Abstract
Recently, we and others reported the discovery of Lichtheimia ramosa (syn. Lichtheimia hongkongensis). We also hypothesized that a proportion of ‘Absidia corymbifera (Lichtheimia corymbifera)’ reported in the literature could be L. ramosa. In this study, we characterized 13 strains that had been reported as ‘A. corymbifera (L. corymbifera)’ in the literature over an 11-year period. Microscopic examination of agar block smear preparations of all 13 strains showed abundant circinate side branches and pleomorphic giant cells with finger-like projections of L. ramosa. ITS1–5.8S–ITS2 rRNA gene cluster (internal transcribed spacer (ITS)) and partial elongation factor-1alpha (EF1α) gene sequencing showed that all 13 strains were clustered with L. ramosa; partial β-actin gene sequencing showed that most of the 13 strains were clustered with L. ramosa; and partial 28S rRNA gene sequencing showed that all 13 strains were clustered with L. ramosa, but one strain of L. corymbifera (HKU25) was also clustered with other strains of L. ramosa. A significant number of reported A. corymbifera (L. corymbifera) infections are L. ramosa infections which are of global distribution. In clinical microbiology laboratories, L. ramosa should be suspected if an Absidia-like mold that possesses abundant circinate side branches on the sporangiophores and pleomorphic giant cells with finger-like projections is observed. ITS and partial EF1α gene sequencing are more reliable than partial β-actin and 28S rRNA gene sequencing for identification of the Lichtheimia species.
Introduction
Mucormycosis is an opportunistic infection caused by ubiquitous molds of the order Mucorales. Important immunosuppressive conditions associated with mucormycosis include diabetes mellitus, hematological malignancies, use of corticosteroids and other immunosuppressive agents in transplant recipients and severe burn patients.Citation1,Citation2 Due to the increasing use of immunosuppressive agents and prolonged survival of these patients, mucormycosis is becoming increasingly common, but mortality remains very high, often above 50%.Citation3 The common clinical manifestations of mucormycosis are rhinocerebral, pulmonary, gastrointestinal and cutaneous, depending on the routes of infection. The most common species of Mucorales associated with mucormycosis belong to the genera Rhizopus, Rhizomucor, Mucor and Absidia, which all belong to the family Mucoraceae.Citation4 Among the Absidia species, the most important species associated with mucormycosis is A. corymbifera.Citation2,Citation5,Citation6,Citation7 According to physiological, phylogenetic and morphological data, it was proposed that three Absidia species, A. corymbifera, A. blakesleeana and A. hyalospora, should be reclassified as a separate family, Lichtheimiaceae fam. nov., and the three species renamed as Lichtheimia corymbifera, Lichtheimia blakesleeana and Lichtheimia hyalospora.Citation8,Citation9 L. blakesleeana was subsequently reduced to a synonym of L. hyalospora.Citation10
Recently, we isolated two fungal strains from two patients with rhinocerebral and gastrointestinal mucormycosis.Citation11 These two strains possessed distinct morphological characteristics that do not fit into patterns of any known fungal species. Moreover, the morphological characteristics of the two strains were similar to another quality control (QC) strain isolated from a patient with cutaneous mucormycosis and sent to our clinical microbiological laboratory from the UK National External Quality Assessment Scheme.Citation11 Phenotypic and genotypic characterization of these three strains revealed that they constitute a novel Lichtheimia species, which we named Lichtheimia hongkongensis.Citation11 A similar observation was also independently reported by another group, by which the novel Lichtheimia species was named Lichtheimia ramosa.Citation12 Evidence from phylogenetic analysis in both studies suggested that L. ramosa (syn. L. hongkongensis) was previously classified as L. corymbifera (A. corymbifera).Citation11,Citation12 Therefore, we suspected that a significant number of isolates reported in the literature and identified as ‘A. corymbifera (L. corymbifera)’ might represent L. ramosa. To test this hypothesis, we requested strains that were reported as ‘A. corymbifera (L. corymbifera)’ in the literature from the corresponding research groups and performed phenotypic and genotypic characterization on them. In addition, we also characterized a L. ramosa strain (CBS124198) described by Garcia-Hermoso et al.Citation12 In this article, we report the results of the characterization and analysis.
Materials and methods
Strains
We sent emails to 55 research groups who had published cases of ‘A. corymbifera (L. corymbifera)’ infections over an 11-year period (1999–2009), requesting for their strains of ‘A. corymbifera (L. corymbifera)’. Among these 55 groups, replies were obtained from 18 groups. Three of these 18 groups have kept and have kindly provided us with their 13 strains of ‘A. corymbifera (L. corymbifera)’ for the present study ().Citation5,Citation6,Citation7 L. ramosa CBS124198Citation10,Citation12 was purchased from The Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre. L. ramosa HKU21 and L. corymbifera HKU25Citation11 were used as the control strains.
Table 1 Clinical sources of the 13 strains analyzed in this study
Phenotypic characterization
All strains were inoculated onto Sabouraud dextrose agar (SDA) for fungal culture. Slides for microscopic examination were prepared using the agar block smear method.Citation13 Semiquantification of enzymatic activities was performed using the API–ZYM test (bioMerieux Vitek, Hazelwood, MO, USA). All tests were performed in triplicate. Requirement of supplemental thiamine for growth was performed according to published protocols.Citation14,Citation15 The effect of different temperatures on growth on SDA and comparison of growth rate on SDA and potato dextrose agar (PDA) at 30 °C were studied using published protocols, with slight modifications.Citation6,Citation16 Sporangiospores were harvested in distilled water from 4-day cultures on SDA. Concentrations of sporangiospores were determined using a hemocytometer. A circular cavity was made at the center of each SDA and PDA plate using a Pasteur pipette. Respective plates were centrally inoculated with 5×102 spores. SDA plates were incubated at temperatures from 23 °C to 50 °C and PDA plates at 30 °C. Radii of circular colonies were measured in four directions after 2 days. All assays were performed in triplicate.
Scanning electron microscopy
Scanning electron microscopy was performed according to our previous publication, with slight modifications.Citation17 Briefly, fungal cells or sporangiospores of L. ramosa CBS124198 were fixed in 2.5% glutaraldehyde (w/v) for 1 h and washed once in 0.1 M sodium cacodylate buffer. Fixed materials were dehydrated through a 20% increment of ethanol concentration every 15 min from 30% to 90%, followed by two subsequent dehydration steps of 15 min each in absolute ethanol. Dehydrated materials in absolute ethanol were critical point dried in a BAL-TEC CPD O30 Critical Point Drier using carbon dioxide as the drying agent. Critical dried materials were mounted on to an aluminum stub and coated with palladium in BAL-TEC SCD 005 scanning electron microscopy (SEM) coating system. Coated materials were examined in Leica Cambridge Stereoscan 440 SEM operating at 12 kV and the specimen stage was tilted at zero degree.
DNA extraction
Fungal DNA extraction was performed as described in our previous publications.Citation18,Citation19,Citation20 Briefly, DNA was extracted from 1 g of fungal cells in 10 ml of distilled water using the DNeasy plant mini kit according to the manufacturer's instructions (QIAGEN, Hilden, Germany). The extracted DNA was eluted in 50 µl of buffer AE, the resultant mixture was diluted 10 times and 1 µl of the diluted extract was used for polymerase chain reaction (PCR).
Internal transcribed spacer (ITS), partial elongation factor-1alpha (EF1α) gene, partial β-actin gene and partial 28S rRNA gene sequencing
PCR amplification and DNA sequencing of the ITS, partial EF1α genes, partial β-actin genes and partial 28S rRNA genes were performed according to published protocols.Citation19,Citation20 Briefly, DNase I-treated distilled water and PCR master mix (which contains deoxynucleoside triphosphates, PCR buffer, and Taq polymerase) were used in all PCR reactions by adding 1 U of DNase I (Pharmacia, Uppsala, Sweden) to 40 µl of distilled water or PCR master mix, incubating the mixture at 25 °C for 15 min, and subsequently at 95 °C for 10 min to inactivate the DNase I. The fungal DNA extract and controls were amplified with 0.5 µM primers (ITS1 5′-TCC GTA GGT GAA CCT GCG G-3′ and ITS4 5′-TCC TCC GCT TAT TGA TAT GC-3′ for ITS, M1 5′-GCT GGT ATC TCC AAG GAT GG-3′ and M2 5′-CGA CGG ACT TGA CTT CRG TGG-3′ for the partial EF1α gene, LPW8614 5′-CAY ACY TTC TAC AAY GAR CTC C-3′ and LPW8615 5′-KGC VAR RAT RGA ACC ACC-3′ for the partial β-actin gene and NL-1 5′-GCA TAT CAA TAA GCG GAG GAA AAG-3′ and NL-4 5′-GGT CCG TGT TTC AAG ACG G-3′ for the partial 28S rRNA gene) (Gibco BRL, Rockville, MD, USA).Citation21,Citation22 The PCR mixture (25 µl) contained fungal DNA, PCR buffer (10 mM Tris-HCl pH 8.3, 50 mM KCl, 2 mM MgCl2 and 0.01% gelatin), 200 µM of each deoxynucleoside triphosphate and 1.0 U Taq polymerase (Applied Biosystem, Foster City, CA, USA). The mixtures were amplified in 40 cycles of 94 °C for 1 min, 55 °C for 1 min and 72 °C for 2 min, and a final extension at 72 °C for 10 min in an automated thermal cycler (Applied Biosystem). DNase I-treated distilled water was used as the negative control. Ten microliters of each amplified product was electrophoresed in 1.5% (w/v) agarose gel, with a molecular size marker (φX174 HaeIII digest; Boehringer Mannheim, Germany) in parallel. Electrophoresis in Tris–borate–ethylenediaminetetraacetic acid buffer was performed at 100 V for 1.5 h. The gel was stained with ethidium bromide (0.5 µg/ml) for 15 min, rinsed and photographed under ultraviolet light illumination.
The PCR product was purified using the QIAquick Gel Extraction kit (QIAGEN). Both strands of the PCR product were sequenced twice with an ABI Prism 3700 DNA Analyzer (Applied Biosystems), using the PCR primers. The sequences of the PCR products were compared with sequences of closely related species in GenBank by multiple sequence alignment using ClustalX 1.83.Citation23
Phylogenetic characterization
Phylogenetic tree construction was performed using the neighbor-joining method with MEGA4.0.2. Six hundred and four nucleotide positions of ITS, 399 nucleotide positions of EF1α, 428 nucleotide positions of β-actin and 609 nucleotide positions of 28S rRNA gene were included in the analysis.
Results
Morphological characteristics
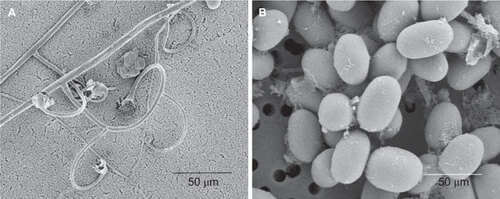
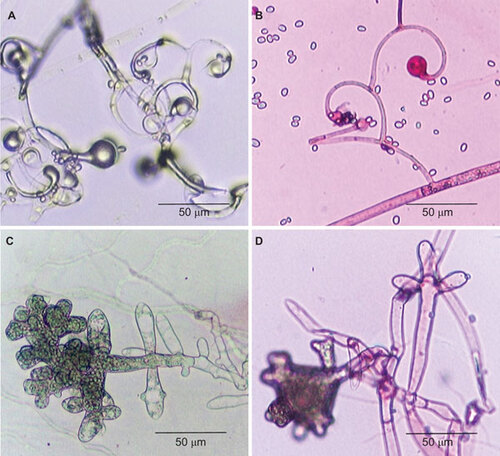
On SDA, all the 13 strains identified as ‘A. corymbifera (L. corymbifera)’ and L. ramosa CBS124198 grew rapidly as cottony colonies and attained diameters of 60–73 mm after incubation at 37 °C for 48 h. Microscopic examination of all the 13 strains and L. ramosa CBS124198 showed non-septate broad hyphae. The sporangiophores were highly branched, with the diameters of the main sporangiophores being 8–10 µm and those of the side branches being 3–5 µm. The sporangiophores terminated in sporangia or the columellar remnants. Similar to L. ramosa HKU21, HKU22 and HKU23,Citation11 most of the side branches of the 13 strains and L. ramosa CBS124198 were characteristically circinate (), although sometimes they were arranged in umbel, usually at the terminals. Rhizoids were not observed. The sporangia (25–35 µm) were hyaline, multispored and pyriform in shape and the columella (15–21 µm) was dome-shaped with a prominent flask-shaped apophysis. Hyaline sporangiospores ((2–3)×(3–4) µm) were formed inside sporangia. Most of them were smooth and ellipsoidal in shape. Similar to L. ramosa HKU21, HKU22 and HKU23,Citation11 abundant pleomorphic giant cells with finger-like projections, usually submerged in agar, were observed in all the 13 strains and L. ramosa CBS124198 ().
Figure 1 Microscopic examination of strain 1 (A and C, lactophenol cotton blue stain) and L. ramosa CBS124198 (B and D, acid fuchsin stain) showing branched sporangiophores with characteristic circinate side branches (A and B) and pleomorphic giant cells with finger-like projections (C and D). CBS, Centraalbureau voor Schimmelcultures.
Enzyme production and requirement of supplemental thiamine for growth
The API-ZYM test for all the 13 strains and L. ramosa CBS124198 showed positive results for alkaline phosphatase, esterase (C4), esterase lipase (C8), leucine arylamidase, acid phosphatase, naphthol-AS-BI-phosphohydrolase, β-glucosidase and N-acetyl-β-glucosaminidase. Thiamine supplementation was not required for the growth of all the 13 strains and L. ramosa CBS124198.
Effect of temperature on growth
All the 13 strains and L. ramosa CBS124198 grew at 23–44 °C but not at 47 °C and 50 °C. Their median rates of growth at 30 °C on SDA and PDA were 43 (range: 38–56) mm/48 h and 27 (range: 25–30) mm/48 h respectively.
Scanning electron microscopy
Scanning electron microscopic examination of L. ramosa CBS124198 revealed the characteristic sporangiophores with circinate side branches and ellipsoid sporangiospores, consistent with the results observed on light microscopy ().
ITS, partial EF1α gene, partial β-actin gene and partial 28S rRNA gene sequencing and phylogenetic characterization
PCR of the ITS region of the 13 strains and L. ramosa CBS124198 showed bands at about 800 bp. Phylogenetic analysis showed that they were clustered with L. ramosa strains (GenBank accession numbers HQ285686, HQ285700, HM104215 and HM104212), L. ramosa HKU21 (GenBank accession number EU852633), L. ramosa HKU22 (GenBank accession number EU852634) and L. ramosa HKU23 (GenBank accession number EU852632) as well as four other strains named A. corymbifera (GenBank accession numbers AY944896, EF136359, AY944897 and AB305110) ().
Figure 3 Phylogenetic trees showing the relationship of the 13 strains (highlighted in bold) and L. ramosa CBS124198 to closely related species, inferred from ITS (602 nucleotide positions), EF1α gene (544 nucleotide positions), β-actin gene (441 nucleotide positions) and 28S rRNA gene (639 nucleotide positions) sequence data by the neighbor-joining method and rooted using Fennellomyces linderi (GQ249890), Pilobolus umbonatus (AF157277), dichotomocladium robustum (EU826396) and Paecilomyces lilacinus (AY213717), respectively. The scale bars indicate the estimated number of substitutions per 20, 100, 50 and 10 bases, respectively. Numbers at nodes indicated levels of bootstrap support calculated from 1000 trees. All names and accession numbers are given as cited in the GenBank database.
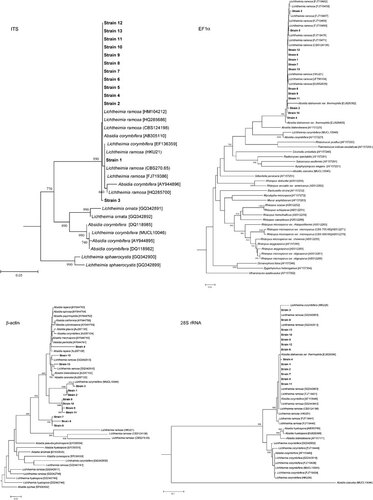
PCR of the partial EF1α genes of the 13 strains and L. ramosa CBS124198 showed bands at about 450 bp. Phylogenetic analysis showed that they were clustered with L. ramosa strains (GenBank accession numbers FJ719479 and FJ719476), L. ramosa HKU21 (GenBank accession number EU852637), L. ramosa HKU22 (GenBank accession number EU852636) and L. ramosa HKU23 (GenBank accession number EU852635) ().
PCR of the partial β-actin genes of the 13 strains and L. ramosa CBS124198 showed bands at about 900 bp. Phylogenetic analysis showed that 10 of the 13 strains and L. ramosa CBS124198 were clustered with L. ramosa HKU21 (GenBank accession number FJ444938), L. ramosa HKU22 (GenBank accession number FJ444939) and L. ramosa HKU23 (GenBank accession number FJ444939) (). Two strains (NOs. 3 and 11) were clustered with L. ramosa (GenBank accession numbers GQ342746 and GQ342747) and another strain (NO. 13) was clustered with L. ramosa (GenBank accession number GQ342845) ().
PCR of the partial 28S rRNA genes of the 13 strains and L. ramosa CBS124198 showed bands at about 500 bp. Phylogenetic analysis showed that they were clustered with L. ramosa HKU21, L. ramosa HKU22 and L. ramosa HKU 23 (). Notably, one strain of L. corymbifera (HKU25), confirmed by phenotypic tests and ITS, partial EF1α gene and partial β-actin gene sequencing, was also clustered with other strains of L. ramosa ().
Discussion
A significant number of reported A. corymbifera (L. corymbifera) infections are L. ramosa infections which are of global distribution. Recently, we reported the discovery of L. ramosa from three patients with mucormycosis.Citation11 The first patient was a liver transplant recipient with rhinocerebral mucormycosis, the second a renal transplant recipient with gastrointestinal mucormycosis and the third a burn patient with cutaneous mycormycosis.Citation11 Since phylogenetic analysis inferred by ITS sequences showed that these three strains of L. ramosa were clustered with at least four sequences named ‘A. corymbifera (L. corymbifera)’ deposited in the GenBank database,Citation11 we suspected that a significant proportion of A. corymbifera (L. corymbifera) infections were actually L. ramosa infections. In this study, among the 13 published strains of A. corymbifera (L. corymbifera) from Spain, France and Qatar we collected and re-characterized, all were unambiguously identified as L. ramosa using both phenotypic and genotypic methods. These 13 strains were obtained from diverse sites, including the respiratory tract, paranasal sinuses, brain, heart, blood and wound (), implying that L. ramosa could be the cause of different clinical forms of mucormycosis. Therefore, we concluded that a significant proportion of infections caused by A. corymbifera (L. corymbifera) were in fact L. ramosa infections. Further basic and clinical studies on L. corymbifera and L. ramosa will reveal any difference between the pathogenicity, epidemiology, anti-fungal susceptibility, clinical disease spectrum and outcome of these two Lichtheimia species.
ITS and partial EF1α gene sequencing are more reliable than partial β-actin gene and partial 28S rRNA gene sequencing for identification of the Lichtheimia species. In our previous study and the present study, all 16 strains of L. ramosa were identified correctly using ITS and partial EF1α gene sequencing ().Citation11 For partial β-actin gene sequencing, some strains of L. ramosa (e.g., strains 3 and 11) were not as closely related to most strains of L. ramosa than the cluster of A. hyalospora (EF030531 and PW1264), L. hyalospora (GQ342748 and GQ342749) and A. blakesleeana (PW1263 and AJ287132) to most strains of L. ramosa (). As for partial 28S rRNA gene sequencing, although all 16 strains of L. ramosa were identified correctly, one strain of L. corymbifera (HKU25) showed clustering with other strains of L. ramosa (). These indicated that the ITS and partial EF1α genes are better targets for identification of Lichtheimia species. It also implied that sequencing multiple genes should be performed for more reliable speciation in the Lichtheimia genus.
This polyphasic approach of phenotypic and genotypic characterization should be used for speciation of ‘difficult-to-identify’ pathogenic fungi. Accurate identification of medically important fungi is the cornerstone to prescription of the correct antifungal drugs to patients with fungal infections. In clinical microbiology laboratories, L. ramosa should be suspected if an Absidia-like mold that possesses abundant circinate side branches on the sporangiophores and pleomorphic giant cells with finger-like projections is observed. Since the presence of circinate side branches on the sporangiophores and pleomorphic giant cells is not exclusively found in L. ramosa though they are more abundantly found in it,Citation10,Citation24 these are not absolute microscopic characteristics of this fungus. With the increasing availability of PCR and DNA sequencing, ITS and partial EF1α gene sequencing should be performed for accurate identification of L. ramosa.
Taxonomy
L. ramosa (syn. Lichtheimia hongkongensis)
MycoBank MB515445
In ‘SDA’ post-dies 2 ad 37 °C, coloniae pilosae, coloniarum 64–85 mm diametros, hyphae latarum aseptatarum. Sporangiophores ramosissimae, sporangiophorerum potissimarum 8–10 µm diametros, sporangiophorerum ramis lateralibus 3–5 µm diametros. Ramis lateralibus plerus circinalibus, interdum umbelliformis. Sine structuris rhizoideis similibus. Sporangiis (25–35 µm) hyalinis, pyriformibus cum sporis numerosis. Collumellis (15–21 µm) tholiformibus cum apophyses ampulliformibus. Sporangiophoris hyalinis, cotidianum teres, ellipticus, et cellulis pleomorphus grandibus, numerosis cum projecturis digitiformibus.
Description of L. ramosa (syn. Lichtheimia hongkongensis)
On SDA, L. ramosa grows rapidly as cottony colonies and attains a diameter of 64–85 mm after incubation at 37 °C for 48 h. Microscopic examination shows non-septate broad hyphae. The sporangiophores are highly branched. The diameters of main sporangiophores are 8–10 µm and those of the side branches are 3–5 µm. The sporangiophores terminate in sporangia or the columellar remnants. Most of the side branches are characteristically circinate, but sometimes they are arranged in umbel, usually at the terminals. The circinate sporangiophores are also evident when young colonies are directly observed under the microscope using a ×10 objective. Rhizoids are not observed. The sporangia (25–35 µm) are hyaline, multispored and pyriform in shape. The columella (15–21 µm) is dome-shaped with a prominent flask-shaped apophysis. Hyaline sporangiospores ((2–3)×(3–4) µm) are formed inside sporangia. Most of them were smooth and ellipsoidal in shape. Characteristically, abundant pleomorphic giant cells with finger-like projections, usually submerged in agar, are observed.
This work is partly supported by the HKSAR Research Fund for the Control of Infectious Diseases of the Health, Welfare and Food Bureau, a Research Grants Council Grant, University Development Fund and the Committee for Research and Conference Grant, The University of Hong Kong. We thank S Alfandari, J Guarro and S J Taj-Aldeen for providing us with their 13 fungal strains.
Notes
Abbreviation: NA, not available.
- Cheng VC, Chan JF, Ngan AH et al.Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol2009;47: 2834–2843.
- Sugar AM.Mucormycosis. Clin Infect Dis1992;14( Suppl 1): S126–S129.
- Saegeman V, Maertens J, Ectors N, Meersseman W, Lagrou K.Epidemiology of mucormycosis: review of 18 cases in a tertiary care hospital. Med Mycol2009;48: 245–254.
- Skiada A, Vrana L, Polychronopoulou H et al.Disseminated zygomycosis with involvement of the central nervous system. Clin Microbiol Infect2009;15( Suppl 5): 46–49.
- Almaslamani M, Taj-Aldeen SJ, Garcia-Hermoso D, Dannaoui E, Alsoub H, Alkhal A.An increasing trend of cutaneous zygomycosis caused by Mycocladus corymbifer (formerly Absidia corymbifera): report of two cases and review of primary cutaneous Mycocladus infections. Med Mycol2009;47: 532–538.
- Alvarez E, Sutton DA, Cano J et al.Spectrum of zygomycete species identified in clinically significant specimens in the United States. J Clin Microbiol2009;47: 1650–1656.
- Tricot S, Gastine T, Sendid B, Wurtz A, de Botton S, Alfandari S.Pulmonary zygomycosis in a patient treated for invasive aspergillosis. Med Mal Infect2006;36: 118–121. French.
- Hoffmann K, Discher S, Voigt K.Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol Res2007;111( Pt 10): 1169–1183.
- Hoffmann K, Walther G, Voigt K.Mycocladus vs. Lichtheimia: a correction (Lichtheimiaceae fam. nov., Mucorales, Mucoromycotina).Mycol Res2009;113: 275–278.
- Alastruey-Izquierdo A, Hoffmann K, de Hoog GS et al.Species recognition and clinical relevance of the zygomycetous genus Lichtheimia (syn. Absidia pro parte, ycocladus). J Clin Microbiol2010;48: 2154–2170.
- Woo PC, Lau SK, Ngan AH et al.Lichtheimia hongkongensis sp nov., a novel Lichtheimia spp. associated with rhinocerebral, gastrointestinal, and cutaneous mucormycosis. Diagn Microbiol Infect Dis2010;66: 274–284.
- Garcia-Hermoso D, Hoirnard D, Gantier JC, Grenouillet F, Dromer F, Dannaoui E.Molecular and phenotypic evaluation of Lichtheimia corymbifera (ex. Absidia corymbifera) omplex isolates associated with human mucormycosis: rehabilitation of L. ramosa, ex synonym of L. corymbifera. J Clin Microbiol2009;47: 3862–3870.
- Woo PC, Ngan AH, Chui HK, Lau SK, Yuen KY.Agar block smear preparation: a novel method of slide preparation for preservation of native fungal structures for microscopic examination and long-term storage. J Clin Microbiol2010;48: 3053–3061.
- Bartnicki-Garcia S, Nickerson WJ.Thiamine and nicotinic acid: anaeerobic growth factors for Mucor rouxii. J Bacteriol1961;82: 142–148.
- Andrade VS, De Barros NB, Fukushima K, De Campos GM.Effect of medium components and time of cultivation on chitin production by Mucor circinelloides (Mucor javanicus IFO 4570)—a factorial study. Rev Iberoam Micol2003;20: 149–153.
- Cao CW, Li RY, Wan Z et al.The effects of temperature, pH, and salinity on the growth and dimorphism of Penicillium marneffei. Med Mycol2007;45: 401–407.
- Woo PC, Tam EW, Chong KT et al.High diversity of polyketide synthase genes and the melanin biosynthesis gene cluster in Penicillium marneffei. FEBS J2010;277: 3750–3758.
- Woo PC, Lau CC, Chong KT et al.MP1 homologue-based multilocus sequence system for typing the pathogenic fungus Penicillium marneffei: a novel approach using lineage-specific genes. J Clin Microbiol2007;45: 3647–3654.
- Woo PC, Lau SK, Ngan AH, Tse H, Tung ET, Yuen KY.Lasiodiplodia theobromae pneumonia in a liver transplant recipient. J Clin Microbiol2008;46: 380–384.
- Woo PC, Leung SY, To KK et al.Internal transcribed spacer region sequence heterogeneity in rhizopus microsporus: implications for molecular diagnosis in clinical microbiology laboratories. J Clin Microbiol2010;48: 208–214.
- White TJ, Bruns T, Lee S, Taylor JW.Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications.New York: Academic Press, Inc., 1990: 315–322.
- Kurtzman CP, Robnett CJ.Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5' end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol1997;35: 1216–1223.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG.The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res1997;25: 4876–4882.
- Schipper MA.Notes on Mucorales—I. Observations on Absidia. Persoonia1990;14: 133–149.