Abstract
Hepatitis B infection, especially by perinatal transmission, is endemic in Asian countries. After the first successful universal hepatitis B virus (HBV) vaccination programme for newborns in Taiwan, it became feasible to prevent HBV transmission and the resultant hepatocellular carcinoma in endemic countries. However, a small subset of vaccinated people have a suboptimal immunological response to vaccination, and the immunity of some young adults who were vaccinated as infants seems to have waned over time. Despite this loss, recent studies suggest that anamnestic anti-HBs antibody responses rapidly resume and eliminate acute HBV infection acquired through sexual contact or blood transfusion, even though the anti-HBs antibody titre has decreased below a protective level. These observations indicate prolonged protection by the HBV vaccine. Therefore, for people with a low infection risk, a universal booster vaccination is not currently recommended, but it should be considered for high-risk groups. However, we still advocate close monitoring of acute hepatitis B among patients who lack a protective level of anti-HBs antibody and suggest a wait-and-see policy to determine the necessity for booster vaccines.Emerging Microbes & Infections (2012) 1, e27; doi:10.1038/emi.2012.28
Introduction
Chronic viral hepatitis is known to have many detrimental health outcomes. Hepatitis B virus (HBV) infection is the major cause of viral hepatitis and affects more than 350 million individuals worldwide. The consequences of chronic hepatitis B infection include liver cirrhosis, liver failure and hepatocellular carcinoma (HCC).Citation1 After researchers elucidated the infection route and the natural clinical course of hepatitis B, they found that perinatal transmission of HBV from carrier mothers to infants plays an important role in spreading the virus and leads to chronic infection beginning in childhood. Perinatal transmission is the major route of hepatitis B transmission in Asia, where the infection is endemic.
The policy on and efficacy of HBV vaccination
To prevent perinatal HBV transmission, HBV vaccination has been advocated since the 1980s, based upon reliable results from clinical trials. Beasley et al.Citation2 and Lo et al.Citation3 demonstrated the effectiveness of both passive and active immunisation to interrupt perinatal HBV transmission. The introduction of safe, effective and highly immunogenic HBV vaccines led to the recommendation for universal immunisation of all newborns in Taiwan from 1984. The first worldwide vaccination programme effectively prevented perinatal HBV transmission and the chronic carrier state, and approximately 85% of infant vaccinees had adequate levels of protective antibody at 18 months of age.Citation4 This programme is extremely successful in the control of HBV infection in Taiwan in several ways. Firstly, the HBsAg carrier rate in children gradually decreased from 11% in 1984 to 1% in 2004.Citation5,Citation6,Citation7,Citation8 Secondly, after the implementation of universal vaccination of newborns, the infant mortality rate from fulminant hepatitis decreased from 5.36 per 100 000 to 1.71 per 100 000.Citation9 Fulminant hepatitis B almost disappeared in children older than 1 year of age.Citation10 Thirdly, the average annual incidence of HCC in children 6–14 years of age significantly declined from 0.70 per 100 000 children between 1981 and 1986 to 0.57 per 100 000 children between 1986 and 1990, and to 0.36 per 100 000 children between 1990 and 1994. The incidence of HCC in children 6–9 years of age also significantly declined from 0.52 per 100 000 children for those born between 1974 and 1984 to 0.13 per 100 000 children for those born between 1984 and 1986, after the universal vaccination programme was implemented.Citation11 This decline in HCC incidence extended to teenagers and young adults 2 decades post vaccination.Citation12,Citation13 This was the first study demonstrating that a vaccine can prevent a human cancer and serve as a potential way to eliminate hepatitis B.Citation14
Poor responders and failure of HBV vaccination
Although the current vaccines are highly effective, there are still some populations with suboptimal immunogenic responses, such as preterm infants, the elderly, smokers, obese individuals, and those with chronic liver or renal diseases, diabetes mellitus (DM) or human immunodeficiency virus (HIV) infection.Citation14 For newborns, HBV vaccination should be given within 24 h of delivery with or without hepatitis B immune globulin (HBIG); otherwise, the protective efficacy will be lower.Citation15 It is known that the hepatitis B vaccine has a lower immunogenicity in preterm infants than in infants born at term, especially those with gestational ages < 34 weeksCitation16 or birth weights < 1800 g.Citation17 For preterm infants of HBsAg-negative mothers, deferring the first vaccination dose by 1 month is recommended to improve the vaccine’s immunogenicity and efficacy.Citation18
Some newborns fail to complete their vaccination courses, and approximately 10% of children born to HBeAg-positive mothers with high viral loads become persistently infected with HBV owing to HBIG or vaccination failure.Citation19 These newborn HBV carriers, along with the existing adult chronic HBV carriers, are still at risk of developing HBV-related complications, such as cirrhosis and HCC, in the following 30 years.
Declining anti-HBV titres in the vaccinated cohorts during long-term follow-up: risk of HBV infection?
After the first dose of the hepatitis B vaccine, adequate protective anti-HBs antibody levels (> 10 IU/L) develop within 1 month in 48% of neonates. Anti-HBs antibody is detected in 91% of neonates 2 months after the second dose, and in 96% at 6 months.Citation20 However, the anti-HBs antibody titre declines rapidly in the first year and then gradually after 1 year.Citation21 The persistence of anti-HBs antibody depends on the initial postvaccination concentration.Citation22 The immunity gained from perinatal HBV vaccination further diminishes in adolescents, approximately 10–15 years after immunisation.Citation23,Citation24 Approximately 62.4% of 15 year olds no longer have protective levels of anti-HBs antibody.Citation23
At the same time, those adolescents are more likely to be exposed to HBV infection than children because of sexual activity, substance abuse, or medical interventions. Therefore, it is important to determine whether HBV infection can emerge in this population and cause clinical problems and whether a routine vaccine booster is required.
HBV infections in previously vaccinated subjects: an unusual clinical course
To better understand the HBV infections that occur in HBV-vaccinated subjects, it is important to follow prospectively individuals who might be exposed to HBV.Citation24 A large-scale longitudinal survey of 18 779 subjects showed that vaccination in infancy provides adequate long-term protection for up to 20 years. Despite occasional exposure to HBV (0.1%–4% anti-HBc seropositive rate), the risk of persistent HBV infection did not increase with age, before adulthood.Citation8
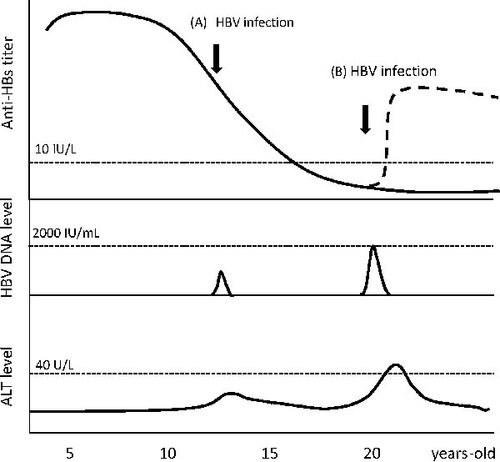
Recently, after a mass screening of 3.7 million blood donations by nucleic acid testing, Stramer et al.Citation25 identified nine occult HBV donors harbouring low levels of HBV viraemia. Six had previously received the HBV vaccine and exhibited low or undetectable anti-HBs antibody titres (0–96 IU/L). Careful tracing indicated that these individuals probably acquired acute HBV infection from their sexual partners, who had high HBV loads (> 106 IU/ml). At follow-up visits, these newly infected individuals only presented with transient HBV viraemia (11–86 IU/ml) for a few months and did not show biochemical hepatitis. A rapid anamnestic escalation of anti-HBs antibody was also observed after HBV infection, which was attributed to the priming by the HBV vaccine about 7–27 years earlier.Citation25 In contrast, unvaccinated blood donors developed clinically significant acute hepatitis B after exposure to HBV via sexual transmission. From this study by Stramer et al., it is clear that vaccinated subjects with anti-HBs antibody titres below the protective level are still susceptible to HBV infection, especially if they are exposed to a high viral load. Interestingly, only transient viraemia and subclinical hepatitis were observed, and none of the vaccinated subjects became HBsAg carriers. Similarly, Liu et al.Citation26 evaluated 327 HBV-naïve blood transfusion recipients and found that four vaccinated children developed transient HBV viraemia (3.4×104 – 3.3×105 HBV DNA copies/ml) within 1 month of receiving blood transfusions contaminated with HBV. These children exhibited only subclinical HBV infection, with minimal alanine aminotransferase (ALT) level elevation, after transfusion. Although their anti-HBs antibody titres were low or undetectable (0–150.4 IU/L) at the time of the blood transfusion, none became HBsAg carriers, and all cleared the HBV infection after 1 month. These two studies suggest that anamnestic anti-HBs antibody responses rapidly resume and eliminate acute HBV after infection resulting from sexual contact or blood transfusion, up to 27 years post vaccination. A dynamic change in the serum anti-HBs antibody titre, HBV DNA and ALT levels during acute HBV infection in an HBV vaccinee is illustrated in . Recently, anti-HBc antibody levels have been shown to reflect an individual’s immune activity to HBV. Whether there is also a dynamic change in anti-HBc antibody levels after acute HBV infection in vaccinated individuals remains to be elucidated.Citation27
The anamnestic effect of anti-HBs antibody
The anamnestic effect of anti-HBs antibody was observed in individuals who received an additional vaccine dose, who showed a rapid and immediate rise in antibody titre.Citation28 Several studies of health volunteers confirmed the immunological memory demonstrated by anti-HBs antibody escalation, with a titre > 10 IU/L in 73%–100% of vaccinees after a booster dose administered 5–12 years after the first dose.Citation29 This immunologic memory following booster vaccination was verified by in vitro assays, demonstrating that memory B lymphocytes were present in vaccinated adults even though these adults exhibited low anti-HBs antibody titres. Such B lymphocytes would maintain the capacity to differentiate and produce anti-HBs IgG upon subsequent stimulation by HBsAg.Citation30 If this immunological memory still provides immunity despite an inadequate level of anti-HBs antibody, then a breakthrough persistent infection is unlikely to occur. From clinical observations, although many vaccinees fail to maintain 10 IU/L of anti-HBs antibody, there have been very few significant breakthrough infections despite HBV exposure.Citation29
The necessity of booster vaccination in target groups
According to the aforementioned evidence, vaccinated individuals rapidly regain protection against HBV transmission through sexual exposure or blood transfusion even if their anti-HBs antibody titre falls below the detection limit. Therefore, for people with a low infection risk, a universal booster vaccination is not currently recommended.
However, there are special groups that require more attention. Zanetti et al.Citation31 studied 1212 children and 446 Air Force recruits in Italy with follow-up for 10 years, when they found a well-preserved immunological memory in 64% of children vaccinated at infancy and in 89% of young adults vaccinated at 12 years of age. HBV infection was rare over the 10 years of the study, and none of the individuals studied became HBsAg carriers, even though their anti-HBs antibody titres declined with time. Thus, the investigators concluded that vaccination protection persists for longer than 10 years after primary vaccination, and booster doses of hepatitis B vaccine may not be needed.Citation31 In a study of 60 children receiving a living-donor liver transplant, recipients with anti-HBs antibody levels > 1000 IU/L after a booster vaccination were all protected from de novo hepatitis B even after transplantation with a liver from an anti-HBc-positive donor.Citation32 The booster for vaccine responders before their liver transplantation yielded good protection. A recent study enrolled 127 college students without protective anti-HBs antibody levels who were administered booster vaccinations. The percentages of individuals exhibiting seroprotective levels of anti-HBs antibody for 7–10 days, 1, 6 and 7 months post vaccination were 20.5%, 75.6%, 94.5%, and 99.2%, respectively. The early booster response predicts higher levels of protection at 1 and 6 months post vaccination. At least one quarter of these HBV vaccinees had lost their immune memory to the HBV vaccine by the time they enter college. A prompt immunological memory to revaccination was present in only 20% of the vaccinees studied. Therefore, at least 2 doses of booster vaccines are recommended for at-risk vaccinated adults without persistent protection.Citation33 However, postvaccination testing of anti-HBs antibody levels is not routinely needed because of the high response rate (> 96%) to vaccination.Citation28
In contrast, in several studies of immunocompromised adults with HIV infection, only 40%–53% of vaccinees developed anti-HBs antibody.Citation34 The hyporesponders to HBV vaccination were patients with chronic renal failure, alcoholism, type 1 DM, or cancer. The vaccine also has been shown to have low immunogenicity in older adults, smokers, males and obese individuals. The poor response to HBV vaccination may be rescued by shifting to intradermal injection, by increasing vaccine dosage and frequency or by improving adjuvants.Citation14 For the hyporesponder populations, a postvaccination test of anti-HBs antibody levels may be needed.Citation29 For populations at high risk of infection, a booster vaccination may be considered after adulthood.
In conclusion, HBV vaccination is an effective way to terminate perinatal and early horizontal transmission of hepatitis B. The antibody levels raised in response to vaccination decline with time, but the protection afforded by vaccination seems to persist for 2 decades or more because of the anamnestic effect of anti-HBs antibody. Therefore, although HBV infections might occur in vaccinated individuals as they reach adulthood, these infections do not seem to be a substantial threat to the population as a whole. However, we advocate close monitoring for acute hepatitis B among these individuals in the future and a wait-and-see policy to determine the necessity for booster vaccines.
References
- Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis2002; 2: 395–403.
- Beasley RP, Hwang LY, Lee GC et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet1983; 2: 1099–1102.
- Lo KJ, Tsai YT, Lee SD et al. Immunoprophylaxis of infection with hepatitis B virus in infants born to hepatitis B surface antigen-positive carrier mothers. J Infect Dis1985; 152: 817–822.
- Hsu HM, Chen DS, Chuang CH et al. Efficacy of a mass hepatitis B vaccination program in Taiwan. Studies on 3464 infants of hepatitis B surface antigen-carrier mothers. JAMA1988; 260: 2231–2235.
- Hsu HY, Chang MH, Chen DS, Lee CY, Sung JL. Baseline seroepidemiology of hepatitis B virus infection in children in Taipei, 1984: a study just before mass hepatitis B vaccination program in Taiwan. J Med Virol1986; 18: 301–307.
- Chen HL, Chang MH, Ni YH et al. Seroepidemiology of hepatitis B virus infection in children: Ten years of mass vaccination in Taiwan. JAMA1996; 276: 906–908.
- Ni YH, Chang MH, Huang LM et al. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med2001; 135: 796–800.
- Ni YH, Huang LM, Chang MH et al. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology2007; 132: 1287–1293.
- Kao JH, Hsu HM, Shau WY, Chang MH, Chen DS. Universal hepatitis B vaccination and the decreased mortality from fulminant hepatitis in infants in Taiwan. J Pediatr2001; 139: 349–352.
- Chen HL, Chang CJ, Kong MS et al. Pediatric fulminant hepatic failure in endemic areas of hepatitis B infection: 15 years after universal hepatitis B vaccination. Hepatology2004; 39: 58–63.
- Chang MH, Chen CJ, Lai MS et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med1997; 336: 1855–1859.
- Chang MH, Chen TH, Hsu HM et al. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res2005; 11: 7953–7957.
- Chang MH, You SL, Chen CJ et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst2009; 101: 1348–1355.
- Chen DS. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol 2009; 50: 805–816.
- Marion SA, TommPastore M, Pi DW, Mathias RG. Long-term follow-up of hepatitis B vaccine in infants of carrier mothers. Am J Epidemiol1994; 140: 734–746.
- Sood A, Singh D, Mehta S, Midha V, Kumar R. Response to hepatitis B vaccine in preterm babies. Indian J Gastroenterol2002; 21: 52–54.
- Freitas da Motta MS, Mussi-Pinhata MM, Jorge SM, Tachibana Yoshida CF, Sandoval de Souza CB. Immunogenicity of hepatitis B vaccine in preterm and full term infants vaccinated within the first week of life. Vaccine2002; 20: 1557–1562.
- Saari TN. Immunization of preterm and low birth weight infants. Pediatrics2003; 112: 193–198.
- Lee CY, Huang LM, Chang MH et al. The protective efficacy of recombinant hepatitis B vaccine in newborn infants of hepatitis B e antigen-positive-hepatitis B surface antigen carrier mothers. Pediatr Infect Dis J1991; 10: 299–303.
- Hwang LY, Beasley RP, Stevens CE, Szmuness W. Immunogenicity of HBV vaccine in healthy Chinese children. Vaccine1983; 1: 10–12.
- Mast EE, Weinbaum CM, Fiore AE et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep2006; 55: 1-33; quiz CE31–34.
- Williams IT, Goldstein ST, Tufa J, Tauillii S, Margolis HS, Mahoney FJ. Long term antibody response to hepatitis B vaccination beginning at birth and to subsequent booster vaccination. Pediatr Infect Dis J2003; 22: 157–163.
- Lu CY, Chiang BL, Chi WK et al. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology2004; 40: 1415–1420.
- Lin YC, Chang MH, Ni YH, Hsu HY, Chen DS. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J Infect Dis2003; 187: 134–138.
- Stramer SL, Wend U, Candotti D et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med2011; 364: 236–247.
- Liu CJ, Lo SC, Kao JH et al. Transmission of occult hepatitis B virus by transfusion to adult and pediatric recipients in Taiwan. J Hepatol2006; 44: 39–46.
- Yuan Q, Song LW, Liu CJ et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients. Gut2012 Jun 14. DOI: 10.1136/gutjnl-2012-302656.
- West DJ. Clinical experience with hepatitis B vaccines. Am J Infect Control1989; 17: 172–180.
- West DJ, Calandra GB. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine1996; 14: 1019–1027.
- Wismans PJ, van Hattum J, de Gast GC et al. The spot-ELISA: a sensitive in vitro method to study the immune response to hepatitis B surface antigen. ClinExpImmunol1989; 78: 75–79.
- Zanetti AR, Mariano A, Romano L et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet2005; 366: 1379–1384.
- Lin CC, Chen CL, Concejero A et al. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant2007; 7: 195–200.
- Jan CF, Huang KC, Chien YC et al. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology2010; 51: 1547–1554.
- Goilav C, Piot P. Vaccination against hepatitis B in homosexual men. A review. Am J Med1989; 87: 21S–25S.