?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Niger Delta region of Nigeria is faced with the problem of drinking water supply. Different methods of water purification were compared and reverse osmosis was considered a choice method for the experiment. Among the region’s brackish water reserves with heavy metal contamination, nickel and cobalt were considered. Model solutions of nickel and cobalt were prepared to mimic the brackish water contamination levels and were purified with cellulose acetate reverse osmosis membrane. Experimental results showed that purifications were more dependent on the overall salt concentrations and not on individual components.
1 Introduction
The Niger Delta region of Nigeria is facing a challenge of portable water supply. The region is bounded by the Atlantic Ocean to the south and has a huge network of water sources and vast reserves of oil and gas. In 2006, the population of the entire region was 31,277,901 people (CitationNational Population Commission, 2010). A large part of this population is widely distributed along coastal communities and disconnected locations with challenging terrain to build water supply infrastructures.
Drilling of boreholes and traditional dug wells to access underground water is meeting a new phase of challenge. Some areas are experiencing an increase in the salt content of their drilled water. This could be attributed to the nature of water aquifers and the rate of exploitation, which results to a reduction in thickness and the effective head of the freshwater wedge leading to salt water intrusion into fresh water aquifers (CitationNwankwoala and Ngah, 2014).
Besides the intrusion of salt water, the indiscriminate discharge of refuse, untreated industrial waste and the proximity of sceptic tanks to drinking water sources are some of the problems faced by the region. It is assumed that all the water sources in the region are contaminated or have the tendency of being contaminated in the future.
Analysis of 140 groundwater samples in different locations from January 2009 to November 2011 conducted by CitationAmadi et al. (2004) confirmed that several samples did not meet the national drinking water standard. Results showed that several samples were contaminated biologically and with heavy metals. Analysis of heavy metal content in a petroleum imparted and non-petroleum imparted river in the region (CitationOwamah, 2013), as shown in and , attests to the prevalence of heavy metals exceeding required concentrations.
Table 1a Concentration of selected heavy metals in water from petroleum imparted river (Warri River) in the Niger Delta region during the dry and rainy season, and corresponding Nigerian standard for drinking water (CitationNigeria Industrial Standard, 2010).
Table 1b Concentration of selected heavy metals from non-petroleum imparted river (Ikpoba River) in the Niger Delta region during the dry and rainy seasons, and corresponding Nigerian standard for drinking water (CitationNigeria Industrial Standard, 2010).
Availability of data from other researched works also corroborate and on the level of water contamination in the Niger Delta region (CitationEmuedo et al. 2014; Etim et al. 2013; Tubonimi et al. 2010).
2 Sources of water pollution
The dominant industry in the Niger Delta region is the petroleum industry. The country’s crude oil contains heavy metals and contamination of water sources with hydrocarbons and heavy metals in part is associated with this industry.
There have been hypothesis on the geological formations that leads to the prevalence of heavy metals. Metals such as nickel and vanadium have been associated with crude oil formation from derivatives of algae and bacteria (CitationBarwise, 1990). Other heavy metal could be as a result of geological activities and mineral accumulation during rock formation and chemical compounds used in drilling crude oil.
Drilling fluid is mainly formulated with heavy metals and corrosion inhibitors. Despite assurances of compliance with regulated requirements, both the handling of these fluids and disposals barely meet the regulatory requirements. The contributions of drilling fluid on the contamination of oil and water sources could be considered remarkable. This is coupled with the frequently recorded accidents and oil spills associated with the oil industry in Nigeria.
An analysed crude oil sample from the Niger Delta, as shown in , shows the presence of heavy metals (mg/l) (CitationAhmad et al. 2010). Blends of various samples taken from other oil fields also attest to the prevalence of heavy metal content in Nigerian crude oil (CitationUdeme and Etim, 2012).
Table 2 Heavy metal content of crude oil sample from the Niger Delta region of Nigeria.
A detailed assessment on the impact of oil spills conducted by the United Nations Environment Programme in Ogoni kingdom of the region revealed that both soil and water were heavily contaminated (CitationUNEP, 2011). Several water samples taken from underground dug well were certified unsafe for drinking. Surface water samples obtained from rivers and lakes were very unsafe. Such contaminations is applicable to several parts of the Niger Delta region.
The continuous spill of crude oil increases the heavy metals and hydrocarbons in water sources, thereby lending importance to this work aimed at finding a better method of water purification from the vast reserves of brackish water.
3 Water purification
Purification methods that would be considered in the Niger Delta region would be concerned with desalinations due to the large reserves of brackish water and fresh water with heavy metal contamination.
Most widely used methods for desalting brackish and salt water are: distillation, electrodialysis/ion exchange, and reverse osmosis. Each of these process has its advantages and disadvantages. While some processes can remove dissolved ions effectively it may not remove organics and vice versa. Others may remove both, but may require continuous monitoring and eventual pretreatment of the influent to keep the process afloat.
Although membrane and distillation processes equally share current desalination production capacity, RO has emerged as the leader in future desalination installations (CitationGreenlee et al., 2009).
Review of capital and operational cost of desalination processes by CitationSeminat (2002) shows a range of overlapping economic benefits for some technologies, with reverse osmosis maintaining a good advantage. Reverse osmosis is ranked among the most appropriate (CitationMohsen and Al-Jayyousi, 1999) technology followed by electrodialysis.
Currently, Nigeria, especially its remote and disconnected communities, is faced with lack of constant electricity and low technical personnel. In connection with this, the choice for desalination would be dictated by the water treatment method of low power consumption, cost, simplicity of the process and easy maintenance, which is achieved using ion exchange and reverse osmosis purification methods.
The Niger Delta region is facing the challenge of environmental degradation. Having produced portable water that meets drinking standards, the disposal of the effluent could add additional cost if treated before disposal. The reverse osmosis system could be designed with recovery rates that will allow easy disposal of the effluents untreated.
More so, the RO system could be modularized to serve disconnected communities.
A comparison of an ion exchange and reverse osmosis methods of purification which have close principles of operation, makes reverse osmosis a choice method (CitationBeardsley et al., 1994).
4 Experimental procedure
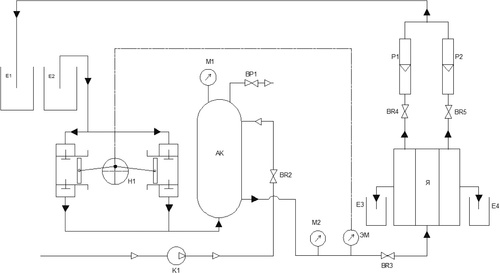
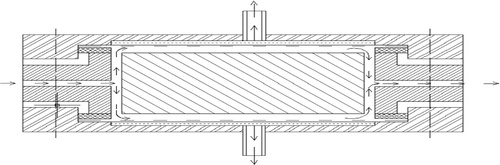
The experiment was carried out on a reverse osmosis pilot plant (). The membrane unit is made of a rectangular chamber with two compartments. This unit is piped with influent uniformly distributed on both sides of the membrane surfaces, while purified solutions exit on both sides of the compartment.
The reverse osmosis membrane used in the experimental process was a cellulose acetate membrane tagged МГА-95П. The membrane has the following characteristics: selectivity based on 0.5% NaCl—95%; maximum working pressure—5 MPa; productivity (at 25 °C)—33 dm3/m2h; pH range—3–8 (retrieved from http://www.vladipor.ru/catalog/show/%26cid=006%26id=1).
4.1 Solution preparations
Water composition in several parts of the Niger Delta region is brackish with heavy metal contamination. Nickel and cobalt were considered and solutions were prepared to mimic brackish water with different contamination levels of nickel and cobalt.
Nickel solution was prepared from a high grade Nickel sulphate 7-water (NiSO4·7H2O), and cobalt solution was prepared from cobalt(II) chloride 5-water (CoCl2·5H2O). Sodium chloride was obtained from high grade sodium chloride salt. Chlorine concentrations in cobalt(II) chloride 5-water and chlorine from sodium chloride salt were maintained at concentrations not exceeding brackish water concentration level.
The first batch of samples were made of NiSO4 with a concentration range of (1.1, 2.2, 3.3, 4.4 mg/l) and CoCl2 (0.1, 0.4, 0.8, 1.2 mg/l). The second batch of samples were made of both CoCl2 and NiSO4 and were also prepared in four different concentrations, while the third samples were made of sodium chloride concentration in the range of brackish water with CoCl2 and NiSO4. Concentrations of the various compounds were determined through their electrical conductance and corresponding charts.
4.2 Process description
The process set-up as shown in , is made of the following units: 1) two containers E1 (for receiving unrecovered solution) and E2 (containing prepared samples) with a capacity of 20 l. 2) A pump unit denoted with H1. 3) A compressor K1. 4) Accumulator AK. 5) A membrane cell unit я with two receivers E3 and E4 attached for purified water. 6) A rotameter P1 and P2. 7) Valves denoted with BR.
The plant was powered and the accumulator valves were opened to allow the flow of compressed air. The compressor was turned on and air compressed into the hydro accumulator. Half of the working pressure was achieved through this compression. When the required pressure was attained, the valve (BR2) was closed and the compressor switched off.
Valve BR3 meant for fluid flow from the pump was opened and the pump was switched on. The solution flowed through the plunger of the pump to the membrane unit comprising of two chambers coupled together. Each half of the cell is rectangular with a dimension of 13 × 6 mm. As shown in , the chamber has a surface that is served with a central-like liquid distributor that effectively splashes the influent on both side of the chamber. On both side of the cell are placed with reverse osmosis membranes with an opening for purified solution (permeates) to flow out of the cell.
The fluid flowed through the membrane unit and the unrecovered solution is collected at E1. For each prepared solution, the entire procedure was observed.
5 Results
Effectiveness of the reverse osmosis separation was monitored base on the selectivity and permeability of the membrane, which was estimated from the amount of water recovered as permeate.
The selectivity of the membrane for each of the solution was determined from the formula below:
where φ—membrane selectivity. Cper—permeate concentration. Cfeed—feed concentration.
Productivity of the membrane was determined from the volume of water recovered from each solution per surface area of membrane per time. The productivity was calculated from the equation below:
where G—productivity, m3/m2s. V—volume of permeate, m3. F—surface area of membrane, m2. τ—time s.
Results of experiment for one-component-model solution (CoCl2 and NiSO4) are presented in . The membrane selectivity reduced with increase in pressure and concentration. At low pressure of 1.5 MPa, the membrane recorded the highest selectivity of 98.8% for CoCl2 and 99.3% for NiSO4. The cellulose acetate membrane is good at rejecting nickel sulphate compared with CoCl2. However, the membrane recorded higher productivity for cobalt(II) chloride solution at high pressure and low concentration. Flux for nickel compound had a minimal decrease compared with other compounds (CitationBakalar et al., 2009).
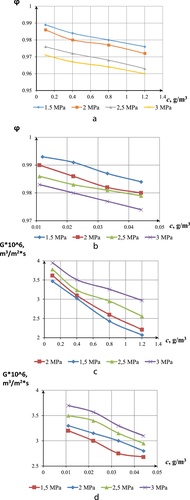
Experiment for solution with two-component-model comprising of cobalt(II) chloride and nickel sulphates are presented in . It involved fixing the concentration of one component while varying the other. Selectivity and productivity were determined at 3 MPa. The membrane recorded the highest selectivity of 98.84% and productivity of 3.49 at low TDS of 1.2 mg/l and at low concentrations of cobalt(II) chloride.
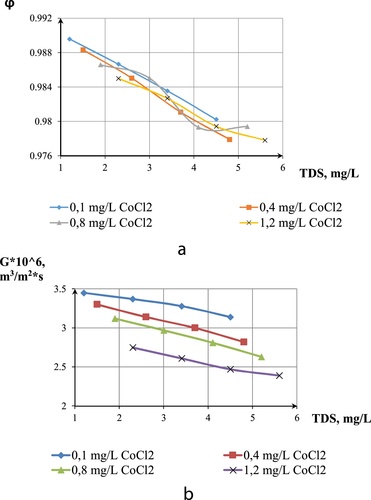
The selectivity for one-component solution and two component solutions showed no remarkable difference even at low to high TDS. Differences were only observed on the membranes productivity. At 3 MPa, the membrane productivity for nickel was 3.95, cobalt—3.7, cobalt and nickel solution—3.45 and three-component solution-2.7 × 106, m3/m2 s. Membrane productivity has shown to reduce in the presence of many salts.
The third-model solution of brackish water was prepared with the following concentrations: NaCl (5000 g/m3) + CoCl2 (0.4 g/m3) + NiSO4 (3 g/m3). The degree of separation was also estimated by electrical conductivity of the solution.
As shown in , an increase in the operating pressure increases salt passage on the membrane, hence the low electrical conductivity of permeate at low pressures. High productivity of the membrane was also achieved at high pressures for the three component-model solution.
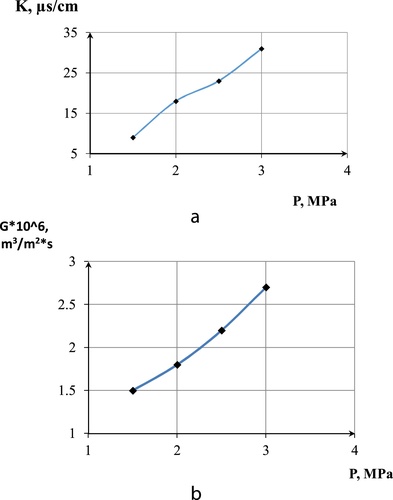
Variations with normal saline water were not remarkable, as results obtained in related research (CitationFeini et al., 2008) showed the same pattern of membrane rejections at various pressures.
Cellulose acetate membranes are known with lower salt rejection and structural compaction at high operating pressure. However, for single and many component solution, the membrane showed high selectivity sufficient for applications in practical purposes, compared to the productivity of different solutions.
6 Conclusions
CitationCellulose acetate reverse osmosis membrane showed good performance in the removal of cobalt and nickel. However, increase in pressure reduces the selectivity and increases permeate. From one-component up to the three-component samples, the membrane purified the samples based on the overall salt concentrations. Reverse osmosis ‘membranes are produced base on sodium chloride salt. However, solutions with unbalanced ion concentrations may have influence on the selectivity and productivity of the membrane.
Nickel and cobalt are transition elements and behaves alike. The membrane could have recognized their combination as one component. This can aid the easy purifications of brackish water contaminated with heavy metals of the same group.
Employing reverse osmosis method of water purification for the several brackish water sources contaminated with nickel and cobalt in the Niger Delta region could be successful if the contamination level are within the range as have been experimented. Several locations with heavy metal concentrations above the experimented range could have a pre-treatment stage to reduce clogging of ions on the membrane pores.
Continuous monitoring of water sources to account for other heavy metal contamination should serve as the basis to guide sample preparations and experiments to access the effectiveness of RO system in the region. Such models, including the considered nickel and cobalt would form a larger model that will consider different ranges and concentrations of heavy metals in future.
To compensate for the lack of constant electricity, an assessment of solar energy should be considered as an option to providing portable water for the rural and disconnected populace.
Notes
Peer review under responsibility of National Water Research Center.
References
- H.AhmadA.I.TsafeA.A.ZuruR.A.ShehuF.A.AtikuA.U.ItodoPhysicochemical and heavy metals value of crude oil samplesInt. J. Nat. Appl. Sci.6120101015
- A.N.AmadiP.I.OlasehindeM.A.Dan-HassanN.O.OkoyeG.G.EzeaguHydrochemical facies classification and groundwater quality studies in Eastern Niger Delta-NigeriaInt. J. Eng. Res. Dev.10200419
- T.BakalarM.BugelL.GajdosovaHeavy metal removal using reverse osmosisActa Montan. Slov.32009250253
- A.J.G.BarwiseRole of nickel and vanadium in petroleum classificationJ. Energy Fuels41990647652
- Beardsley, S.S, Coker, S.D., Whipple, S.S., 1994. The Economics of Reverse Osmosis and Ion Exchange. WATERTECH Expo 94. Dow Liquid Separations, Texas.
- Cellulose acetate reverse osmosis membrane, n.d. Retrieved from http://www.vladipor.ru/catalog/show/%26cid=006%26id=1.
- O.A.EmuedoG.O.AnoliefoC.O.EmuedoOil pollution and water quality in the Niger Delta: implications for the sustainability of the mangrove ecosystemGlob. J. Hum. Soc. Sci.14201419
- E.E.EtimR.OdohA.U.ItodoS.D.UmohU.LawalWater quality index for the assessment of water quality from different sources in Niger Delta region of NigeriaFront. Sci.3320138995
- L.FeiniZ.GuoliangM.QinZ.HongziPerformance of nanofiltration and reverse osmosis membranes in metal effluent treatmentChin. J. Chem. Eng.162008441445
- L.F.GreenleeD.F.LawlerB.D.FreemanB.MarrotP.MoulinReverse osmosis desalination: water sources, technology, and today’s challengesWater Res. J.43200923172348
- M.S.MohsenO.R.Al-JayyousiBrackish water desalination: an alternative for water supply enhancement in JordanDesalination1241999163174
- National Population Commission, 2010. 2006 Population and Housing Census of the Federal Republic of Nigeria: Priority Tables, Vol. 1, NPC, Abuja.
- Nigerian Industrial Standard, 2010. Nigeria Standard for Drinking Water Quality in the Federal Republic of Nigeria, NIS, Abuja.
- H.O.NwankwoalaS.A.NgahGroundwater resources of the Niger Delta: quality implications and management considerationsInt. J. Water Resour. Environ. Eng.62014155163
- H.I.OwamahHeavy metals determination and assessment in a petroleum impacted river in the Niger Delta Region of NigeriaJ. Pet. Environ. Biotechnol.42013135
- R.SeminatDesalination: present and futureWater Int.2520025456
- J.K.I.TubonimiA.OmuboO.S.HerbertAssessment of water quality along Amadi Creek in Port Harcourt, NigeriaSci. Afr.912010150162
- J.D.UdemeI.U.EtimPhysicochemical studies of Nigeria’s crude oil blendJ. Pet. Coal5432012243251
- UNEPEnvironmental Assessment of Ogoni Land2011UNEPKenya