Abstract
It is now privately acknowledged that there may be little if any perceptible impact of the national Bacille Calmette–Guerin (BCG) vaccination program on disease prevalence, despite the extensive coverage of the newborn infant population and likely benefit in the early years of life. A better preventive vaccine than BCG is now being sought by Chinese researchers. Urgency has been added to the control problem by the emergence of multidrug-resistant tuberculosis (TB). Furthermore, expensive second-line drugs seem unlikely to be made available by the government to treat drug-resistant cases, so attention in addition has turned to the potential of immunotherapy as an adjunct to chemotherapy. Research trends are summarized here.Emerging Microbes & Infections (2012) 1, e7; doi:10.1038/emi.2012.3
Keywords:
Introduction
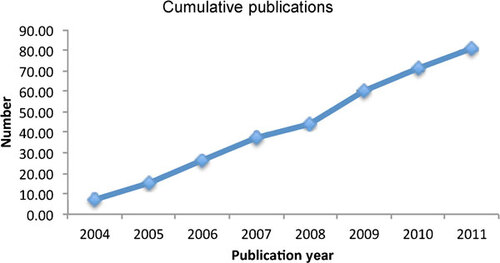
There has been a striking upsurge in tuberculosis (TB) vaccine research in China in recent years. More than 80 papers reporting such research have been published since 2004 (), many in English Language Journals. This has been driven and funded largely by Chinese Government agencies, since the scale of the TB problem and the difficulties in implementing fully effective control measures were recognized.Citation1,Citation2,Citation3 There seemingly has been little commercial interest. In considering the published output, we can note that there are at least two additional restricting factors limiting the progress of the field in China: few research groups have access to facilities for safely working with animals infected with a dangerous pathogenic organism such as the tubercle bacillus; infrastructure for undertaking vaccine clinical trials to international standards is sparse. In consequence, the universal problem of how to select among the promising approaches and candidate vaccines is particularly acute in China. Nevertheless, the basic research problems are being tackled with some enthusiasm and creativity, and the new 5-year program to improve TB control that was announced by the Chinese Ministry of Health on 1 April 2009 will have an impact. The antigens investigated to date and the locations of research groups are listed in and , respectively.
Table 1 TB antigens tested.
Table 2 The leading cities for TB vaccine research.
TB immunity
A substantial body of work has gone into characterizing the immune responses to be found in TB infection and following vaccination. The twin purposes of identifying candidate antigens for use in vaccines and diagnostic tests and of defining immunological markers for latent infection and disease states drive these studies. These studies have not always been followed up with tests of protective or therapeutic efficacy in animal infection models.
The increased immunogenicity of mouse dendritic cells transfected with antigen heat shock protein 65 (hsp65) has been tested in WuhanCitation4 and in Hong Kong, and differences have been found in the polarizing effect of intracellular Mycobacterium bovis Bacille Calmette–Guerin (BCG) vaccine bacteria on antigen presentation by human adult and cord blood dendritic cells.Citation5 Various forms of recombinant BCG expressing early secreted antigen 6 of Mycobacterium tuberculosis (ESAT6),Citation6 or antigen 85B (Ag85B) and antigen Rv3425,Citation7 or Ag85B and ESAT6 with tumor-necrosis factor-α,Citation8 or ESAT6 and human granulocyte macrophage-colony stimulating factor (GM-CSF)Citation9 have shown enhanced immunogenicity. Recombinant M. smegmatis expressing M. tuberculosis culture filtrate protein 10 (CFP10)/ESAT6 fusion protein was found stimulatory for macrophage inducible nitric oxide synthetase.Citation10 Recombinant M. smegmatis and BCG strains have been made that can express cloned antigens at a range of different levels under the control of modified FurA gene promoters.Citation11 Other BCG recombinants expressing ESAT6,Citation12 ESAT6/interleukin 2 (IL-2)Citation13 or Ag85B/ESAT6 fusionsCitation14 have been made.
A range of known antigens has been tested for immunogenicity as mixtures, fusion proteins and peptides. These include chimeric Ag85B/ESAT6 with adjuvants monophosphoryl lipid A (MPL) and trehalose 6,6'-dimycolate,Citation15 hsp16.3 with dimethyl-dioctadecyl-ammonium bromide/MPL (DDA/MPL) adjuvant,Citation16 fusion protein M. tuberculosis protein 64 (MPT64)–ESAT6,Citation17 resuscitation-promoting factor B (Rv1009),Citation18 or CFP10, ESAT6 or RpfE (Rv2450) with nitrocellulose,Citation19,Citation20 or Rv3772Citation21 and Rv3425Citation22 with incomplete Freund’s adjuvant; or Ag85B/MPT64190–198/Mtb8.4 plus a novel adjuvant of DDA with BCG extract.Citation23 In-silico analysis of putative MHC Class 1-restricted epitopes present in antigens encoded within the region of difference 1 (RD-1) to RD-16 regions of M. bovis genome has revealed potential high-affinity HLA bindersCitation24 and profiles of human humoral responses to 38-kDa, MTB48, CFP10/ESAT6 antigens have been defined.Citation25 Screening of human immune sera against an expression library of M. tuberculosis open reading frames revealed three novel antigens among the top 20 most strongly recognized: Rv1987, Rv3807c and Rv3887c.Citation26
More than a dozen studies have used DNA vaccination as a means of delivering antigens in immunogenicity tests. Many have shown Th1-biased immunogenicity without additional adjuvanting, for example with Ag85B,Citation27,Citation28 Ag85B/ESAT6 fusion,Citation29 ESAT6/CFP10 fusion,Citation30 Mtb8.4/38-kDa/Ag85B fusionCitation31 and epitopes from ESAT6, Ag85A, CFP10 and Ag85B inserted within hsp65.Citation32 Targeting the expressed product for degradation via the ubiquitin pathway has been used to enhance MHC Class 1 presentation of epitopes from MPT64 and 38-kDa,Citation33 MPT64,Citation34 and ESAT6.Citation35,Citation36 Liposome associated Ag85A DNA vaccine was shown to be immunogenic with oral delivery.Citation37 Enhanced responses to encoded antigens have been obtained by additionally encoding cytokines, such as interleukin 21 (IL-21), with Ag85ACitation38 or Ag85A/ESAT6,Citation39 and GM-CSF with Ag85A.Citation40 Inclusion of DNA expressing IL-12 enhanced prime/boost responses to BCG and to plasmids expressing Ag85A and ESAT6.Citation41 ESAT6 DNA priming and protein boosting has also been shown to give enhanced Th1 responses.Citation42,Citation43,Citation44
TB prophylaxis
Many antigen preparations have been tested for their capacity to protect against challenge infection with virulent M. tuberculosis. Subcutaneous ESAT6/CFP10,Citation19 or ESAT6/MPT64Citation17,Citation45 fusion proteins on nitrocellulose protected mice against H37Rv challenge, but not as effectively as BCG. hsp65/IL-2 fusion protein was found to elicit better protection than hsp65 given with DDA/MPL adjuvant and protection was equivalent to that produced by BCG.Citation46 Similarly, either hspX, a dormancy associated antigen, or synthetic epitope 91–104 gave protection equivalent to BCG when given with DDA/MPL.Citation47 Ag85B/MPT64190–198/Mtb8.4 fusion protein with DDA adjuvant boosted protection against challenge in BCG primed mice.Citation48 A similar fusion protein in which Mtb8.4 was replaced by hspX gave a similar boost, but boosting with a mixture of the two fusion proteins was even better.Citation49 A fusion protein of ESAT6 and Ag85A also significantly boosted protection against H37Rv in mice.Citation50
Recombinant BCG expressing the Ag85B/MPT64190–198/Mtb8.4 fusion protein gave slightly better protection to mice against H37Rv challenge than parent BCG or BCG expressing rAg85B alone.Citation51 Recombinant BCG expressing a fusion protein of human interleukin (hIL)-12p70 and ESAT6 showed increased immunogenicity but less protective effect.Citation52 Recombinant Salmonella typhimurium that both expressed ESAT6/Ag85B fusion protein and delivered it as a DNA vaccine when given orogastrically gave protection similar to subcutaneous BCG and the combination was superior to either vaccine alone.Citation53
Protection by DNA vaccination has been tested by many groups. The earliest reports indicated protection in mice superior to BCG when a divalent construct expressing both Ag85B and MPT64,Citation54 or a mixture of plasmids expressing Ag85B, MPT64, MPT63 and ESAT6Citation54,Citation55 was used. The protection given by a mixture of three plasmids expressing MPT83, Ag85B and ESAT6 was enhanced by including DDA adjuvantCitation56 and an encoded fusion protein of Ag85B and MPT64 was superior to the separately encoded antigens.Citation57,Citation58 Encapsulation in poly(lactide-co-glycolide) microspheres with DDA enhanced the protective efficacy in mice of DNA-encoding Ag85B/MPT64/MPT83 fusion antigen,Citation59 and strikingly the DNA mixed with DDA was superior to BCG in protecting cattle against challenge.Citation60 Inclusion of a plasmid expressing IL-2 improved protection by this plasmidCitation61 or by plasmid expressing Mtb8.4.Citation62 Plasmids expressing Ag85B,Citation28,Citation63 Ag85B/ESAT6Citation29,Citation64 or fusion proteins Mtb8.4/38-kDa/Ag85BCitation31 have given protection similar to BCG in mouse model challenge infections. A mixture of plasmids encoding Ag85B, MPT64, MPT70 and TB10.4 boosted protection by BCG,Citation65 as did a plasmid expressing a CFP21/MPT64 fusion protein.Citation66 Ag85B or Ag85A were superior to ESAT6 when compared separately for protection as DNA vaccines.Citation67 DNA expressing a fusion of MPB64/Ag85B/ESAT6 was superior to a mixture of plasmids expressing the separate antigens and gave protection equivalent to BCG.Citation68 The protective effect of DNA expressing hsp65 against BCG challenge was enhanced by incorporating epitopes of ESAT6, Ag85A/B and CFP10 within the hsp65 backbone.Citation32 The protective effect of hsp65 DNA against H37Rv challenge was increased by expression as a fusion with hIL-2, but did not surpass that of BCG.Citation69 Expression of Ag85B fused to bovine herpes virus 1 VP22 protein, which facilitates dissemination of antigen to adjacent cells, resulted in protection against H37Rv challenge in mice that was better than protection by BCG.Citation70 Few studies have been conducted with animals other than mice: a mixture of Ag85B, hspX and CFP10/ESAT6 fusion together with CpG and AlOH as adjuvant was immunogenic in mice but gave little protection against challenge with H37Rv in guinea pigs;Citation71 in contrast, combined DNA vaccines encoding antigens Ag85B, MPT64 and MPT83 given with DDA appeared to be better than BCG in protecting cattle.Citation72
TB therapy
Interest in therapeutic vaccination has been sustained by clinical studies of a commercial Chinese product, M. vaccae extract. Recent meta-analyses of published data concluded that this product gave significant benefit in preventing TB in people at high risk (13 studies),Citation73 but there was only a minor benefit from treating new TB cases (54 studies).Citation74 In a unique and contrasting approach, a recombinant M. smegmatis delivering DNA expressing human granulysin and murine IL-12 was recently found to be therapeutic against H37Rv infection.Citation75
Most research into therapeutic vaccines for TB has focused on the use of naked DNA vaccination. hsp65 DNA had a significant therapeutic effect against H37Rv in mice and the fusion hsp65/IL-2 was significantly better.Citation76 Treatment of infected mice with a DNA vaccine expressing a fusion protein of mycobacterial hsp70 and leukocyte cluster of differentiation antigen 80 substantially reduced acid-fast bacteria and pathology in liver and spleen, whereas BCG had no effect.Citation77 Although treatment with DNA expressing Ag85B was therapeutic, treatment with DNA expressing MPT64 was not, and the mixture was less effective than the Ag85B vaccine on its own,Citation78 and inclusion of DNA expressing IL-12 gave a slight additional benefit.Citation79 Studies of treatment of multidrug-resistant TB in mice have given encouraging results. Mice infected with a clinical isolate resistant to isoniazid and rifampicin responded well to treatment with the drugs plus DNA expressing chimeric Ag85A/ESAT6,Citation80 and appeared to respond better to treatment with the drugs plus DNA expressing Ag85A than to the DNA vaccine alone;Citation81 Ag85A DNA alone was at least as effective as Ag85A plus rifampicin in treating mice infected with a strain resistant to rifampicin and isoniazid, Ag85A/ESAT6 DNA was less effectiveCitation82 and Ag85A DNA worked in combination with pyrazinamide.Citation83 An immunogenic mixture of DNA vaccines expressing Ag85B, MPT64 and MPT83 has been found to work as a potent adjunct to isoniazid plus pyrazinamide therapy in miceCitation84 and to be therapeutic in cattle infected with M. bovis, reducing both pathology and bacterial numbers; a mixture expressing Ag85B, MPT64 and hsp65 was similarly effective.Citation72 Inclusion of immunostimulatory nucleotide motifs in the gene transcript enhanced the therapeutic efficacy of a plasmid expressing hsp65 when tested against H37Rv in mice.Citation85
Conclusions
It is evident in considering this body of recent TB vaccine research in China that much of it has been tactical in nature, establishing credentials both nationally and internationally, and building new research bases. Additionally, there are creative and insightful studies of particular relevance to the needs of China. Researchers are now able to exploit cutting edge technologies in designing new TB vaccines and are increasingly able to test the vaccines in relevant animal models of infection. The evidence that DNA vaccines can provide effective therapy for TB in cattleCitation72 may be a significant pointer to the future. The potential benefits of adding immunotherapies/therapeutic vaccines to TB chemotherapy have been recognized in China, but both preventive and therapeutic approaches against human TB await development of clinical trial capacity for their proper assessment.
References
- Announcement of the national tuberculosis prevention and control plan (2001–2010). State Council of the People’s Republic of China, General Office: Beijing, China, 2001. Document No. 75.
- Zhao FZ, Zhao Y, Liu XQ. Tuberculosis control in China. Tuberculosis2003; 83: 15–20.
- Wang LD, Liu JJ, Chin DP. Progress in tuberculosis control and the evolving public-health system in China. The Lancet2007; 369: 691–696.
- Wei YZ, Liu SW, Xia F. Initial research of dendritic cell vaccine against tuberculosis. Med J Wuhan Univ2005; 26: 747–752.
- Liu EM, Law HK, Lau YL. Mycobacterium bovis bacillus Calmette–Guerin treated human cord blood monocyte-derived dendritic cells polarize naive T cells into a tolerogenic phenotype in newborns. World J Pediat2010; 6: 132–140.
- Wang LM, Shi CH, Fan XL, Xue Y, Bai YL, Xu ZK. Expression and immunogenicity of recombinant Mycobacterium bovis bacillus Calmette–Guerin strains secreting the antigen ESAT-6 from Mycobacterium tuberculosis. Chin Med J (Engl)2007; 120: 1220–1225.
- Wang JL, Qie YQ, Zhu BD et al. Evaluation of a recombinant BCG expressing antigen Ag85B and PPE protein Rv3425 from DNA segment RD11 of Mycobacterium tuberculosis in C57BL/6 mice. Med Microbiol Immunol (Berl)2009; 198: 5–11.
- Shen HB, Wang C, Yang EZ et al. Novel recombinant BCG coexpressing Ag85B, ESAT-6 and mouse TNF-alpha induces significantly enhanced cellular immune and antibody responses in C57BL/6 mice. Microbiol Immunol2010; 54: 435–441.
- Yang XL, Bao L, Deng YH. A novel recombinant Mycobacterium bovis bacillus Calmette–Guerin strain expressing human granulocyte macrophage colony-stimulating factor and Mycobacterium tuberculosis early secretory antigenic target 6 complex augments Th1 immunity. Acta Biochim Biophys Sin2011; 43: 511–518.
- Li Y, Bao L, Zhang HD, Shang ZL, Zhong Q, Li YS. Expression of M. tuberculosis CFP-10/ESAT-6 fusion gene in M. smegmatis and its immunogenicity. Chin J Med Microbiol2006; 26: 1076–1079.
- Fan XY, Ma H, Guo J et al. A novel differential expression system for gene modulation in Mycobacteria. Plasmid2009; 61: 39–46.
- Zhang LX, Wu XQ, Dong EJ. Construction and identification of ESAT6 recombinant BCG vaccine. J Pract Med2009; 25: 517–520.
- Fan XF, Wang LM, Lu XY, Tu ZG, Shi CH, Xu ZK. Cloning and expression of the fusion protein of interleukin-2 and ESAT6 in Mycobacterium bovis Bacillus Calmette Guerin strain. Chin Med J (Engl)2005; 118: 762–765.
- Shi CH, Xu ZK, Zhu DS, Li Y, Bai YL, Xue Y. Screening and construction of recombinant BCG strains expressing the Ag85B–ESAT6 fusion protein. Chin J Tuberculosis Respir Dis2005; 28: 254–257.
- Xu Y, Wang B, Chen J et al. Chimaeric protein improved immunogenicity compared with fusion protein of Ag85B and ESAT-6 antigens of Mycobacterium tuberculosis. Scand J Immunol2006; 64: 476–481.
- Shi CH, Zhang TF, Zhu DS et al. The Immunological response induced by Mycobacterium tuberculosis heat shock protein 16.3 and its synthetic peptide in mice. Chin J Tuberculosis Respir Dis2009; 32: 603–607.
- Shi CH, An JZ, Tang XF et al. Immune responses and protective efficacy induced in mice by Mycobacterium tuberculosis MPT64–ESAT-6 fusion protein. J 4th Mil Med Univ2006; 27: 769–771.
- Fan AL, Shi CH, Su MQ et al. Immunological properties of Rv1009 domain from Mycobacterium tuberculosis. Chin J Lab Med2007; 31: 1282–1286.
- Zhang H, Shi CH, Xue Y, Bai YL, Wang LM, Xu ZK. Immune response and protective efficacy induced by fusion protein ESAT6–CFP10 of M. tuberculosis in mice. Chin J Cell Molec Immunol2006; 22: 443–446.
- Xue Y, Bai YL, Gao H, Wang LM, Xu ZK. Immune responses induced by Mycobacterium tuberculosis Rv2450 protein in mice. Chin J Cell Molec Immunol2007; 23: 921–923.
- Zhang M, Xu Y, Qie YQ et al. Humoral and cellulr immune responses in mice induced by trehalose-6-phosphate phosphatase from Mycobacterium tuberculosis. J Microbes Infect2006; 1: 144–148.
- Wang JL, Qie YQ, Zhang HM et al. PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a novel immunodominant antigen of Mycobacterium tuberculosis induces humoral and cellular immune responses in mice. Microbiol Immunol2008; 52: 224–230.
- Song NN, Wang BX, Shi DS et al. Dimethyldioctyl ammonium bromide-BCG polysaccharide nucleic acid adjuvant enhanced the immunogenicity of a Mycobacterium tuberculosis subunit vaccine. Chin J Tuberculosis Respir Dis2009; 32: 513–516.
- Wang JL, Zhang HM, Wang HH. Analysis of predicted CD8+ T cell epitopes from proteins encoded by the specific RD regions of Mycobacterium tuberculosis for vaccine development and specific diagnosis. Mol Biol Rep2010; 37: 1793–1799.
- Wu XQ, Yang YR, Zhang JX et al. Humoral Immune Responses against the Mycobacterium tuberculosis 38-Kilodalton, MTB48, and CFP-10/ESAT-6 Antigens in Tuberculosis. Clin Vacc Immunol2010; 17: 372–375.
- Li YQ, Zeng JM, Shi JF et al. A Proteome-scale identification of novel antigenic proteins in Mycobacterium tuberculosis toward diagnostic and vaccine development. J Proteome Res2010; 9: 4812–4822.
- You L, Zhao Y, Gao CH. Construction of Ag85B DNA vaccine for Mycobacterium tuberculosis and its immunogenicities. Chin J Tuberculosis Respir Dis2001; 24: 736–739.
- Hu J, Wu XQ, Wang LH, Zheng Y. The construction, immunogenicity and protective efficacy of tuberculosis Ag85B DNA vaccine. Sci Technol Eng2006; 6: 1765–1771.
- Shi CH, Wang XW, Zhang H, Xu ZK, Li Y, Yuan LT. Immune responses and protective efficacy induced by 85B antigen and early secreted antigenic target-6 kDa antigen fusion protein secreted by recombinant bacille Calmette–Guerin. Acta Biochim Biophys Sin2007; 39: 290–296.
- Zhang H, Shi CH, Wang LM, Xue Y, Bai YL, Xu ZK. Immunogenicity of DNA vaccine expressing ESAT6–CFP10 fusion protein of Mycobacterium tuberculosis. J 4th Mil Med Univ2007; 28: 489–492.
- Liu JB, Zhu ZY, Wang HB, Y.X, ZhangCF, ZhangY. Construction and immunoprotection of the TB DNA vaccine expressing Mycobacterium tuberculosis Mtb8.4–38000–Ag85B fusion protein. Immunological J2006;22: 47–50.
- Gao HF, Yue Y, Hu LK, Xu W, Xiong SD. A novel DNA vaccine containing multiple TB-specific epitopes casted in a natural structure (ECANS) confers protective immunity against pulmonary mycobacterial challenge. Vaccine2009; 27: 5313–5319.
- Wang QM, Sun SH, Hu ZL et al. Epitope DNA vaccines against tuberculosis: spacers and ubiquitin modulates cellular immune responses elicited by epitope DNA vaccine. Scand J Immunol2004; 60: 219–225.
- Wang QM, Sun SH, Kang L. Construction of ubiquitin-Mycobacterium tuberculosis PMT64 fusion gene DNA vaccine and its cellular immunological efficacy in mice. Acad J 2nd Mil Med Univ2007; 29: 117–121.
- Wang QM, Yin M, Zhang JC, Hu JQ, He Y, Sun SH. Ubiquitin and ESAT6 antigen fusion gene DNA vaccine induced stronger cellular immune response in mice. Acad J 2nd Mil Med Univ2007; 28: 261–265.
- Wang QM, Kang L, Wang XH. Improved cellular immune response elicited by a ubiquitin-fused ESAT-6 DNA vaccine against Mycobacterium tuberculosis. Microbiol Immunol2009; 53: 384–390.
- Wang DA, Xu J, Feng YH et al. Liposomal oral DNA vaccine (mycobacterium DNA) elicits immune response. Vaccine2010; 28: 3134–3142.
- Dou J, Tang Q, Zhao FS et al. Comparison of immune responses induced in mice by vaccination with DNA vaccine constructs expressing mycobacterial antigen 85A and interleukin-21 and bacillus Galmette–Guerin. Immunol Invest2008; 37: 113–127.
- Li JL, Yu FL, Wang YQ et al. Constructing nucleic acid vaccine expressing fusion antigens of Mycobacterium tuberculosis Ag85A and ESAT-6, and interleukin 21 as well as studying its immune effect in mice. Lett Biotech2009; 20: 765–768.
- Dou J, Tang Q, Yu FL et al. Investigation of immunogenic effect of the BCG priming and Ag85A–GM-CSF boosting in Balb/c mice model. Immunobiol2010; 215: 133–142.
- Bao L, Gao L, Bao Y. Immunogenicity of interleukin 12 and DNA vaccine prime-BCG boost against Mycobacterium tuberculosis. Int J Infect Dis2010; 14: e446.
- Wang QM, Sun SH, Hu ZL, Yin M, Xiao CJ, Zhang JC. Improved immunogenicity of a tuberculosis DNA vaccine encoding ESAT6 by DNA priming and protein boosting. Vaccine2004; 22: 3622–3627.
- Li ZM, Song D, Zhang HY et al. Improved humoral immunity against tuberculosis ESAT-6 antigen by chimeric DNA prime and protein boost strategy. DNA Cell Biol2006; 25: 25–30.
- Li ZM, Zhang HY, Fan XY et al. DNA electroporation prime and protein boost strategy enhances humoral immunity of tuberculosis DNA vaccines in mice and non-human primates. Vaccine2006; 24: 4565–4568.
- Bai Y, Xue Y, Gao H et al. Expression and purification of Mycobacterium tuberculosis ESAT-6 and MPT64 fusion protein and its immunoprophylactic potential in mouse model. Protein Expr Purif2008; 59: 189–196.
- Shi C, Yuan S, Zhang H, Zhang T, Wang L, Xu Z. Cell-mediated immune responses and protective efficacy against infection with Mycobacterium tuberculosis induced by hsp65 and hIL-2 fusion protein in mice. Scand J Immunol2009; 69: 140–149.
- Shi C, Zhang H, Zhang T et al. New alternative vaccine component against Mycobacterium tuberculosis-heat shock protein 16.3 or its T-cell epitope. Scand J Immunol2009; 70: 465–474.
- Luo Y, Wang BX, Hu LN et al. Fusion protein Ag85B–MPT64190–198–Mtb8.4 has higher immunogenicity than Ag85B with capacity to boost BCG-primed immunity against Mycobacterium tuberculosis in mice. Vaccine2009; 27: 6179–6185.
- Li Q, Yu H, Zhang Y et al. Immunogenicity and protective efficacy of a fusion protein vaccine consisting of antigen Ag85B and HspX against Mycobacterium tuberculosis infection in mice. Scand J Immunol2011; 73: 568–576.
- Lu J, Wang C, Zhou ZG et al. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin Dev Immunol2011; 2011: 617892.
- Qie YQ, Wang JL, Liu W et al. More vaccine efficacy studies on the recombinant bacille Calmette–Guerin Co-expressing Ag85B, Mpt64190–198 and Mtb8.4. Scand J Immunol2009; 69: 342–350.
- Deng YH, Bao L, Yang XL. Evaluation of immunogenicity and protective efficacy against Mycobacterium tuberculosis infection elicited by recombinant Mycobacterium bovis BCG expressing human Interleukin-12p70 and Early Secretory Antigen Target-6 fusion protein. Microbiol Immunol2011; 55: 798–808.
- Wang QL, Pan Q, Ma YF et al. An attenuated Salmonella-vectored vaccine elicits protective immunity against Mycobacterium tuberculosis. Vaccine2009; 27: 6712–6722.
- Tian X, Cai H, Zhu YX. Protection of mice with a divalent tuberculosis DNA vaccine encoding antigens Ag85B and MPT64. Acta Biochim Biophys Sin (Shanghai)2004; 36: 269–276.
- Cai H, Tian X, Hu XD et al. Immunogenicity and protective efficacy study using combination of four tuberculosis DNA vaccines. Sci China Ser C-Life Sci2003; 46: 495–498.
- Cai H, Tian X, Hu XD, Zhuang YH, Zhu YX. Combined DNA vaccines formulated in DDA enhance protective immunity against tuberculosis. DNA Cell Biol2004; 23: 450–456.
- Luo XD, Zhu DY, Chen Q, Jiang Y, Jiang S, Yang CS. A study of the protective effect of the DNA vaccine encoding tubercle antigen 85B with MPT64 in mice challenged with Mycobacterium tuberculosis. Chin J Tuberculosis Respir Dis2004; 27: 611–616.
- Luo XD, Zhu DY, Jiang S, Chen Q, Jiang Y. The estimation of protective efficacy of the fusion gene vaccine encoding tubercle antigen 85B and MPT64 in mice challenged with Mycobacterium tuberculosis. Natl Med J Chin2004; 84: 687–691.
- Cai H, Hu XD, Yu DH, Li SX, Tian X, Zhu YX. Combined DNA vaccine encapsulated in microspheres enhanced protection efficacy against Mycobacterium tuberculosis infection of mice. Vaccine2005; 23: 4167–4174.
- Cai H, Tian X, Hu XD, Li SX, Yu DH, Zhu YX. Combined DNA vaccines formulated either in DDA or in saline protect cattle from Mycobacterium bovis infection. Vaccine2005; 23: 3887–3895.
- Cai H, Yu DH, Tian X, Zhu YX. Coadministration of interleukin 2 plasmid DNA with combined DNA vaccines significantly enhances the protective efficacy against Mycobacterium tuberculosis. DNA Cell Biol2005; 24: 605–613.
- Zhu ZY, Xie Y, Wang HB et al. Protective efficacy of co-immunization of T DNA vaccine pVS84 and plasmid pIL2 equivalent to that of BCG. Chin J Zoonoses2006; 22: 318–321.
- Liu JB, Zhu ZY, Wang HB, Xie Y, Zhang CF, Zhang Y. Construction of the eukaryotic expression plasmid pVS85B expressing the protective antigen Ag85B of Mycobacterium tuberculosis and its immunoprotective studies. Curr Immunol2006; 26: 121–126.
- Shi CH, Wang XW, Zhang H, Zhang TF, Wang LM, Xu ZK. Immune responses and protective efficacy of the gene vaccine expressing Ag85B and ESAT6 fusion protein from Mycobacterium tuberculosis. DNA Cell Biol2008; 27: 199–207.
- Li M, Yu DH, Cai H. DNA prime-BCG boost vaccination strategy improved the protective efficacy against M-tuberculosis H37Rv in mice. Prog Biochem Biophys2007; 34: 746–753.
- Wang C, Chen ZH, Fu RL et al. A DNA vaccine expressing CFP21 and MPT64 fusion protein enhances BCG-induced protective immunity against Mycobacterium tuberculosis infection in mice. Med Microbiol Immunol (Berl)2011; 200: 165–175.
- Fan XL, Gao Q, Fu RL. Differential immunogenicity and protective efficacy of DNA vaccines expressing proteins of Mycobacterium tuberculosis in a mouse model. Microbiol Res2009; 164: 374–382.
- Liu SG, Gong Q, Wang CL et al. A novel DNA vaccine for protective immunity against virulent Mycobacterium bovis in mice. Immunol Lett2008; 117: 136–145.
- Wang LM, Bai YL, Shi CH et al. Immunogenicity and protective efficacy of a DNA vaccine encoding the fusion protein of mycobacterium heat shock protein 65 (Hsp65) with human interleukin-2 against Mycobacterium tuberculosis in BALB/c mice. APMIS2008; 116: 1071–1081.
- Yao WH, Liu SW, Qu XJ, Xiao SB, Liu Y, Liu JY. Enhanced immune response and protection efficacy of a DNA vaccine constructed by linkage of the Mycobacterium tuberculosis Ag85B-encoding gene with the BVP22-encoding gene. J Med Microbiol2009; 58: 462–468.
- Chen L, Xu M, Wang ZY et al. The development and preliminary evaluation of a new Mycobacterium tuberculosis vaccine comprising Ag85b, HspX and CFP-10:ESAT-6 fusion protein with CpG DNA and aluminum hydroxide adjuvants. FEMS Immunol Med Microbiol2010; 59: 42–52.
- Hu XD, Chen ST, Yu DH, Li SX, Cai H. Immunotherapy with combined DNA vaccines is an effective treatment for M. bovis infection in cattle. Vaccine2009; 27: 1317–1322.
- Yang XY, Chen QF, Cui XH, Yu Y, Li YP. Mycobacterium vaccae vaccine to prevent tuberculosis in high risk people: a meta-analysis. J Infect2010; 60: 320–330.
- Yang XY, Chen QF, Li YP, Wu SM. Mycobacterium vaccae as adjuvant therapy to antituberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. Plos One2011; 6: e23826.
- Yang C, He YL, Zhang L et al. GLS/IL-12-modified Mycobacterium smegmatis as a novel anti-tuberculosis immunotherapeutic vaccine. Int J Tuberc Lung Dis2009; 13: 1360–1366.
- Shi CH, Zhang H, Wang LM et al. Therapeutic efficacy of a tuberculosis DNA vaccine encoding heat shock protein 65 of Mycobacterium tuberculosis and the human interleukin 2 fusion gene. Tuberculosis2009; 89: 54–61.
- Shi XL, Li HW, Zhong S. Study on the role of the chimerichsp70/CD80 DNA vaccine for treating infection of Mycobacterium tuberculosis. Chin J Infect Dis2004; 22: 30–33.
- Zhu DY, Jiang S, Luo XD. Therapeutic effects of Ag85B and MPT64 DNA vaccines in a murine model of Mycobacterium tuberculosis infection. Vaccine2005; 23: 4619–4624.
- Jiang S, Zhu DY, Luo XD, Jiang Y, Chen Q. Therapeutic effects of DNA vaccines in a murine model of Mycobacterium tuberculosis infection. Zhonghua Jie He He Hu Xi Za Zhi; 28: 305–309. Chinese.
- Liang Y, Wu XQ, Zhang JX. Therapeutic effects of chimeric Ag85A/ESAT-6 DNA vaccines combined with chemotherapy in a mouse model infected with multi-drug resistant Mycobacterium tuberculosis. J Chin Antituberc Assoc2007; 29: 382–385.
- Liang Y, Wu XQ, Zhang JX. Therapeutic effects of Ag85A DNA vaccines combined with chemotherapy in a mouse model of MDR Mycobacterium tuberculosis infection. Guangdong Med J2007; 28: 1398–1400.
- Liang Y, Wu XQ, Zhang JX et al. The treatment of mice infected with multi-drug-resistant Mycobacterium tuberculosis using DNA vaccines or in combination with rifampin. Vaccine2008; 26: 4536–4540.
- Liang Y, Wu X, Zhang J et al. Treatment of multi-drug-resistant tuberculosis in mice with DNA vaccines alone or in combination with chemotherapeutic drugs. Scand J Immunol2011; 74: 42–46.
- Yu DH, Hu XD, Cai H. Efficient tuberculosis treatment in mice using chemotherapy and immunotherapy with the combined DNA vaccine encoding Ag85B, MPT-64 and MPT-83. Gene Ther2008; 15: 652–659.
- Wu J, Ma H, Qu Q et al. Incorporation of immunostimulatory motifs in the transcribed region of a plasmid DNA vaccine enhances Th1 immune responses and therapeutic effect against Mycobacterium tuberculosis in mice. Vaccine2011; 29: 7624–7630.