?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study presents an evaluation of Egyptian diatomite as a low cost adsorbent for the removal of heavy metal under different conditions (pH, weight of diatomite and contact time). The adsorption of heavy metals was investigated under various pH values ranged from 2 to 8 at 25 °C. The obtained results indicate that at low pH (2–4), the removal efficiency of diatomite for heavy metal increased slightly as the pH, adsorbent dose and contact time increased, while at pH > 4, the percentage of metal ions adsorbed decreased with increasing pH due to precipitation of heavy metals. At pH equal 4, with using 2 g L−1 of diatomite and 75 min as contact time, the maximum adsorption capacity of diatomite was obtained. The high adsorption capacity of diatomite makes it a suitable low cost material for the removal of different heavy metals from aqueous solutions.
1 Introduction
The removal of heavy metals ions from the environment is a big concern due to their association with health hazards for all living organisms due to its toxicity and non-biodegradable nature (CitationEl-Bayaa et al., 2009; CitationXue et al., 2009). The municipal and industrial solid wastes are another important source of pollution, which are considered a main source of the heavy metals like Hg, Cd, Fe, Mn, Pb and the treatment of these wastes must be agreed with the environmental standard before it is reused or returned to surface water (CitationAli and El-Sayed, 2017). There are many techniques have been developed such as chemical precipitation, reverse osmosis, ion exchange, and adsorption to remove heavy metals and other hazard materials from wastewaters (CitationShi et al., 2009). Among all of the previous techniques, the adsorption on clay and other chemical substances is considered to be a particularly more effective process specially by using low cost and environment friendly martial for the removal of heavy metals from industrial wastewaters and aqueous solutions (CitationLin and Juang, 2002; CitationSwayampakula et al., 2009).
Table 4 Removal efficiency of different heavy metals concentrations using diatomite clay (1 g L−1).
The most common sorption methods are using of natural clay minerals and activated carbon which has high metal adsorption capacity (CitationAyala et al., 2008). Although activated carbon is effective in the removal of metal ions from wastewater, it is expensive and requires chelating agents to enhance its performance, thus increasing treatment cost (CitationOliveira et al., 2005). Some researchers have focused on using low cost, highly efficient sorbents for pollutants, and the sorption behavior of several natural materials and chemical products has also been studied (CitationIjagbemi et al., 2009). Some of these materials are clay minerals (CitationSolener et al., 2008), agricultural bi-products, some aquatic plants, and microorganisms (CitationIjagbemi et al., 2009). Most of these investigations have shown that natural materials can act as good sorbents for hazard materials including heavy metals (CitationAli and El-Sayed, 2017), while the types and availability of clay minerals also are important, because they are involved in choosing which pollutants will be treated. Egypt has huge reserves of diatomite deposits in Gebel Elow El Masakheet at south west El-Fayoum Governorate. These deposits vary in the quality grade from high, moderate to low (CitationIbrahim and Selim, 2011). The diatomite deposits of the El-Fayoum area are fine and regular in lamination developed through seasonal fluctuation of sedimentation. The deposits are overlain in some parts by detrital materials such as blown sands and semi-consolidated sandy clay. Because diatomaceous deposits occur in the form of several disconnected outcrops extending over about 30 km from Qasr Elsagha to Kom Oshim in the east, some authors concluded that they were formed in separate lakes.
Diatomite is a siliceous sedimentary rock composed of an amorphous form of silica (SiO2·nH2O) containing a small amount of microcrystalline material. It has a unique combination of physical and chemical properties such as high porosity, high permeability, small particle size, large surface area, and low thermal conductivity. Due to these properties the diatomite has been successfully used for cleaning drinking water or industrial and sewage waste water. Sometimes it is blended with aluminum chloride or ferric chloride to enhance the filtration processes (CitationMarquez et al., 2004; CitationQdais and Moussa, 2004).
The removal of hazardous contaminants from wastewater using natural zeolite was performed by CitationAli et al. (2017). Their results indicate that, the uptake of clinoptilolite for ammonium was ranged from 70 to 92%, while it ranged from 70 to 99% for heavy metals at various conditions. The removal efficiency of zinc from aqueous solution with different adsorbents was investigated by CitationBhattacharya et al. (2006), the obtained results showed that the adsorption percentage of zinc increased by increasing the concentration of the adsorbents to reach maximum uptake at 98% and pH between 5 and 7.
This study aims to investigate and evaluate the removal capacities of diatomite for some metal ions such as aluminum (Al+3), barium (Ba+2), cadmium (Cd+2), chromium (Cr+3), copper (Cu+2), iron (Fe+2), lead (Pd+2), manganese (Mn+2), nickel (Ni+2), and zinc (Zn+2). The adsorption experiments were performed in laboratory, using metal ions initial concentration, pH of solution, and contact time as variables.
2 Materials and methods
2.1 Identification of clay
Diatomite sample was obtained by the Egyptian Mineral Resources Authority from Kom Oshim in the east of El-Fayoum Governorate (). The diatomite sample was crushed using roller mills and the small sizes were selected to be prepared for the batch study, while the large size was used for physical and chemical identification. The characterizations of diatomite were performed using X-ray (model X Pert PRO). All the previous analysis were done including electroscaning microscope (ESM) at the Laboratories of The Egyptian Mineral Resources Authority.
2.2 Diatomite preparation for the study
A representative sample of diatomite was prepared for the batch experiment by washing with distilled water to remove any non-adhesive impurities and then, dried in oven at 80 °C for 24 h to remove any moisture. The diatomite sample was grinded to 0.25–0.5 mm size to be used for the batch study (CitationReddy and Dastgheibi, 2014).
2.3 Heavy metals preparation
Metals stock standard of multi element (Al, Ba, Cd, Cr, Cu, Fe, Pb, Mn, Ni and Zn, 1000 mg L−1 Merck Brand, product ID: 1.11355.0100-100 mL), has been used to prepare various concentrations of heavy metals (0.5, 1, and l0 mg L−1) with deionized water (CitationAPHA, 2012). The desired pH varying from 2 to 8 were obtained by adding negligible drops of sodium hydroxide (NaOH) or hydrochloric acid (HCl) to the solution.
2.4 Batch study
The experiments were performed in duplicate, while the blank and other concentrations of heavy metals (1.0, and 10 mg L−1) were measured as the quality control samples.
The equilibrium state for removing of heavy metals by diatomite clay was obtained by adding the constant weight of absorbent (0.5, 1.0 and 2.0 g) to 100 mL of initial concentrations of prepared aqueous solution of heavy metals (0.5, 1, and 10 mg L−1), with constant agitation shaking at100 rpm and 25 °C to enhance the interaction of the diatomite particles and heavy metals solution (CitationLasheen et al., 2017). It was observed that the equilibrium between absorbed heavy metals ions and adsorbent was after 2 h (the heavy metals concentrations were measured for all samples after every 15 min until reach to equilibrium state at which the heavy metals uptake is constant with insignificant changes). However, to ensure that the absorption process reached to complete equilibrium the experiment was kept for 4 h. After that the samples were filtered using filter paper and the concentration of different heavy metals ions were measured by ICP-MS (model NexIon 300D), and the removal was calculated using Eq. (Equation1(1)
(1) ):
(1)
(1)
where C0 is the initial concentration of heavy metals ions (mg L−1) and Ce is the equilibrium concentration of the metal ions (mg L−1).
3 Results and discussion
3.1 Characterizations of Egyptian diatomite
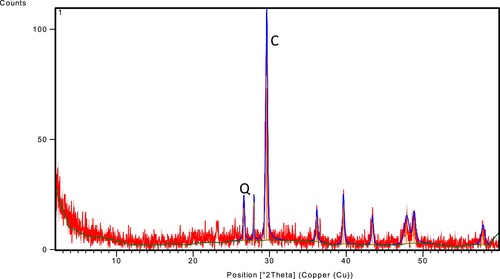
The chemical composition of diatomite plays an important role in metals removal. In this study, the physical properties of the Egyptian diatomite is presented in , while the X-ray diffraction analysis indicates that diatomite contains calcite, quartz and clay minerals as shown in and . presents the electroscaning microscope (ESM) for the studied diatomite particles.
Table 1 The physicochemical properties of Kom Oshim diatomite at El Fayoum Governorate.
Table 2 The chemical characteristics of the natural sorbent.
The presence of calcite gives an advantage to diatomite as adsorbent because, it is widely used as sorbent material by formation of metal hydroxide or metal carbonate as reported by CitationAl-Wakeel (2009), and also quartz is one of the most common minerals found in the earth's crust. Its structure shows unique physicochemical characteristics therefore it can be used as sorbent substance (CitationIbrahim and Selim, 2011). In this study, it was found that the formation of metal hydroxide and silicone dioxide due to presence of calcite and quartz respectively leads to increase the metals uptake properties of diatomite.
3.2 Effect of pH
The adsorption capacity of heavy metals on the adsorbents surface is controlled by the pH of aqueous solution. Therefore, the effect of various pH values at 25 °C was investigated in the current study. The batch experiment was conducted in acidic conditions (pH = 2) to ensure adsorption between diatomite particles and pollutants, due to in alkaline media heavy metals start to precipitate (forming metal oxides and hydroxides), and also in higher pH conditions the diatomite particles is slightly unstable as a results of presence of silica that can be precipitate or dissolve in alkaline condition. So the batch experiments were conducted at pH equal to 2, to keep and preserves the chemical stability of the diatomite. The pH of the solution influence the chemical groups on to the adsorbent surfaces, where the following equations describe the process (CitationSud et al., 2008):
(2)
(2)
(3)
(3)
(4)
(4)
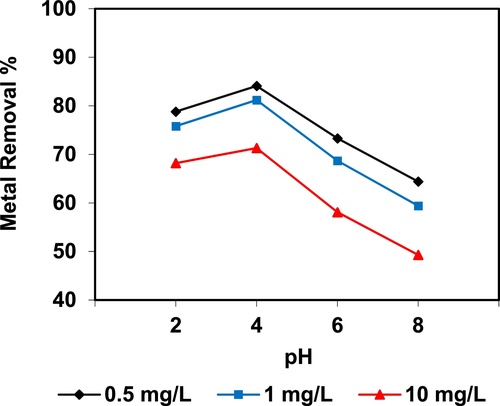
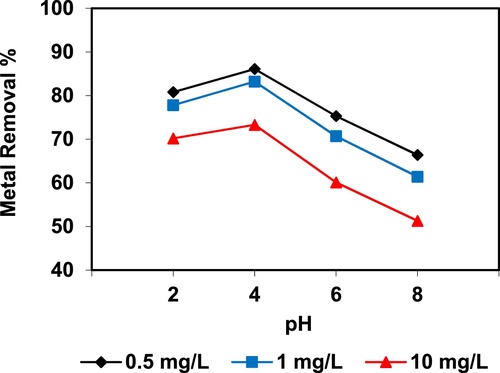
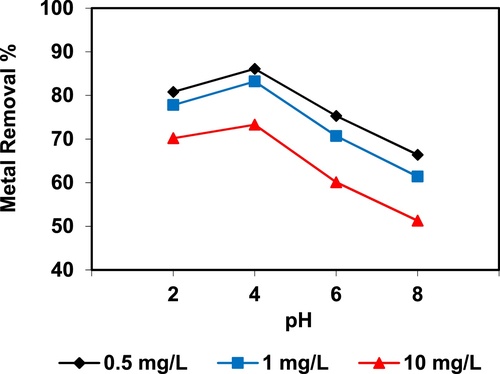
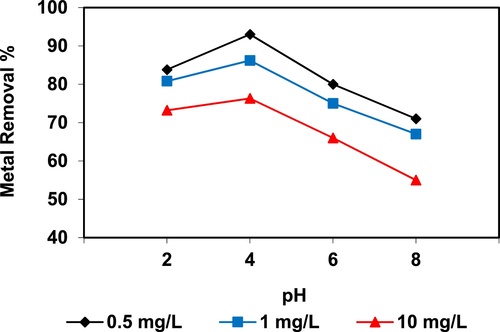
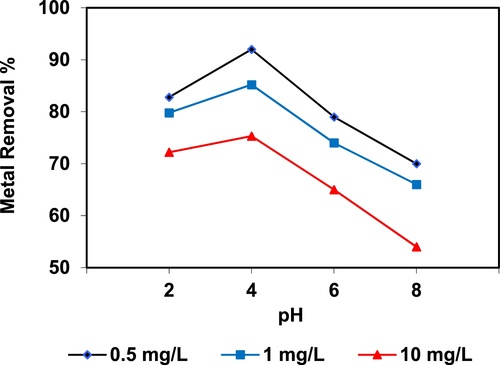
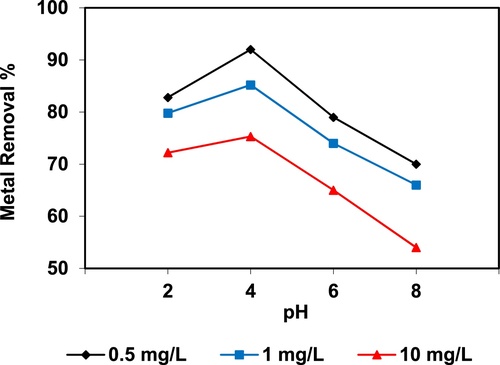
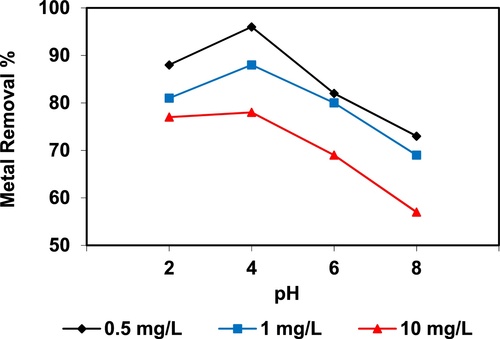
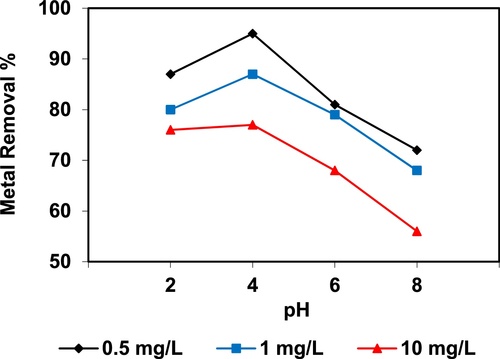
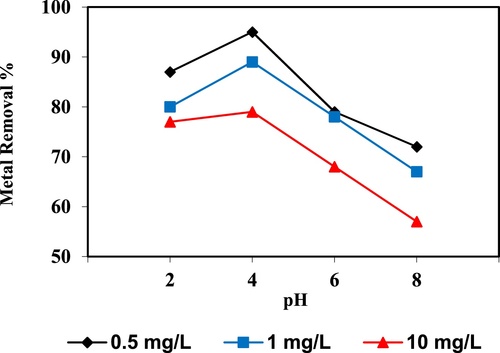
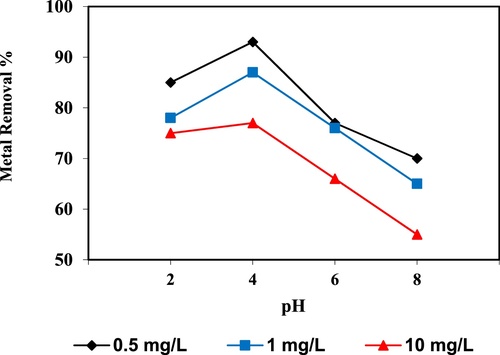
In the current study, the removal of metal ions as a function of pH is illustrated in – and –. The figures displayed a general trend of increasing heavy metals removal with increasing pH values until maximized at pH = 4. The results indicate that the slow metal uptake shown by diatomite at low pH values (pH = 2), which can be explained by increasing the positive charge (protons) density on the surface sites and thus, electrostatic repulsion occurred between the heavy metal ions (M2+) and the edge groups with positive charge (Si–OH2+) on the surface of diatomite, which is agreed with by CitationJohnson and Hallberg (2005), while when pH of the solution varies from 2.0 to 4.0, the adsorption efficiency is increased, because the heavy metals solution is exposed to the negative charges on the surface of diatomite and strongly attracting the metal ions. The highest adsorption percentage for heavy metal ions was observed at pH equal to 4. According to the obtained data, diatomite is the efficient clay with the selectivity sequence given as Pb > Mn > Ni > Zn > Cr > Fe > Cu > Cd > Ba > Al, due to the correlation between the ionic radii of heavy metals with external and internal pore diameters of diatomite. The obtained results were agreed with the study performed by CitationYusan et al. (2012) for using diatomite as adsorbent for heavy metals.
Table 3 Removal efficiency of different heavy metals concentrations using diatomite clay (2 g L−1).
Table 5 Removal efficiency of different heavy metals concentrations using diatomite clay (0.5 g L−1).
3.3 Effect of adsorbent dosage
Adsorbent dosage is an important parameter because of its strong effect on the capacity of the diatomite at a given initial concentration of the pollutants. The effect of various doses of diatomite (0.5, 1.0 and 2 g L−1) on heavy metals removal was studied in the current study at 25 °C and the results are listed in –. The obtained data regarding removal efficiency of heavy metals is increased from 55.2% to 99.7% by increasing the amount of the adsorbent between 0.5 and 2 g L−1, this is because at low diatomite content, all surface sites are exposed for metal ions and the surface reaches saturation faster, which means that the adsorption of metals had the highest values using 2 g L−1 of the diatomite. Regarding the data, the removal efficiency was recorded 99.7% for Cr, Pb and Zn, while the lowest value was recorded for Fe 99% using 2 g L−1 of the adsorbent. Therefore, the amount of 2 g L−1 was selected as the optimum dosage of the used diatomite under study. CitationBilgin and Tulun (2015) studied the removal of lead ions from wastewater using diatomite. The maximum obtained removal efficiency was 98.09% by using 1.5 g L−1 diatomite for 20 min, which is close to the results of the current study.
3.4 Effect of contact time
The contact time is important factor that influence the adsorption of heavy metal ions onto diatomite. During the current study, the batch experiment investigate the effect of contact time on interaction between diatomite particles and metal ions at 25 °C. The results are presented in –. The obtained results indicate that the removal percentage started rapid and then became slower which can be explained by, at the beginning of the batch experiment, the large surface area of diatomite is ready to interact with heavy metals (the adsorption capacity was high because all sites on the adsorbent were vacant and heavy metals concentration was high) however with time the uptake rate was slowed due to reduction in sorption sites. As soon as the surface of adsorption sites became filled with metal ions, the uptake rate slightly decreased and controlled by the ability of metal ions can moved from outside to inside sites of the diatomite particles (CitationYu et al., 2000).
In this study, removal efficiency for Ba, Cd, Pb, Mn, Ni and Zn indicates that it remained almost unchanged during the experiment when the time increased from 15 to 70 min and recorded (99%–99.7%). The results revealed that the optimum contact time for heavy metals removal is at 75 min, after that the adsorption percentage of metal ions gradually decreased with increase in contact time. The obtained results are agreed with that found by CitationRiza et al. (2010); CitationSheng et al. (2009) and CitationVarank et al. (2014) which investigate the removal of heavy metal ions from aqueous solutions by natural adsorbant.
4 Conclusions
Natural diatomite collected from El-Fayoum area has been investigated for the removal of different metal ions from aqueous solutions. The batch study was preformed at various conditions such as pH, adsorbent dose and contact time. The maximum percent removal of many heavy metal ions were observed at pH 4 and significantly decreased at higher pH values, while metal ions removal is positively influenced by an increase in the adsorbent dosage and contact time.
5 Recommendation
Diatomite rocks are already found in governorate of El-Fayoum, South West of Cairo with large amount, while the present study give an evidence that using of diatomite as low cost adsorbents for hazard material uptake from wastewater is efficient, so it is recommended to use diatomite particles on large scale as a single unit in a treatment plant for industrial wastewater, and then the treated water must be compared with the international guidelines to check its ability to be reused.
Acknowledgements
This research was performed through using of ICP-MS (model NexIon 300D) funded by Science & Technology Development Fund (STDF), project code 5186/2013.
Special appreciation and thanks to Dr. Mohamed Ramadan (The Egyptian Mineral Resources Authority) who bring the diatomite from El-Fayoum Governorate and helped me well to prepare it for this study. I would especially like to thank Prof. Dr. Maha Ali (Deputy Director and Operation Manager of CLEQM) you have been a tremendous mentor for me.
Notes
Peer review under responsibility of National Water Research Center.
References
- M.M.AliE.E.El-SayedM.Z.KamelRemoval of hazardous contaminants from wastewater using natural zeoliteJ. Water Res. Photon1382017333347
- M.M.AliE.E.El-SayedCapability of natural bentonite for removing organic and inorganic pollutants from wastewaterJ. Water Res. Photon1382017361370
- M.I.Al-WakeelCharacterization and process development of the Nile diatomaceous sedimentInt. J. Miner. Process.922009128136
- APHA (American Public Health Association) 2012: Standard Methods for Examination of Water and Wastewater, 22th ed. Washington, D.C. 3125–B.
- J.AyalaJ.L.VegaR.AlvarezJ.LoredoRetention of heavy metal ions in bentonites from Grau Region (Northern Peru)Environ. Geol.53200813231330
- A.BhattacharyaS.MandalS.DasAdsorption of Zn (II) from aqueous solution by using different adsorbentsChem. Eng. J.123120064351
- M.BilginS.TulunUse of diatomite for the removal of lead ions from water: thermodynamics and kineticsBiotechnol. Biotechnol. Equip.2942015696704
- A.El-BayaaN.BadawyE.A.AlKhalikEffect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineralJ. Hazard. Mater.1702200912041209
- S.S.IbrahimA.Q.SelimEvaluation of Egyptian diatomite for filter aid applicationsPhysicochem. Probl. Miner. Process.4720112011113122
- C.O.IjagbemiM.H.BaekD.S.KimMontmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutionsJ. Hazard. Mater.16612009538546
- D.B.JohnsonK.B.HallbergAcid mine drainage remediation options: a reviewSci. Total Environ.3382005314
- M.R.LasheenI.Y.El-SherifS.T.El-WakeelD.Y.SabryM.F.El-ShahatHeavy metals removal from aqueous solution using magnetite Dowex 50WX4 resin nanocompositeJMES822017503511
- S.H.LinR.S.JuangHeavy metal removal from water by sorption using surfactant-modified montmorilloniteJ. Hazard. Mater9232002315326
- G.MarquezM.RibeiroJ.VenturaJ.LabrinchaRemoval of nickel from aqueous solutions by clay-based bedsCeram. Int.3012004111119
- E.OliveiraS.MontanherA.AndradeJ.NobregaM.RollembergEquilibrium studies for the sorption of chromium and nickel from aqueous solutions using raw rice branProcess Biochem.4011200534853490
- H.A.QdaisH.MoussaRemoval of heavy metals from wastewater by membrane processes: a comparative studyDesalination16422004105110
- K.R.ReddyT.X.S.DastgheibiRemoval of heavy metals from urban stormwater runoff using different filter materialsJ. Environ. Chem. Eng.22014282292
- A.K.RizaI.AlacabetN.C.KilicRemoval of metal ions from aqueous solution by diatomiteHacettepe J. Biol. Chem.38220108593
- G.ShengS.WangJ.HuY.LuJ.LiY.DongX.WangAdsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperatureColloid Surf. A: Physicochem. Eng. Asp.33912009159166
- W.Y.ShiH.B.ShaoH.LiM.A.ShaoS.DuProgress in the remediation of hazardous heavy metal-polluted soils by natural zeoliteJ. Hazard. Mater.1701200916
- M.SolenerS.TunaliA.S.OzcanA.OzcanT.GedikbeyAdsorption characteristics of lead(II) ions onto the clay/poly(methoxyethyl)acrylamide (PMEA) composite from aqueous solutionsDesalination22312008308322
- D.SudG.MahajanM.KaurAgricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—a reviewBioresour. Technol.9914200860176027
- K.SwayampakulaV.M.BodduS.K.NadavalaK.AbburiCompetitive adsorption of Cu(II), Co(II) and Ni(II) from their binary and tertiary aqueous solutions using chitosan-coated perlite beads as biosorbentJ. Hazard. Mater.17022009680689
- G.VarankA.DemirM.S.BilgiliS.TopE.SekmanS.YaziciH.S.ErkanEquilibrium and kinetic studies on the removal of heavy metal ions with natural low-cost adsorbentsEnviron. Protect. Eng.40320144361
- Y.XueH.HouS.ZhuCompetitive adsorption of copper(II), cadmium(II), lead(II) and zinc(II) onto basic oxygen furnace slagJ. Hazard. Mater.16212009391401
- B.YuY.ZhangA.ShuklaS.S.ShuklaK.L.DorrisThe removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copperJ. Hazard. Mater.80120003342
- S.YusanC.GokS.ErenturkS.AytasAdsorptive removal of thorium(IV) using calcined and flux calcined diatomite from Turkey: evaluation of equilibrium, kinetic and thermodynamic dataAppl. Clay Sci.672012106116