984). Subsequently the vials were sealed and 5 mL inoculum of bacteria cultivated in Postgate medium was added by a sterile syringe. The change in color from yellowish to colorless indicated the growth of the consortium (CitationSingh et al., 2011). All tests were performed in duplicates; average values were reported. Later optimized parameters were applied for treatment of synthetic wastewater. The reactors were kept in BOD incubator at optimized conditions for 7 days under static conditions. Sampling from the reactors was performed at 0 h and day 7 of incubation.
2.4 Analysis for various parameters
Liquid samples were centrifuged using a Hettich Rotofix 32 centrifuge at 3000 rpm for 10 min, prior to determination of COD, Cr (VI) and sulfate in the supernatant. Spectralab COD Digester (2015 M) and COD Titrator (CT-15) were used to determine soluble chemical oxygen demand in the supernatant. Total residual chromium was quantified by Atomic Absorption Spectrophotometer (Shimadzu AA-6300, Japan). The sulfate in the diluted sample was measured using standard methods of APHA-1995. pH was also measured according to the standard methods recommended by (CitationAPHA, 1995). The removal (%) of heavy metal was determined using the following equation:(1)
(1) where Ci is the initial heavy metal concentration; Cf is the final heavy metal concentration.
3 Results and discussion
Effectiveness of the process was evaluated through diagnosis of sulfate, metals ions and COD bioreduction in simulated wastewater. Formation of black precipitate is indicative of sulfide as a reduction of sulfate (SO42−) to sulfide (S2−) (CitationHerbert and Gilbert, 1984). In batch biosorption study, experiments were conducted for the optimization of different parameters such as pH, temperature, Cr (VI) concentration, and HRT. The results are explained in the following sections.
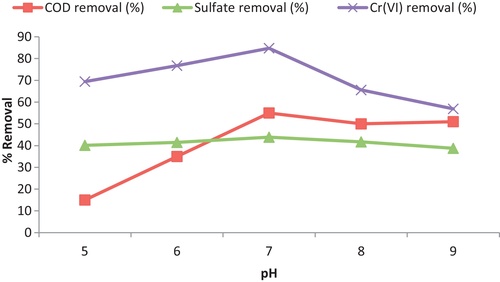
3.1 Effect of pH
The pH had a key effect on the biogenic sulfide ions in solution and effected the reduction of Cr (VI). The experiments were conducted at constant initial Cr (VI) concentration of 50 mg/L at temperature 37 °C, by varying pH from 5 to 9. As evident from the results, at pH 7, maximum COD removal was 55% on day 7. As shown in , there was a progressive increase in biosorption of Cr (VI) with increase in pH up to 7.0 and later decreasing trend of Cr (VI) removal was investigated with further increase in pH. The maximum removal percentage of Cr (VI) and sulfate was found to be 84.78% and 49.9% respectively at pH 7; then a reduction in efficiency of sulfate removal was observed above pH 7.0. These results are coincided with that found by CitationPagnanelli et al. (2012). They found that pH 7.6 was the optimum value for removal of Cr (VI) contaminated waters by sulfate-reducing bacteria. CitationBratkova et al. (2013) reported similar findings as maximum rate of sulfate reduction was achieved at pH 7.25. It is reported that optimum pH for growth of SRB is between pH 5.5 and 9 (CitationBarton, 1995). Mostly thermophilic bacteria grow near neutral pH from 6 to 8 but pH value exceeding the bounds is liable to cause deactivation of SRB activity (CitationWiddel, 1988; CitationZhao et al., 2011).
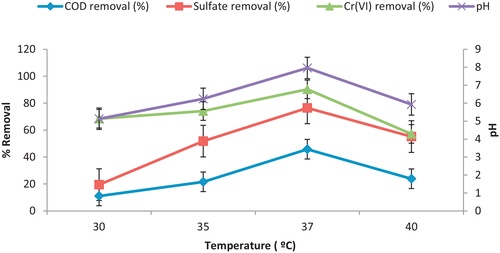
3.2 Effect of temperature
Optimum temperature was obtained for the isolated consortia by conducting experiments at temperature ranging from 30 to 40 °C for 7 days of incubation at constant initial metal ion concentration Cr (VI) of 50 mg/L. As shown in , maximum reduction in COD and sulfate was observed to be 45.8% and 76.5% respectively. The utmost removal of Cr (VI) was 90.3% at an optimum temperature of 37 °C and pH 7.9 but with further increase in temperature, the removal efficiency was decreased. Thus it can be concluded from the results that at 37 °C, maximum growth of SRB and maximum reduction in parameters such as COD, sulfate and metal ion concentration will be obtained. CitationZhou et al. (2013) outlined similar findings as 37 °C was found to be the optimum temperature for sulfate reduction and Cu (II) removal from wastewater.
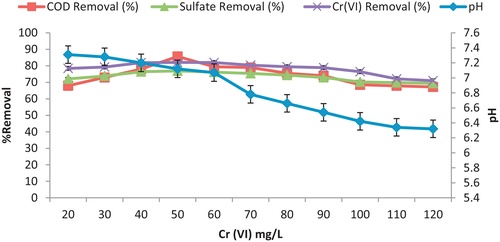
3.3 Effect of heavy metal dosing
Initially, increasing dose of chromium (VI) up to 50 mg/L increased the microbial growth indicated that chromium acts as essential nutrient for growth. Beyond 50 mg/L of Cr (VI), microbial growth was inhibited due to its toxicity. The activity and growth of bacteria were strongly affected by metals as it interferes with nucleic acids and enzyme active sites (CitationSani et al., 2001). Many enzymes and co-enzymes depend on a minimal amount of certain traces of metals for their activation and activity. When present in large amounts, they cause an inhibitory or toxic effect to micro-organisms (CitationLi and Fang, 2007). Thus 50 mg/L concentration was considered as the optimum metal loading dose for further experiments. The maximum metal and COD removal efficiency for Cr (VI) at 50 mg/L were determined to be 82.1% and 85.7% but it decreased with further increase of metal ion dosing as shown in . The maximum sulfate removal efficiency was observed to be 76.9% due to more H2S production but it decreased with further metal ion dosing beyond 50 mg/L. This may be due to the increase in the number of metal ion competing for available binding sites in the biosorbent and also due to lack of binding sites for the complexation at higher concentration level.
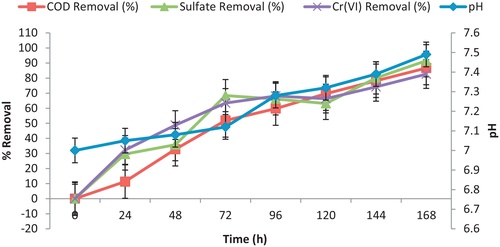
3.4 Effect of hydraulic retention time (HRT)
Hydraulic retention time (HRT) is the key factor that affects proficiency of bioreactors and has been reported extensively (CitationRockhold et al., 2002; CitationKaksonen et al., 2004; CitationSingh et al., 2011). A shorter HRT may not allow adequate time for SRB activity to neutralize acidity and precipitate metals. A longer HRT may imply depletion of either the available organic matter source or the sulfate source for SRB (CitationDvorak et al., 1992). The reactors were set up for 7 days at optimum conditions temperature 37 °C with sodium lactate as carbon source. As shown in , it could be concluded that pH of the reactor fluctuates between 7.0 and 7.49, which was optimum for the activity of the SRB. The continuous COD reduction was observed and 86.7% was achieved after 168 h of incubation period. Cr (VI) and sulfate removal were noticed to be 82.7% and 91.5%, respectively on day 7 of incubation period.
3.5 Treatment of simulated water under optimum conditions
The treatment of simulated wastewater was performed under the optimized condition of Cr (VI) 50 ppm, temperature 37 °C and pH 7 for 7 days. The two reactors each of 1000 mL capacity were used in this study for Cr (VI) removal from simulated wastewater. Out of these two reactors one was set as a control and was run parallel to check the removal efficiency without microbial growth (SRB). 100 mL SRB innoculum was added into the 900 mL mixture in 1:1 ratio (v/v) of Postgate growth media and wastewater in the reactors. The wastewater treatment study was carried out in duplicate and the mean value was reported. One reactor was inoculated with 100 mL SRB inoculum, while the second was kept as control having Postgate media only. The anaerobic conditions were created by maintaining all reactors air-tight. The reactors were coupled with aspirated bottles filled with labeled water level. Decreased water level in aspirated bottle indicated production of gas which was due to growth of SRB. The level of the water was continuously monitored while water level in control reactor remained the same. The reactors were kept in BOD incubator at optimized conditions i.e. temperature 37 °C with Cr (VI) 50 mg/L for seven days under static conditions. Sampling from reactors was performed at the time of incubation (0 hr) and after seven days. The increase in pH of effluent from 6.35 to 7.82 indicated growth of SRB due to formation of hydroxyl ions. At this pH, SRB were capable of removing 95.3% sulfate, 81.9% COD and 89.2% Cr (VI) from the simulated wastewater in an anaerobic bioreactor operated under alkaline condition with lactate as organic carbon source. The microorganisms present in the wastewater convert this sulfate into biogenic sulfides which helps in heavy metal precipitation.
4 Conclusion
The study concludes that the maximum removal of chromium was found to be 89.2% in simulated wastewater with initial 50 mg/L of Cr (VI) loading and sodium lactate as carbon source for 7 days of HRT. Treatment of synthetic wastewater under optimized conditions resulted in high removal efficiency for Cr (VI), sulfate and COD because SRB utilized organic substrate from wastewater as a carbon source. The obtained results confirmed that consortium of SRB was found suitable for development of an efficient and economic biosorbent for removal of sulfate and heavy metals from wastewater.
Conflict of interest
Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Notes
References
- A.AgrawalV.KumarB.D.PandeyRemediation options for the treatment of electroplating and leather tanning effluent containing chromium: a reviewMiner. Process. Extr. Metall. Rev.27200699130
- APHA (American Public Health Association) American Water Works Association (AWWA) Water Environment Federation (WEF)Standard Methods for the Examination of Water and Wastewaternineteenth ed.1995 Washington, DC
- H.BaiY.KangH.QuanY.HanJ.SunY.FengBioremediation of copper-containing wastewater by sulfate reducing bacteria coupled with ironJ. Environ. Manage.1292013350356
- A.BaralR.D.EngelkenChromium-based regulations and greening in metal finishing industries in the USAEnviron. Sci. Pol.52002121133
- L.L.BartonSulfate-Reducing Bacteria1995Plenum PressNew York/London150180
- S.M.BertolinoI.C.B.RodriguesR.Guerra-SaS.F.AquinoV.A.LeaoImplications of volatile fatty acid profile on the metabolic pathway during continuous sulfate reductionJ. Environ. Manage.10320121523
- J.BoonstraR.V.LierG.JanssenH.DijkmanC.J.N.BuismanBiological treatment of acid mine drainageR.AmilsA.BallesterBiohydrometallurgy and the Environment Toward the Mining of the 21st Century, Proceedings of the International Biohydrometallurgy SymposiumMadrid1999559567
- S.BratkovaB.KoumanovaV.BeschkovBiological treatment of mining wastewaters by fixed-bed bioreactors at high organic loadingBioresour. Technol.1372013409413
- K.CirikN.DursunE.SahinkayaO.CinarEffect of electron donor source on the treatment of Cr (VI)-containing textile wastewater using sulfate-reducing fluidized bed reactors (FBRs)Bioresour. Technol.1332013414420
- B.DhalH.N.ThatoiN.N.DasB.D.PandeyChemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a reviewJ. Hazard. Mater.250/2512013272291
- N.M.DoganC.KantarS.GulcanC.J.DodgeB.C.YilmazM.A.MazmanciChromium (VI) bioremoval by pseudomonas bacteria: role of microbial exudates for natural attenuation and biotreatment of Cr (VI) contaminationEnviron. Sci. Technol.45201122782285
- D.H.DvorakR.S.HedinH.M.EdenbornP.E.McIntireTreatment of metal contaminated water using bacterial sulfate reduction: results from pilot-scale reactorsBiotechnol. Bioeng.401992609616
- G.M.GaddMicrobial influence on metal mobility and application for bioremediationGeoderma1222004109119
- B.N.HerbertP.D.GilbertIsolation and growth sulphate reducing bacteriaMicrobiol. Methods Environ. Biotechnol.1984235253
- A.H.KaksonenJ.J.PlumbP.D.FranzmannJ.A.PuhakakaEffects of hydraulic retention time and sulphide toxicity on ethanol and acetate oxidation in sulphate reducing metal-precipitating fluidized-bed reactorBiotechnol. Bioeng.862004332343
- B.KiranA.KaushikEquilibrium sorption study of Cr (VI) from multimetal systems in aqueous solutions by Lyngbya putealisEcol. Eng.3820129396
- B.KiranK.ThanasekaranAn indigenous cyanobacterium, Lyngbya putealis, as biosorbent: optimization based on statistical modelEcol. Eng.422012232236
- C.L.LiH.H.P.FangInhibition of heavy metals on fermentative hydrogen production by granular sludgeChemosphere672007668673
- J.R.LloydMicrobial reduction of metals and radionuclidesFEMS Microbiol. Rev.272003411425
- M.E.LosiC.AmrheinW.T.FrankenbergerJr.Environmental biochemistry of chromiumRev. Environ. Contam. Toxicol.136199491131
- A.LuptakovaM.KusnierovaSulfate-reducing bacteria in biohydrometallurgyV International Conference Metallurgy, Refractories and EnvironmentVysoke Tatry2002199204
- M.MartinsI.A.C.PereiraSulfate-reducing bacteria as new microorganisms for biological hydrogen productionInt. J. Hydrogen Energy3820131229412301
- S.MonaA.KaushikC.P.KaushikBiosorption of reactive dye by waste biomass of Nostoc linckiaEcol. Eng.3710201115891594
- F.PagnanelliC.C.ViggiA.CibatiD.UccellettiL.ToroaC.PalleschiBiotreatment of Cr (VI) contaminated waters by sulphate reducing bacteria fed with ethanolJ. Hazard. Mater.199/2002012186192
- J.R.PostgateThe Sulphate-Reducing Bacteriasecond ed.1984Cambridge University PressUK
- M.L.RockholdR.R.YarwoodM.R.NiemetP.J.BottomleyJ.S.SelkerConsiderations for modelling bacterial-induced changes in hydraulic properties of variably saturated porous mediaAdv. Water Resour.252002477495
- B.SahaC.OrvigBiosorbents for hexavalent chromium elimination from industrial and municipal effluentsCoord. Chem. Rev.254201029592972
- E.SahinkayaN.DursunB.OzkayaA.H.KaksonenUse of landfill leachate as a carbon source in a sulfidogenic fluidized-bed reactor for the treatment of synthetic acid mine drainageMiner. Eng.4820135660
- R.K.SaniB.M.PeytonL.T.BrownCopper-induced inhibition of growth of Desulfovibrio desulfuricans G20: assessment of its toxicity and correlation with those of zinc and leadAppl. Environ. Microbiol.67200147654772
- R.SinghN.R.BishnoiA.KirroliaR.KumarSynergism of Pseudomonas aeruginosa and Fe0 for treatment of heavy metal contaminated effluents using small scale laboratory reactorBioresour. Technol.12720134958
- R.SinghA.KumarA.KirroliaR.KumarN.YadavN.R.BishnoiR.K.LohchabRemoval of sulphate, COD and Cr (VI) in simulated and real wastewater by sulphate reducing bacteria enrichment in small bioreactor and FTIR studyBioresour. Technol.1022011677682
- V.S.SteedM.T.SuidanM.GuptaT.MiyaharaC.M.AchesonG.D.SaylesDevelopment of a sulfate-reducing biological process to remove heavy metals from acid mine drainageWater Environ. Res.722000530535
- E.VaiopoulouP.GikasEffects of chromium on activated sludge and on the performance of wastewater treatment plants: a reviewWater Res.462012549570
- F.WiddelMicrobiology and ecology of sulfate- and sulfur-reducing bacteriaA.J.B.ZehnderBiology of Anaerobic Microorganisms1988Wiley InterscienceNew York469585
- A.M.ZayedN.TerryChromium in the environment: factors affecting biological remediationPlant Soil2492003139156
- C.Q.ZhaoQ.H.YangW.Y.ChenH.LiH.ZhangIsolation of a sulfate reducing bacterium and its application in sulfate removal from tannery wastewaterAfr. J. Biotechnol.1020111196611971
- Q.ZhouY.ChenM.YangW.LiL.DengEnhanced bioremediation of heavy metal from effluent by sulfate-reducing bacteria with copper–iron bimetallic particles supportBioresour. Technol.1362013413417