Abstract
Explosive outbreaks of infectious diseases occasionally occur without immediately obvious epidemiological or microbiological explanations. Plague, cholera and Streptococcus pyogenes infection are some of the epidemic-prone bacterial infections. Besides epidemiological and conventional microbiological methods, the next-generation gene sequencing technology permits prompt detection of genomic and transcriptomic profiles associated with invasive phenotypes. Horizontal gene transfer due to mobile genetic elements carrying virulence factors and antimicrobial resistance, or mutations associated with the two component CovRS operon are important bacterial factors conferring survival advantage or invasiveness. The high incidence of scarlet fever in children less than 10 years old suggests that the lack of protective immunity is an important host factor. A high population density, overcrowded living environment and a low yearly rainfall are environmental factors contributing to outbreak development. Inappropriate antibiotic use is not only ineffective for treatment, but may actually drive an epidemic caused by drug-resistant strains and worsen patient outcomes by increasing the bacterial density at the site of infection and inducing toxin production. Surveillance of severe S. pyogenes infection is important because it can complicate concurrent chickenpox and influenza. Concomitant outbreaks of these two latter infections with a highly virulent and drug-resistant S. pyogenes strain can be disastrous.
Introduction
There are currently 74 species under the genus Streptococcus.Citation1 The type species of the genus, Streptococcus pyogenes, is one of the most virulent species causing human infections. S. pyogenes is a prototype bacterium that causes exotoxin-mediated infections. It produces a plethora of exotoxins, superantigens and cell wall-associated proteins resulting in diverse clinical manifestations, ranging from classical pyogenic infections, to toxic shock syndrome and post-infectious immune-mediated sequelae. Despite the fact that systemic infections, such as meningitis and endocarditis, are rare nowadays, streptococcal pyoderma and pharyngotonsillitis remain common infections with a heavy global burden of disease.Citation2 The past two decades have also witnessed a resurgence of several infective syndromes of S. pyogenes, most notably necrotizing skin and soft tissue infections and scarlet fever. Here we review the bacteriology, epidemiology and clinical manifestations of S. pyogenes infection and scarlet fever with respect to the genesis of outbreaks and their management.
Bacteriology and microbial factors
S. pyogenes is a catalase-negative aerobic Gram-positive coccus arranged in chains. The species is almost synonymous with Lancefield's group A Streptococcus, with the exception of the occasional strains of Streptococcus dysgalactiae and Streptococcus anginosus that may possess the group A antigen.Citation3 Differentiation from other group A streptococci is generally simple using the pyrrolidonylarylamidase and Voges–Proskauer reaction.Citation4
In addition to the Lancefield's antigen, the M and T surface antigens are also important in the classification of S. pyogenes. The M protein is a fibrillar protein located at the cell wall surface and is encoded by the emm gene. Functionally, in the absence of opsonizing antibodies, the M protein is anti-phagocytic, inhibits deposition of complements, interacts with a large number of host proteins, possesses pro-inflammatory activities and contribute to mucosal adhesion.Citation5 Molecular mimicry of M proteins is also implicated in the pathogenesis of post-streptococcal glomerulonephritis and acute rheumatic fever. Antibodies towards M proteins confer type-specific immunity in humans, but there is little heterologous immunity to other M types and the protective opsonizing activity of antibodies appears to be restricted to closely related strains even within the same M types.Citation6,Citation7 The persistence of type-specific antibodies after natural infection is variable; some decline in antibody titer with time occurs, but in some individuals, the antibodies can persist for as long as 32 years.Citation8 M protein typing has been used for over 60 years and has proved to be very useful in distinguishing strains with certain tissue tropism. With the increasing number of M protein types being described over the year, a new system based on emm gene sequence analysis is adopted. Currently, there are 139 named emm types (with 941 subtypes) and 326 unnamed sequence types.Citation9 The M typing correlates strongly with the tissue tropism of individual S. pyogenes strains. The presence of multiple M types and possible strain-restricted protective immunity means that re-infections due to S. pyogenes is inevitable and this also impacts the development of M protein-based vaccines.
Streptococcal genomes are noted for a high degree of plasticity. At least 16 complete genomes of S. pyogenes have been published, covering M types 1, 2, 3, 4, 5, 6, 12, 18, 28 and 49.Citation10,Citation11,Citation12,Citation13,Citation14,Citation15,Citation16,Citation17,Citation18,Citation19 The size of sequenced S. pyogenes genomes is about 1.88 Mbp (range: 1.82–1.94 Mbp), with a G+C content of about 38.6%. One important feature of the S. pyogenes genome is that its genetic variation is largely determined by the presence of prophages or prophage-like elements. Each genome contains on the average 5 such elements (range: 3–8) which together make up about 10% of the total genome. These prophage-like elements frequently carry virulence mechanisms such as exotoxin, adhesin, and superantigen genes.Citation20 Besides genetic changes due to mobile genetic elements, spontaneous mutations of the genes encoding the operon of the CovRS two-component system can alter the transcriptomic pattern of 10% of all the genes in the genome of S. pyogenes M1T1 to an invasive transcriptomic profile. Such mutations can lead to strong upregulaton of many virulence associated genes encoding the hyaluronic acid capsule synthesis, streptolysin O, streptococcal inhibitor of complement, nicotinamide adenine dinucleotide glycohydrolase (NAD glycohydrolase), interleukin-8 protease and DNase Sda1 which allows S. pyogenes to escape killing by neutrophils through the degradation of the DNA-based neutrophil extracellular traps.Citation21,Citation22 Some other mutations of CovRS operon can decrease the expression of the streptococcal pyrogenic exotoxin B (SpeB) which is a broad-spectrum secreted cysteine protease. SpeB can cleave many virulence factors produced by S. pyogenes. The downregulaton of SpeB will stop the degradation of the plasminogen activator streptokinase, M1 surface protein and host plasminogen and therefore the accumulation of plasmin activity on the bacterial cell surface. This will enable the dissemination of the bacterium from the skin or mucosal surfaces to normally sterile anatomical sites.Citation23
Horizontal gene transfer has been found to be a major mechanism in generating emm gene diversity through intragenic and intergenic recombination.Citation24 Such gene transfer events occur not only within the S. pyogenes species, but also among other streptococcal species such as S. dysgalactiae and group G Streptococcus.Citation25 S. dysgalactiae subsp. equisimilis, which is closely related to S. pyogenes phylogenetically, also possesses the emm gene and causes infections classically associated with S. pyogenes, such as streptococcal toxic shock syndrome, post-streptococcal glomerulonephritis and acute rheumatic fever.Citation26 In addition to the emm gene diversity, horizontal gene transfer also confers new antibiotic resistance mechanisms (such as quinolone and sulphonamide resistance) and virulence mechanisms.Citation27,Citation28,Citation29 A recent study also demonstrated the presence of S. pyogenes virulence genes in S. dysgalactiae subsp. dysgalactiae.Citation30 Although further proofs are necessary, it has been suggested that generation of new subclones of the bacterium through these phages and horizontal gene transfer could account for the sudden occurrence of epidemics and changes in the virulence of S. pyogenes.Citation11,Citation29
Epidemiology and environmental factors
Asymptomatic pharyngeal carriage of S. pyogenes occurs in 3%–26% of healthy children, and 3%–17% of children younger than 5 years of age.Citation31 Among children with sore throat, 23%–58% of them have S. pyogenes being detected (17%–24% in children younger than 5 years).Citation31 Screening performed during outbreak investigations in long-term care facilities and military training camps showed that the prevalence of S. pyogenes colonization was around 16%–17%,Citation32,Citation33 and up to 12%–88% among children in schools and day care centres during community outbreaks.Citation34,Citation35 The risk of transmission of S. pyogenes from index cases to contacts depends on the duration of exposure and the distance from the index case.Citation36 In one study, the risk of colonization was significantly higher if the contact time was more than 24 h per week (27% versus 1.8% for contacts having ≥24 h versus 12–24 h per week, respectively).Citation36 About 12% of asymptomatic household contacts may carry the same strain of bacterium.Citation37
The sites of colonization include the mucosal surfaces and, to a lesser extent, the skin. The oropharyngeal mucosa is the main site of colonization, but other locations such as the gastrointestinal tract and lower female genital tract can also be colonized. Person-to-person transmission involves respiratory droplets and direct contact. The bacterium can readily be found in the air and inanimate environment (such as dust and linen) where there are patients with S. pyogenes infections,Citation38,Citation39,Citation40,Citation41 though environmental objects such as blankets and dusts appeared to be unimportant sources of human infection, presumably due to a reduction in infectivity after desiccation.Citation40,Citation41 However, since S. pyogenes may survive on the inanimate objects for up to 4 weeks,Citation42 environmental hygiene and disinfection should be strictly observed especially within institutions and families.
S. pyogenes is considered to be a human-adapted pathogen. The bacterium has occasionally been found in companion animals, but acquisition from household humans might have occurred and pets are not considered to be an important source of human infection.Citation43
One peculiarity in the epidemiology of S. pyogenes is the occurrence of foodborne transmission, often resulting in common source outbreaks. Foodborne streptococcal pharyngitis and occasionally scarlet fever have long been recognized. Unpasteurized milk used to be a common food vehicle, which was often derived from cows with clinical mastitis.Citation44,Citation45,Citation46 As S. pyogenes is considered to be a sole human pathogen, transmission of the bacterium from humans to the cattle was believed to be the source of mastitis.Citation45 Milk-borne epidemics have become less common with the widespread adoption of pasteurization, but other food items can still be contaminated by asymptomatically colonized food handlers (through hands or respiratory secretions) resulting in outbreaks with high attack rates.Citation47,Citation48,Citation49 Eggs and salads are often involved in these outbreaks, as a result of contamination by food handlers and inappropriate holding temperatures after food preparation. In contrast to pharyngitis resulting from droplet transmission, foodborne cases tend to have a high attack rate (50%–90%) and shorter incubation periods.Citation49
S. pyogenes is the streptococcal species most commonly associated with epidemics, and outbreaks are characterized by the diversity of clinical syndromes, and their occurrence in both the community (such as within families, schools, day-care centres and prisons) and health-care institutions. Injecting drug users are at high risks of developing S. pyogenes infections, which can range from pyoderma to pneumonia and other invasive diseases.Citation50 S. pyogenes outbreaks in military facilities have been reported frequently, which may manifest as pyoderma, ecthyma, streptococcal pharyngitis, acute rheumatic fever and pneumonia.Citation32,Citation51,Citation52 In long-term care facilities, such outbreaks can be particularly devastating with high mortality rates because of the large number of residents who may have other concurrent medical illnesses, and can linger on for several months.Citation33
The epidemiology of major syndromes caused by S. pyogenes has seen some dramatic changes in the past decades. The incidence of acute rheumatic fever has decreased in most developing countries in the first three quarters of the twentieth century. Although a variety of explanations have been postulated, a change in the prevalence of rheumatogenic M types of S. pyogenes is possibly an important factor contributing to the decline.Citation53 However, a resurgence of acute rheumatic fever occurred in the mid-1980s in the United States which was not restricted to the underprivileged of the community.Citation54 In many communities, the resurgence is associated with the reappearance of mucoid strains of S. pyogenes type emm18.1.Citation55 From the late 1980s to 1990s, the epidemiology of S. pyogenes was dominated by a surge of invasive infections with high case-fatality rates, including necrotizing skin and soft tissue infections (such as necrotizing fasciitis and myositis) and streptococcal toxic shock syndrome in Europe and North America.Citation56
One of the most recent resurgence of S. pyogenes infections is scarlet fever. The disease was rampant before the twentieth century, causing frequent widespread epidemics or localized outbreaks and carrying high mortality.Citation57,Citation58 In the nineteenth-century England and Wales, for example, epidemics occurred with regularity every 5–6 years, and appeared to be correlated with years of low rainfall.Citation59,Citation60 In temperate countries, the disease is more prevalent in autumn and winter, and is more commonly seen in children (especially under 10 years of age), although adults are also susceptible to the development of scarlet fever.Citation61,Citation62 Scarlet fever outbreaks are particularly problematic in schools with attack rates over 20%–30%, because of the close proximity of a large number of susceptibility of children in a relatively confined environment. School outbreaks can last for weeks and in the pre-antibiotic era, had persisted for about 10 months.Citation63
Scarlet fever outbreaks became less frequently reported in the twentieth century, but are increasingly recognized in the past decade. Scarlet fever outbreaks have been reported in Vietnam (2009, over 23 000 cases); Guernsey, the United Kingdom (2011, 6 cases); Valencia, Spain (2011, 40 cases); Shanghai, China (2011, over 40 cases); Guangdong, China (2011, over 841 cases); Northwest Territories, Canada (2012, over 100 cases); Kansas, USA (2012); and Hong Kong (2011, 1534 cases, ongoing in 2012 at the time of writing).Citation64,Citation65
The appearance of outbreaks and changes in the epidemiology could be contributed by changes in herd immunity, genetic mutations or replacement by new circulating S. pyogenes strains. Data on seroprevalence and level protective immunity in the community are sparse and difficult to obtain because of the multitude of M types and possible strain-specific immunity. In a genome-wide study of S. pyogenes M3 strains which causes an epidemic of invasive disease in Canada, a four-amino acid duplication at the extreme N terminus of the M protein was found to be a consistent feature of the isolates, as well as the predominance of subclones carrying an SpeA-encoding prophage, the latter presumably increased the fitness and virulence of the strains.Citation66 A recent analysis showed that even relatively small accumulations of genetic polymorphism can lead to substantial alterations in the transcriptome and extracellular secretome of the bacterium, which may lead to altered virulence and changing epidemiology of the infections.Citation67 On the other hand, although virulence markers such as the Streptococcus invasive locus (sil) could be associated with invasive diseases, the prevalence of the markers could be the same among both invasive and non-invasive isolates.Citation68 Therefore, while alterations in the virulence genes may contribute to the changing epidemiology of S. pyogenes, they may interact with other epidemiological and host factors in shaping the epidemiology of diseases.
Changes in the predominant emm clones in the community can also account for fluctuations in the incidence of S. pyogenes infections.Citation69 It has also been shown that the main driver for the increase in the prevalence of group A streptococcal infections may not be related to emergence of new hypervirulent strains; rather, widespread transmission and a high prevalence in the community is the more important factor.Citation70 Changes in the disease rate in the community is also related to strain displacements on a wide geographical scale.Citation71
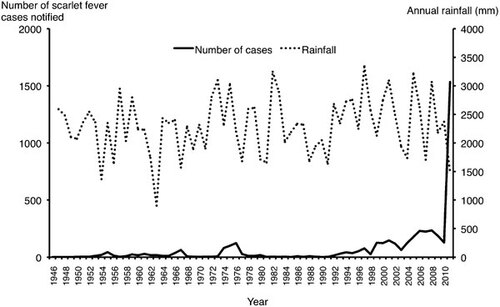
Genetic changes have been detected in S. pyogenes strains causing the 2011 epidemic of scarlet fever in Hong Kong. Scarlet fever has been a notifiable disease in Hong Kong since the 1940s. The annual notification of scarlet fever had remained low with only a peak incidence of 124 cases in 1976 (2.8 cases per 100 000 population). However, since the late 1990s, there appeared to be a gradual increase in the number of cases reported (). In March 2011, there was an abrupt epidemic of scarlet fever in the community, with 1534 cases being reported in 2011 (annual incidence of 21.58 cases per 100 000 population) with at least three fatalities and several severe cases requiring intensive care. The majority of the strains belongs to the emm12 type, which is also commonly encountered globally and in nearby regions such as China and Taiwan.Citation72,Citation73 Pulsed-field gel electrophoresis of 22 emm12 strains isolated from scarlet fever patients in 2011 showed that they are polyclonal in nature.Citation74
Figure 1 Annual reported cases of scarlet fever yearly rainfall in Hong Kong (1946–2011). Data from Department of Health, Hong Kong and Hong Kong Observatory. Rainfall information was not available for the year 1946 due to the Second World War.
Whole-genome analysis of an emm12 strain isolated from a fatal patient (the HKU16 strain) revealed a circular genome of 1 908 100 bp with G+C content of 38.5% (similar to previously reported genome size of S. pyogenes).Citation74 As in MGAS9249 and MGAS2096 (two previously sequenced emm12 genomes), HKU16 also possesses the speC and spd1 genes, but the superantigen gene ssa is present in HKU16 and not in the other two genomes. However, the Φ9429.1 prophage which carries the speC and spdI genes is absent in HKU16. Instead, HKU16 contains a novel prophage termed ΦHKU.vir which also harbours ssa. In addition, the genome of HKU16 differs from MGAS9429 and MGAS2096 by a large genomic inversion encompassing 81% of the genome. The HKU16 genome also contains two novel genomic insertions of the sizes 64.9 kb and 46.4 kb. The 64.9 kb insert contains a Tn916-type transposon embedded within another putative conjugative transposon. This integrative and conjugative element, ICE-emm12, carries the ermB (macrolide–lincomycin–streptogramin resistance) and tetM (tetracycline resistance) genes, as well as a MATE-type efflux pump and multi-drug ABC-type transporter. The 46.4 kb insertion is a prophage containing the ssa (streptococcal superantigen), speC (pyrogenic exotoxin C) and spd1 (DNase) genes. Phenotypically, HKU16 demonstrates a significantly higher level of adherence to HEp-2 human epithelial cells as compared to the hypervirulent clone M1T1 strain 5448, but a similar level of resistance to killing by human neutrophils and lethality to mice. How relevant are such laboratory findings to the clinical and epidemiological situations require further investigations.
The presence of these unique genetic markers in HKU16 was examined in other contemporary and historical S. pyogenes strains in Hong Kong.Citation74 Among 36 emm12 scarlet fever strains in 2011, 86.4% of them possessed ICE-emm12 and 81.8% had ΦHKU.vir. Among non-scarlet fever-associated 2011 S. pyogenes emm12 strains (n=7), ICE-emm12 and ΦHKU.vir were present in 5.7% and 4.3% of the strains, respectively. In emm12 strains isolated from 2005 to 2010 (n=18), 66.7% and 72.2% of them were positive for ICE-emm12 and ΦHKU.vir respectively.
The reasons for the 2011 outbreak of scarlet fever in Hong Kong are still unknown. Although it is tempting to attribute the epidemic to such novel genetic findings, the biological significance of these changes has to be confirmed by further studies. In addition to the virulence mechanisms of such new strains, one should closely scrutinize for any potential enhancement in interpersonal transmission or environmental survival. The earliest time of appearance of this strain in Hong Kong, whether such genetic alterations per se are sufficient to generate an epidemic, and whether bacteriological factors interact with other environmental factors are unknown. The presence of emm12 S. pyogenes isolates carrying ICE-emm12 and ΦHKU.vir from 2005 onwards appears to be consistent with a moderate increase in scarlet fever cases since that time (). It would be informative to see if historical strains from 1999 to 2002 also carried these genetic markers, during which period there was a modest rise in the incidence of scarlet fever. Interestingly, 2011 was also the driest year in Hong Kong since 1963,Citation75 which echoes earlier findings of the relationship between scarlet fever and rainfall.
It is almost a dogma that different strains of S. pyogenes are associated with different propensity to cause acute rheumatic fever and post-streptococcal glomerulonephritis. The emm/M type of the infecting strains bears correlation with the potential to cause either one of these sequelae. M proteins of rheumatogenic strains bear epitopes that cross-react with heart, synovium and brain tissues, which results in autoimmune damages in susceptible hosts.Citation76 Acute rheumatic fever typically follows pharyngotonsillitis, while glomerulonephritis more often follows cutaneous infections (such as impetigo), though it can also complicate respiratory infections. Various streptococcal antigens have been proposed to have a causal role in glomerulonephritis, with SpeB likely to be an important antigen.Citation77,Citation78 M types 1, 3, 5, 6, 14, 18, 19, 24, 27 and 29 are commonly rheumatogenic, while M types 1, 2, 4, 12, 15, 18, 25, 42, 49, 55, 56, 57, 59, 60 and 61 are the nephritogenic types.Citation53,Citation78,Citation79 There are, however, geographic variations in that some less commonly encountered M types could be important causes of non-suppurative sequelae in some parts of the world. Examples include the association of M types 71, 92, 93, 98, 103 and 112 with acute rheumatic fever in Hawaii, M types 48 and 73 with glomerulonephritis in Trinidad, and M type 63 with glomerulonephritis in China.Citation80,Citation81,Citation82
It is perhaps remarkable that the 2011–2012 outbreak of scarlet fever in Hong Kong has not been followed by a major epidemic of acute rheumatic fever. The reason is unknown, but possibly due to the fact that the M12 is not classically a rheumatogenic strain,Citation76 though there had been previous associations between M12 and acute rheumatic fever.Citation55,Citation83 Heightened awareness among clinicians with earlier use of appropriate antibiotics may have contributed to preventing the development of acute rheumatic fever.Citation84
Clinical diseases and host factors
The clinical manifestations of S. pyogenes infection range from asymptomatic carriage to fulminant and fatal diseases. Infections have been classified into: (i) streptococcal toxic shock syndrome; (ii) other invasive infections, defined as the isolation of S. pyogenes in normally sterile sites but not meeting the criteria for streptococcal toxic shock syndrome; (iii) scarlet fever; (iv) non-invasive infections, including cutaneous and mucosal infections; and (v) non-suppurative sequelae.Citation85
The commonest sites of group A streptococcal infection are the upper respiratory tract and the skin and soft tissues. Streptococcal pharyngitis and tonsillitis can be followed by local and distant suppurative complications (such as peritonsillar and retropharyngeal abscesses) or non-suppurative sequelae (acute rheumatic fever being commoner than post-streptococcal glomerulonephritis after respiratory tract infections). Soft tissue infections range from superficial pyoderma such as impetigo, to erysipelas, cellulitis and more devastating necrotizing infections including necrotizing fasciitis and myositis. Other infections such as meningitis and puerperal sepsis are less commonly seen nowadays. Bacteraemia can complicate infections in any organ system. The presence of skin pathologies should alert one to the potential complication by invasive diseases.Citation86 An antecedent episode of varicella is one of the most important risk factors for invasive S. pyogenes infections in children, which often presents as necrotizing fasciitis.Citation86,Citation87 In adults, risk factors include various comorbidities, such as diabetes mellitus, malignancies, alcoholism, use of immunosuppressive agents and steroids, chronic heart, lung, liver or renal diseases, HIV infection and injection drug use.Citation37,Citation86,Citation87,Citation88,Citation89 The presence of gastrointestinal symptoms should alert the clinicians to the potential development of severe group A streptococcal diseases.Citation90
Pneumonia is not a frequent manifestation of S. pyogenes infection in recent years. However, during the latest 2009 pandemic influenza, S. pyogenes has re-emerged as a cause of bacterial superinfection. Clusters of invasive group A streptococcal infection was noted in England, France and the United States, often with high case-fatality rates.Citation91,Citation92 A concurrent viral respiratory illness has also been suggested as a possible contributing factor in a nursing home outbreak of invasive S. pyogenes infection.Citation93 Thus, as in the cases of S. pneumoniae and S. aureus, S. pyogenes acts synergistically with influenza viruses and possibly other respiratory viruses in causing secondary bacterial infections. Enhanced adherence and internalization of S. pyogenes to host cells in the presence of influenza virus infection may explain the synergism.Citation94,Citation95,Citation96
Any form of S. pyogenes infection may potentially lead to systemic manifestations due to release of toxins or superantigens. Scarlet fever typically follows an episode of upper respiratory tract infection, but may also complicate skin and soft tissue or wound infections, as well as varicella in children.Citation97 The incubation period is typically short, commonly 2–3 days (range: 1–6 days), with a sudden onset of fever, headache, malaise, and evidence of tonsillitis and pharyngitis.Citation98 The tongue is furry with enlarged papillae (white strawberry tongue), later becoming red and inflamed (red strawberry tongue). The rash—called punctate erythema—appears on day 2 of illness, consisting of fine, raised, red spots (which may become confluent) on a background of erythema.Citation98,Citation99 It spreads from the face (where there is usually no puncta but often characterized by a circumoral pallor) downwards to the neck and upper trunk, and then to the limbs, sometimes with a sandpaper texture of the skin. Pastia's sign, which are deep red, linear exanthem, is commonly observed on the antecubital fossa, but sometimes also seen on other areas of skin folds. The eyes are usually not catarrhal. Resolution of the rash completes in about a week's time, and is often followed by peeling of the skin. Toxic and septic forms of scarlet fever have also been described in which there is extremely prominent tonsillitis with or without ulcerations, profuse purulent rhinorrhoea, and the rash is often dusky, petechial or haemorrhagic.Citation98 Streptococcal toxic shock syndrome is another systemic inflammatory disease with rash, hypotension and evidence of end organ impairment (kidney, liver, lung and coagulopathy). It often occurs in otherwise immunocompetent individuals with concurrent skin and soft tissue infections and bacteraemia.
Treatment and prevention
Penicillin remains the drug of choice for S. pyogenes infections despite over 60 years of use. Nonetheless, bacteriological treatment failure and sometimes clinical failure is well reported. Many postulations have been put forward to explain the phenomenon, including intracellular survival of the bacterium, coexistence of beta lactamase-producing bacteria, and penicillin tolerance.Citation100 Penicillin tolerance in S. pyogenes has been described since the 1980s, but the clinical significance of this phenomenon remains disputable.Citation101 S. pyogenes is also susceptible to most other beta lactams such as the cephalosporins. When penicillin V is used for the treatment of streptococcal pharyngitis, the standard regimen is a 10-day course. When oral cephalosporins such as cefuroxime axetil is used, a 5-day course give similar or slightly superior bacteriological response as compared to penicillin V.Citation102
Uncomplicated S. pyogenes infections generally respond well to treatment with beta lactams. For the treatment of severe infections, three other modalities of treatment should also be considered. Firstly, urgent surgical interventions remain the cornerstone in treating surgical emergencies such as necrotizing soft tissue infections. Secondly, in addition to penicillins, an antibiotic that inhibits protein synthesis is often added, which is believed to reduce in vivo toxin synthesis by the bacterium. Clindamycin is the most commonly used antibiotic in this setting. In fully susceptible strains, clindamycin is superior to penicillins in suppressing the production of streptococcal pyrogenic exotoxins (SpeA and SpeB) and superantigens in vitro.Citation103,Citation104 The extent of release of SpeA from S. pyogenes is higher at lower penicillin concentrations.Citation105 The usefulness of this strategy has been threatened in recent years with the growing prevalence of clindamycin-resistant S. pyogenes isolates. In addition to being ineffective as an antibacterial agent, exposure of these clindamycin-resistant strains to clindamycin actually increases the amount of toxins produced.Citation106 The optimal antimicrobial strategy for infections caused by clindamycin-resistant strains is uncertain, though linezolid may be considered in severe infections since it also reduces release of SpeA in vitro.Citation107 Fluoroquinolones and rifampicin does not significantly reduce exotoxin production in vitro.Citation108
Thirdly, intravenous immunoglobulin has been widely used in severe S. pyogenes infections, such as toxic shock syndrome and necrotizing soft tissue infections. The mechanism of action of intravenous immunoglobulin probably involves neutralization of toxins and its immunosuppressive activities. Although not all studies have demonstrated unequivocal clinical benefits, the use of intravenous immunoglobulin should still be considered especially in toxic shock syndromes because of its high mortality.Citation109,Citation110
The absence of any commercially available vaccine and the bacterium's genetic plasticity due to mobile genetic elements are two key factors which contribute to the occurrence of repeated explosive outbreaks of scarlet fever or other S. pyogenes invasive infections. Control and preventive measures against such outbreaks should target towards reduction of transmission and prevention of severe complications. The following measures should therefore be considered.
Surveillance for infections due to S. pyogenes
Although invasive group A streptococcal infections are part of the infectious disease surveillance system in some countries, such data are not available in most countries. Passive laboratory surveillance of positive cultures in normally sterile body fluid and throat swab can help to monitor the prevalence of the infection in the community. If resources permit, monitoring of the prevalence of emm types would be helpful for early identification of emergence of new strains.
Exclusion of sick patients from school or work
During outbreaks of scarlet fever, infected children should be excluded from schools until he or she has received at least 24 h of an effective antibiotic and the patient is improving.Citation111,Citation112 While the 24-h recommendation is generally sufficient, it should be noted that in some cases positive cultures of S. pyogenes may last longer than 24 h, and more prolonged isolation may be necessary in the health-care setting.Citation113 For health-care workers, the 24-h recommendation also applies for asymptomatic carriers, while a longer period of exclusion is needed for symptomatic cases or those with skin lesions.Citation113
Hand hygiene, droplet and contact precautions
These should be reinforced, especially in school or institutional settings. For young children who may not be able comply with the recommended infection control measures, supervision by teachers or care-givers is essential. Infection control measures are equally important for health-care workers in view of the known nosocomial transmission of invasive group A streptococcal infections to patients and health-care workers.Citation114 Adherence to droplet and contact precautions with appropriate isolation (single room or cohort) and use of personal protective equipment is important, not only to protect the health-care workers themselves but to prevent nosocomial spread of the infection.Citation113 Lapses in proper infection control precautions have resulted in health care-associated S. pyogenes outbreaks.Citation33,Citation93
Environmental hygiene
Although previous volunteer studies have not consistently demonstrated direct transmission of S. pyogenes from fomites, proper maintenance of environmental hygiene remains a prudent measure to take because of the prolonged survival of S. pyogenes in the environment and that peak seasons of scarlet fever often coincide with other viral infections such as influenza, for which fomites do play a role in transmission. Previous outbreak investigations of streptococcal pyoderma in a military training establishment showed that environmental contamination with S. pyogenes was common, and that contact with contaminated objects contributed to the dissemination of the bacterium.Citation51 Disinfection can be achieved with cleaning followed by the use of 1000 ppm sodium hypochlorite.Citation113
Early and accurate diagnosis
Differentiation from other viral upper respiratory tract is important because S. pyogenes is treatable with antibiotics and early antibiotic treatment will reduce the risk of non-suppurative sequelae such as rheumatic fever and possibly suppurative complications. However, in the absence of the classical clinical signs of scarlet fever, differentiation from viral pharyngitis is not always possible. Scoring systems such as the Breese score and Centor score have been used to aid clinical diagnosis,Citation115,Citation116 and the combination fever ≥38 °C, absence of cough, tonsillar exudates and anterior cervical lymphadenopathy are considered to be suggestive of streptococcal pharyngitis. However, no scoring systems are 100% sensitive or specific. A bacterial culture of the throat swab remains the gold standard for laboratory confirmation, but the test may not be readily available in primary health-care settings. Rapid antigen detection tests are useful adjuncts if culture is not available. The specificity of these tests are generally over 95%, but the sensitivity ranges from 70%–90% compared to culture.Citation117 As a balance between prompt treatment versus unnecessary antibiotic prescription, these point-of-care tests can be useful to supplement clinical diagnosis where microbiology laboratory support is unavailable.Citation118
Antibiotic misuse and abuse
The relationship between outpatient antibiotic prescription rates and the prevalence of antibiotic resistance in the community is well established.Citation119,Citation120 In many countries, there is a disturbing trend to prescribe broader spectrum antibiotics in the primary care setting.Citation119 In most parts of the world, upper respiratory tract infections constitute the commonest reason for antibiotic prescription in the primary care setting.Citation121 While it is generally recommended that children and adults with common cold, acute rhinosinusitis, acute cough or bronchitis, acute pharyngitis (except those suggestive of streptococcal infection clinically) and many cases of acute otitis media do not require immediate antibiotic treatment,Citation122 antibiotics are still very commonly prescribe for a variety of reasons, including various patient- and doctor-related factors.Citation123 Common reasons for prescription include uncertainties in diagnosis and management, the need to meet patient expectations and pressures, and fear of medicolegal consequences.Citation123,Citation124 To avoid unnecessary antibiotic prescriptions, education, training and monitoring by various professional bodies are essential for both clinicians and patients. On the practical side, apart from the no-prescribing strategy for obvious diagnoses that do not require antibiotics, a delayed-prescribing strategy can also be used for some patients.Citation122
In contrast to beta lactam susceptibility, resistance to macrolides, lincosamides and fluoroquinolones is rising among S. pyogenes isolates in many parts of the world.Citation125,Citation126,Citation127 Under such circumstances, the macrolides and clindamycin should not be used as first line antibiotics for the treatment of S. pyogenes infections. Unfortunately, the use of macrolides in primary health-care settings is very common and frequently being overused even in the absence of any history of beta lactam allergy.Citation128 In many countries, the newer macrolides (azithromycin and clarithromycin) are among the commonest antibiotics prescribed for respiratory tract infections because of the broad spectrum of coverage (including atypical bacterial pathogens), ease of administration (once daily dosing) and relatively free from adverse reactions.Citation129 The level of macrolide consumption is correlated with the prevalence of macrolide-resistant bacteria, including S. pneumoniae and S. pyogenes.Citation130,Citation131 The association is especially strong with the use of long-acting macrolides such as azithromycin in some studies, presumably due to extended exposure of the bacteria to a low level of antibiotic.Citation132
The widespread use of macrolides has four important repercussions. Firstly, inappropriate use of antibiotics will adversely affect the colonization resistance at the musosal surfaces, which reduces the antagonism to colonization by potential pathogens including S. pyogenes.Citation133 Secondly, a large proportion of patients will not received adequate antibiotic coverage for S. pyogenes infection. In Hong Kong, 25.6% of the isolates collected between 2005 and 2008 were resistant to macrolides.Citation134 Recent studies from China showed that over 95% of the S. pyogenes isolates were resistant to macrolides and clindamycin.Citation135,Citation136 This means not only are the macrolides unable to control the active infection, but also fail to prevent complications such as acute rheumatic fever.Citation137 One has to note that the problem of macrolide resistance is not just a problem with S. pyogenes and S. pneumoniae, but is also increasingly prevalent among other respiratory pathogens such as Haemophilus influenzae, Staphylococcus aueus and Mycoplasma pneumoniae, with up to 92% of M. pneumoniae strains in China being resistant to macrolides.Citation138,Citation139 Thirdly, the use of a resistant antibiotic may actually increase the level of toxin secretion from S. pyogenes. Therefore, giving the wrong antibiotics may actually do more harm than not giving antibiotics initially and adopting a delayed-prescription strategy. Fourthly, macrolide resistance in S. pyogenes is not evenly distributed among all emm types. Certain clones or emm types are more likely to harbor macrolide resistance.Citation140,Citation141 If such clones concomitantly possess a higher transmissibility and/or virulence for invasive disease, widespread use of macrolides may then drive an epidemic of severe group A streptococal disease.
Early use of appropriate antibiotics for empirical treatment
Unless there are other contraindications (such as severe allergies to beta lactam antibiotics), penicillins (including penicillin V and amoxicillin) or cephalosporins (if a broader spectrum of coverage is clinically indicated) should remain the first-line antibiotics if S. pyogenes infection is suspected clinically.Citation142 Macrolides and clindamycin should never be the sole agents for empirical treatment of group A streptococcal infections, unless the susceptibility of the infecting strain to these antibiotics is confirmed by laboratory testing. In patients with a history of allergy to penicillin, depending on the nature and severity of the hypersensitivity reactions, the severity of disease and spectrum of coverage needed, suitable alternative antibiotics for empirical coverage include cephalosporins, carbapenems, vancomycin and linezolid. On the other hand, one should be cautious not to shift the misuse or abuse of macrolides to other classes of antibiotics, especially in the primary care setting.
Varicella and influenza vaccination
Although they do not directly protect against S. pyogenes infections, these vaccines should be considered where indicated. Antecedent chickenpox and influenza are known to be predisposing factors for severe group A streptococcal infection, including necrotizing soft tissue infections and pneumonia.Citation92,Citation95,Citation96,Citation143,Citation144 Protective effects of influenza vaccination against group A streptococcal illness have also been demonstrated in animals and humans.Citation145,Citation146,Citation147 Protection against these co-pathogens is most valuable when community outbreaks of S. pyogenes infections coincide with the influenza season or when the incidence of chickenpox is also high.
Prudent use of non-steroidal anti-inflammatory drugs (NSAID)
There is currently no evidence that the use of non-steroidal anti-inflammatory drugs predisposes to the development of scarlet fever. However, case series, in vitro and animal studies suggested a possible link between non-steroidal anti-inflammatory drug's use and predisposition to severe S. pyogenes disease, especially skin and soft tissue infections and toxic shock syndrome.Citation148 Although results from epidemiological studies did not show a consistent relationship between the two entities, these drugs should be avoided patients with suspected or confirmed S. pyogenes infections whenever possible in view of the theoretical risk.Citation148,Citation149,Citation150,Citation151,Citation152
Antibiotic prophylaxis
The occurrence of intra-familial and health care-associated outbreaks of invasive group A streptococcal infections has prompted the consideration of antibiotic prophylaxis to close contacts.Citation86,Citation153,Citation154,Citation155 While there is evidence that close contacts have a higher risk of developing severe disease than the general population, and that antibiotic prophylaxis does reduce the risk of intra-familial transmission, especially when cephalosporins are used,Citation37,Citation156,Citation157 routine chemoprophylaxis has not been recommended as the standard practice.Citation113 The only exception would be a direct percutaneous injury by or mucosal exposure to infective materials.Citation113 No controlled trials have been performed to quantify the effectiveness of chemoprophylaxis. Benzathine penicillin has been used successfully to control outbreaks of S. pyogenes infection in military situations, though this practice will unlikely be applicable to the general community except perhaps in extraordinary circumstances.Citation32,Citation158 Education and monitoring of close contact for early symptoms of infection are important, especially within 30 days after the diagnosis in the index patient.Citation159 Chemoprophylaxis may be offered to the household if there are two or more cases of invasive disease within the 30-day period.Citation113
Resurgent diseases: Scarlet fever's unwelcome comeback
Outbreaks of scarlet fever, a once-common and dangerous childhood disease, have increased over the past decade. Kwok-Yung Yuen and Samson Wong of the University of Hong Kong reviewed the causes and management of these outbreaks, which are caused by specific strains of Streptococcus pyogenes, the same bacterium that causes strep throat. The researchers report that genetic factors that may make some strains more deadly, including transfer of genes for virulence and drug resistance from one bacterium to another, and large-scale genomic rearrangements. Environmental factors may also play a role, including high-density living, environmental persistence of bacteria and amount of annual rainfall. Yuen and Wong's review highlights the need for early diagnosis, effective quarantine, surveillance for new strains and careful use of antibiotics. The work will also contribute to improved identification and management of future outbreaks.
- Euzéby JP. List of Prokaryotic names with Standing in Nomenclature—Genus Streptococcus. Available at http://www.bacterio.cict.fr/s/streptococcus.html (accessed 2 January 2012).
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis2005; 5: 685–694.
- Brandt CM, Haase G, Schnitzler N, Zbinden R, Lütticken R. Characterization of blood culture isolates of Streptococcus dysgalactiae subsp. equisimilis ossessing Lancefield's group A antigen. J Clin Microbiol1999; 37: 4194–4197.
- Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev2002; 15: 613–630.
- Oehmcke S, Shannon O, Mörgelin M, Herwald H. Streptococcal M proteins and their role as virulence determinants. Clin Chim Acta2010; 411(17/18): 1172–1180.
- De Malmanche SA, Martin DR. Protective immunity to the group A Streptococcus may be only strain specific. Med Microbiol Immunol1994; 183: 299–306.
- Eriksson BK, Villasenor-Sierra A, Norgren M, Stevens DL. Opsonization of T1M1 group A Streptococcus: dynamics of antibody production and strain specificity. Clin Infect Dis2001; 32: e24–e30.
- Lancefield RC. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med1959; 110: 271–292.
- Centers for Disease Control and Prevention. Streptococcus pyogenes emm sequence database. Available at http://www.cdc.gov/ncidod/biotech/strep/types_emm103-124.htm (accessed 31 January 2012).
- Banks DJ, Porcella SF, Barbian KD et al. Progress toward characterization of the group A Streptococcus metagenome: complete genome sequence of a macrolide-resistant serotype M6 strain. J Infect Dis2004; 190: 727–738.
- Beres SB, Sylva GL, Barbian KD et al. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci USA2002; 99: 10078–10083.
- Beres SB, Richter EW, Nagiec MJ et al. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci USA2006; 103: 7059–7064.
- Ferretti JJ, McShan WM, Ajdic D et al. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci USA2001; 98: 4658–4663.
- Green NM, Zhang S, Porcella SF et al. Genome sequence of a serotype M28 strain of group A Streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J Infect Dis2005; 192: 760–770.
- Holden MT, Scott A, Cherevach I et al. Complete genome of acute rheumatic fever-associated serotype M5 Streptococcus pyogenes strain Manfredo. J Bacteriol2007; 189: 1473–1477.
- McShan WM, Ferretti JJ, Karasawa T et al. Genome sequence of a nephritogenic and highly transformable M49 strain of Streptococcus pyogenes. J Bacteriol2008; 190: 7773–7785.
- Nakagawa I, Kurokawa K, Yamashita A et al. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res2003; 13( 6A): 1042–1055.
- Smoot JC, Barbian KD, van Gompel JJ et al. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc Natl Acad Sci USA2002; 99: 4668–4673.
- National Center for Biotechnology Information (NCBI). Available at http://www.ncbi.nlm.nih.gov/genome?term=txid1314%5borgn (accessed 31 January 2012)
- Vojtek I, Pirzada ZA, Henriques-Normark B, Mastny M, Janapatla RP, Charpentier E. Lysogenic transfer of group A Streptococcus superantigen gene among streptococci. J Infect Dis2008; 197: 225–234.
- Sumby P, Barbian KD, Gardner DJ et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA2005; 102: 1679–1684.
- Walker MJ, Hollands A, Sanderson-Smith ML et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med2007; 13: 981–985.
- Aziz RK, Pabst MJ, Jeng A et al. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol Microbiol2004; 51: 123–134.
- Dowson CG, Barcus V, King S, Pickerill P, Whatmore A, Yeo M. Horizontal gene transfer and the evolution of resistance and virulence determinants in Streptococcus. Soc Appl Bacteriol Symp Ser1997; 26: 42S–51S.
- Simpson WJ, Musser JM, Cleary PP. Evidence consistent with horizontal transfer of the gene (emm12) encoding serotype M12 protein between group A and group G pathogenic streptococci. Infect Immun1992; 60: 1890–1893.
- Jensen A, Kilian M. Delineation of Streptococcus dysgalactiae, its subspecies, and its clinical and phylogenetic relationship to Streptococcus pyogenes. J Clin Microbiol2012; 50: 113–126.
- Jönsson M, Ström K, Swedberg G. Mutations and horizontal transmission have contributed to sulfonamide resistance in Streptococcus pyogenes. Microb Drug Resist2003; 9: 147–153.
- Pletz MW, McGee L, Van Beneden CA et al. Fluoroquinolone resistance in invasive Streptococcus pyogenes isolates due to spontaneous mutation and horizontal gene transfer. Antimicrob Agents Chemother2006; 50: 943–948.
- Sumby P, Porcella SF, Madrigal AG et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis2005; 192: 771–782.
- Rato MG, Nerlich A, Bergmann R et al. Virulence gene pool detected in bovine group C Streptococcus dysgalactiae subsp. dysgalactiae solates by use of a group A S. pyogenes virulence microarray. J Clin Microbiol2011; 49: 2470–2479.
- Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics2010; 126: e557–e564.
- Crum NF, Russell KL, Kaplan EL et al. Pneumonia outbreak associated with group a Streptococcus species at a military training facility. Clin Infect Dis2005; 40: 511–518.
- Deutscher M, Schillie S, Gould C et al. Investigation of a group A streptococcal outbreak among residents of a long-term acute care hospital. Clin Infect Dis2011; 52: 988–994.
- Holmström L, Nyman B, Rosengren M, Wallander S, Ripa T. Outbreaks of infections with erythromycin-resistant group A streptococci in child day care centres. Scand J Infect Dis1990; 22: 179–185.
- Cockerill FR 3rd, MacDonald KL, Thompson RL et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA1997; 277: 38–43.
- Weiss K, Laverdière M, Lovgren M, Delorme J, Poirier L, Béliveau C. Group A Streptococcus carriage among close contacts of patients with invasive infections. Am J Epidemiol1999; 149: 863–868.
- Davies HD, McGeer A, Schwartz B et al. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N Engl J Med1996; 335: 547–554.
- Brown WA, Allison VD. Infection of the air of scarlet-fever wards with Streptococcus pyogenes. J Hyg (Lond)1937; 37: 1–13.
- Edward DG. Haemolytic streptococci in the dust of hospital wards, and their relationship to infection: a report to the Medical Research Council. J Hyg (Lond)1944; 43: 256–265.
- Rammelkamp CH Jr, Morris AJ, Catanzaro FJ, Wannamaker LW, Chamovitz R, Marple EC. Transmission of group A streptococci. III. The effect of drying on the infectivity of the organism for man. J Hyg (Lond)1958; 56: 280–287.
- Perry WD, Siegel AC, Rammelkamp CH Jr, Wannamaker LW, Marple EC. Transmission of group A streptococci. I. The role of contaminated bedding. Am J Hyg1957; 66: 85–95.
- Wagenvoort JH, Penders RJ, Davies BI, Lütticken R. Similar environmental survival patterns of Streptococcus pyogenes strains of different epidemiologic backgrounds and clinical severity. Eur J Clin Microbiol Infect Dis2005; 24: 65–67.
- Wilson KS, Maroney SA, Gander RM. The family pet as an unlikely source of group A beta-hemolytic streptococcal infection in humans. Pediatr Infect Dis J1995; 14: 372–375.
- Scamman CL. Milk-borne septic sore throat and scarlet fever. Am J Public Health Nations Health1929; 19: 1339–1346.
- Juel Henningsen E, Ernst J. Milk epidemic of angina, originating from a cow with mastitis and due to Streptococcus pyogenes (Lancefield group A). J Hyg (Lond)1938; 38: 384–391.
- Camps FE, Miller Wood JL. An outbreak of epidemic sore-throat of milk-borne origin. Lancet1936; 228: 756–760.
- Levy M, Johnson CG, Kraa E. Tonsillopharyngitis caused by foodborne group A Streptococcus: a prison-based outbreak. Clin Infect Dis2003; 36: 175–182.
- Falkenhorst G, Bagdonaite J, Lisby M et al. Outbreak of group A streptococcal throat infection: don't forget to ask about food. Epidemiol Infect2008; 136: 1165–1171.
- Katzenell U, Shemer J, Bar-Dayan Y. Streptococcal contamination of food: an unusual cause of epidemic pharyngitis. Epidemiol Infect2001; 127: 179–184.
- Lamagni TL, Neal S, Keshishian C et al. Epidemic of severe Streptococcus pyogenes infections in injecting drug users in the UK, 2003–2004. Clin Microbiol Infect2008; 14: 1002–1009.
- Cruickshank JG, Lightfoot NF, Sugars KH et al. A large outbreak of streptococcal pyoderma in a military training establishment. J Hyg (Lond)1982; 89: 9–21.
- Wasserzug O, Valinsky L, Klement E et al. A cluster of ecthyma outbreaks caused by a single clone of invasive and highly infective Streptococcus pyogenes. Clin Infect Dis2009; 48: 1213–1219.
- Shulman ST, Stollerman G, Beall B, Dale JB, Tanz RR. Temporal changes in streptococcal M protein types and the near-disappearance of acute rheumatic fever in the United States. Clin Infect Dis2006; 42: 441–447.
- Bisno AL. The resurgence of acute rheumatic fever in the United States. Annu Rev Med1990; 41: 319–329.
- Miner LJ, Petheram SJ, Daly JA et al. Post-Streptococcal Syndrome Study Team. Molecular characterization of Streptococcus pyogenes isolates collected during periods of increased acute rheumatic fever activity in Utah. Pediatr Infect Dis J2004; 23: 56–61.
- Lamagni TL, Efstratiou A, Vuopio-Varkila J, Jasir A, Schalén C; Strep-EURO. The epidemiology of severe Streptococcus pyogenes associated disease in Europe. Euro Surveill2005; 10: 179–184.
- Richardson BW. Facts relating to scarlet fever. Assoc Med J1853; 1: 502–507.
- Rolleston JD. The history of scarlet fever. Br Med J1928; 2: 926–929.
- Brownlee J. The relationship between rainfall and scarlet fever. Proc R Soc Med1923; 16(Sect Epidemiol State Med): 30–34.
- Duncan CJ, Duncan SR, Scott S. The dynamics of scarlet fever epidemics in England and Wales in the 19th century. Epidemiol Infect1996; 117: 493–499.
- Anonymous. Epidemiology of scarlet fever. Br Med J1944; 1: 118–119.
- Perks EM, Mayon-White RT. The incidence of scarlet fever. J Hyg (Lond)1983; 91: 203–209.
- Glover JA, Griffith F. An outbreak of scarlet fever at a preparatory school. Lancet1930; 216: 815–817.
- Promedmail. Available at http://www.promedmail.org (accessed 31 January 2012)
- Centre for Health Protection, Department of Health. Number of notifications for notifiable infectious diseases in 2011. Hong Kong. Available at http://www.chp.gov.hk/en/data/1/10/26/43/455.html (accessed 19 February 2012).
- Beres SB, Sylva GL, Sturdevant DE et al. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc Natl Acad Sci USA2004; 101: 11833–11838.
- Carroll RK, Beres SB, Sitkiewicz I et al. Evolution of diversity in epidemics revealed by analysis of the human bacterial pathogen group A Streptococcus. Epidemics2011; 3( 3/4): 159–170.
- Billal DS, Hotomi M, Shimada J et al. Prevalence of Streptococcus invasive locus (sil) and its relationship with macrolide resistance among group A Streptococcus strains. J Clin Microbiol2008; 46: 1563–1564.
- Chiou CS, Wang YW, Chen PL, Wang WL, Wu PF, Wei HL. Association of the shuffling of Streptococcus pyogenes clones and the fluctuation of scarlet fever cases between 2000 and 2006 in central Taiwan. BMC Microbiol2009; 9: 115.
- Rogers S, Commons R, Danchin MH et al. Strain prevalence, rather than innate virulence potential, is the major factor responsible for an increase in serious group A Streptococcus infections. J Infect Dis2007; 195: 1625–1633.
- Metzgar D, McDonough EA, Hansen CJ et al. Local changes in rates of group A Streptococcus disease and antibiotic resistance are associated with geographically widespread strain turnover events. Virulence2010; 1: 247–253.
- Kao CH, Chen PY, Huang FL et al. Clinical and genetic analysis of invasive and non-invasive group A streptococcal infections in central Taiwan. J Microbiol Immunol Infect2005; 38: 105–111.
- Jing HB, Ning BA, Hao HJ et al. Epidemiological analysis of group A streptococci recovered from patients in China. J Med Microbiol2006; 55( Pt 8): 1101–1107.
- Tse H, Bao JY, Davies MR et al. Molecular characterization of the 2011 Hong Kong scarlet fever outbreak. J Infect Dis.May 2012. doi: 10.1093/infdis/jis362.
- Hong Kong Observatory. The Year's Weather—2011. Available at http://www.weather.gov.hk/wxinfo/pastwx/ywx2011.htm (accessed 19 February 2012).
- Stollerman GH. Rheumatic fever. Lancet1997; 349: 935–942.
- Rodríguez-Iturbe B, Batsford S. Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney Int2007; 71: 1094–1104.
- Cunningham MW. Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol2008; 609: 29–42.
- Tewodros W, Kronvall G. M protein gene (emm type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. J Clin Microbiol2005; 43: 4369–4376.
- Reid HF, Bassett DC, Gaworzewska E, Colman G, Poon-King T. Streptococcal serotypes newly associated with epidemic post-streptococcal acute glomerulonephritis. J Med Microbiol1990; 32: 111–114.
- Erdem G, Mizumoto C, Esaki D et al. Group A streptococcal isolates temporally associated with acute rheumatic fever in Hawaii: differences from the continental United States. Clin Infect Dis2007; 45: e20–e24.
- Zheng MH, Jiao ZQ, Zhang LJ et al. Genetic analysis of group A Streptococcus isolates recovered during acute glomerulonephritis outbreaks in Guizhou Province of China. J Clin Microbiol2009; 47: 715–720.
- Martin JM, Barbadora KA. Continued high caseload of rheumatic fever in western Pennsylvania: possible rheumatogenic emm types of Streptococcus pyogenes. J Pediatr2006; 149: 58–63.
- Robertson KA, Volmink JA, Mayosi BM. Antibiotics for the primary prevention of acute rheumatic fever: a meta-analysis. BMC Cardiovasc Disord2005; 5: 11.
- The Working Group on Severe Streptococcal Infections. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA1993; 269: 390–391.
- Lepoutre A, Doloy A, Bidet P et al. Microbiologists of the Epibac Network. Epidemiology of invasive Streptococcus pyogenes infections in France in 2007. J Clin Microbiol2011; 49: 4094–4100.
- O'Brien KL, Beall B, Barrett NL et al. Epidemiology of invasive group a Streptococcus disease in the United States, 1995–1999. Clin Infect Dis2002; 35: 268–276.
- O'Grady KA, Kelpie L, Andrews RM et al. The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Med J Aust2007; 186: 565–569.
- Tyrrell GJ, Lovgren M, Kress B, Grimsrud K. Invasive group A streptococcal disease in Alberta, Canada (2000 to 2002). J Clin Microbiol2005; 43: 1678–1683.
- Khateeb OM, Osborne D, Mulla ZD. Gastrointestinal symptomatology as a predictor of severe outcomes of invasive group A streptococcal infections. Epidemiol Infect2010; 138: 534–541.
- Zakikhany K, Degail MA, Lamagni T et al. Increase in invasive Streptococcus pyogenes and Streptococcus pneumoniae infections in England, December 2010 to January 2011. Euro Surveill2011; 16: pii:19785.
- Jean C, Louie JK, Glaser CA et al. Invasive group A streptococcal infection concurrent with 2009 H1N1 influenza. Clin Infect Dis2010; 50: e59–e62.
- Thigpen MC, Thomas DM, Gloss D et al. Nursing home outbreak of invasive group A streptococcal infections caused by 2 distinct strains. Infect Control Hosp Epidemiol2007; 28: 68–74.
- Sanford BA, Davison VE, Ramsay MA. Fibrinogen-mediated adherence of group A Streptococcus to influenza A virus-infected cell cultures. Infect Immun1982; 38: 513–520.
- Okamoto S, Kawabata S, Nakagawa I et al. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J Virol2003; 77: 4104–4112.
- Hafez MM, Abdel-Wahab KS, El-Fouhil DF. Augmented adherence and internalization of group A Streptococcus pyogenes to influenza A virus infected MDCK cells. J Basic Microbiol2010; 50( Suppl 1) : S46–S57.
- Rolleston JD. Concurrent scarlet fever and chicken-pox. Proc R Soc Med1911; 4(Sect Study Dis Child): 14–16.
- Christie AB. Epidemiology and Clinical Practice. In: Infectious Diseases. 2nd ed. Edinburgh: Churchill Livingstone, 1974.
- Warrack JS. The differential diagnosis of scarlet fever, measles, and rubella. Br Med J1918; 2: 486–488.
- Pichichero ME, Casey JR. Systematic review of factors contributing to penicillin treatment failure in Streptococcus pyogenes pharyngitis. Otolaryngol Head Neck Surg2007; 137: 851–857.
- Steininger C, Allerberger F, Gnaiger E. Clinical significance of inhibition kinetics for Streptococcus pyogenes in response to penicillin. J Antimicrob Chemother2002; 50: 517–523.
- Adam D, Scholz H, Helmerking M. Comparison of short-course (5 day) cefuroxime axetil with a standard 10 day oral penicillin V regimen in the treatment of tonsillopharyngitis. J Antimicrob Chemother2000; 45: S23–S30.
- Mascini EM, Jansze M, Schouls LM, Verhoef J, Van Dijk H. Penicillin and clindamycin differentially inhibit the production of pyrogenic exotoxins A and B by group A streptococci. Int J Antimicrob Agents2001; 18: 395–398.
- Sriskandan S, McKee A, Hall L, Cohen J. Comparative effects of clindamycin and ampicillin on superantigenic activity of Streptococcus pyogenes. J Antimicrob Chemother1997; 40: 275–277.
- Goscinski G, Tano E, Thulin P, Norrby-Teglund A, Sjölin J. Release of SpeA from Streptococcus pyogenes after exposure to penicillin: dependency on dose and inhibition by clindamycin. Scand J Infect Dis2006; 38( 11/12): 983–987.
- Minami M, Kamimura T, Isaka M, Tatsuno I, Ohta M, Hasegawa T. Clindamycin-induced CovS-mediated regulation of the production of virulent exoproteins streptolysin O, NAD glycohydrolase, and streptokinase in Streptococcus pyogenes. Antimicrob Agents Chemother2010; 54: 98–102.
- Coyle EA, Cha R, Rybak MJ. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin a release. Antimicrob Agents Chemother2003; 47: 1752–1755.
- Tanaka M, Hasegawa T, Okamoto A, Torii K, Ohta M. Effect of antibiotics on group A Streptococcus exoprotein production analyzed by two-dimensional gel electrophoresis. Antimicrob Agents Chemother2005; 49: 88–96.
- Darenberg J, Ihendyane N, Sjölin J et al.; StreptIg Study Group. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003; 37: 333–340.
- Kaul R, McGeer A, Norrby-Teglund A et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis1999; 28: 800–807.
- Health Protection Agency. Guidance on infection control in schools and other childcare settings. United Kingdom, 2010. Available at http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947358374 (accessed 2 January 2012).
- National Health and Medical Research Council. Recommended minimum exclusion periods for infectious conditions for schools, pre-schools and child care centres. Australia, 2005. Available at http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ch43poster4.pdf (accessed 2 January 2012).
- Steer JA, Lamagni T, Healy B et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect2012; 64: 1–18.
- Daneman N, McGeer A, Low DE et al. ; Ontario Group A Streptococcal Study Group. Hospital-acquired invasive group a streptococcal infections in Ontario, Canada, 1992–2000. Clin Infect Dis2005; 41: 334–342.
- Breese BB. A simple scorecard for the tentative diagnosis of streptococcal pharyngitis. Am J Dis Child1977; 131: 514–517.
- Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making1981; 1: 239–246.
- Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev2004; 17: 571–580.
- Wong MC, Chung CH. Group A streptococcal infection in patients presenting with a sore throat at an accident and emergency department: prospective observational study. Hong Kong Med J2002; 8: 92–98.
- Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet2005; 365: 579–587.
- Van de Sande-Bruinsma N, Grundmann H, Verloo D et al. ; European Antimicrobial Resistance Surveillance System Group; European Surveillance of Antimicrobial Consumption Project Group. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis2008; 14: 1722–1730.
- Rautakorpi UM, Huikko S, Honkanen P et al.; MIKSTRA Collaborative Study Group. The Antimicrobial Treatment Strategies (MIKSTRA) program: a 5-year follow-up of infection-specific antibiotic use in primary health care and the effect of implementation of treatment guidelines. Clin Infect Dis2006; 42: 1221–1230.
- Tan T, Little P, Stokes T; Guideline Development Group. Antibiotic prescribing for self limiting respiratory tract infections in primary care: summary of NICE guidance. Br Med J2008; 337: a437.
- Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother2011; 66: 2215–2223.
- Kumar S, Little P, Britten N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. Br Med J2003; 326: 138.
- Pires R, Ardanuy C, Rolo D et al. Emergence of ciprofloxacin-nonsusceptible Streptococcus pyogenes isolates from healthy children and pediatric patients in Portugal. Antimicrob Agents Chemother2010; 54: 2677–2680.
- Malhotra-Kumar S, van Heirstraeten L, Lammens C, Chapelle S, Goossens H. Emergence of high-level fluoroquinolone resistance in emm6 Streptococcus pyogenes and in vitro resistance selection with ciprofloxacin, levofloxacin and moxifloxacin. J Antimicrob Chemother2009; 63: 886–894.
- Montes M, Tamayo E, Orden B, Larruskain J, Perez-Trallero E. Prevalence and clonal characterization of Streptococcus pyogenes clinical isolates with reduced fluoroquinolone susceptibility in Spain. Antimicrob Agents Chemother2010; 54: 93–97.
- Hinnerskov M, Therkildsen JM, Cordoba G, Bjerrum L. Macrolide overuse for treatment of respiratory tract infections in general practice. Dan Med Bull2011; 58: A4356.
- Vojvodić Ž. Antimicrobial use and indication-based prescribing among general practitioners in Eastern Croatia: comparison with data from the European Surveillance of Antimicrobial Consumption project. Croat Med J2010; 51: 524–533.
- Bergman M, Huikko S, Pihlajamäki M et al. ; Finnish Study Group for Antimicrobial Resistance (FiRe Network). Effect of macrolide consumption on erythromycin resistance in Streptococcus pyogenes in Finland in 1997–2001. Clin Infect Dis2004; 38: 1251–1256.
- Bergman M, Huikko S, Huovinen P, Paakkari P, Seppälä H; Finnish Study Group for Antimicrobial Resistance (FiRe Network). Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother2006; 50: 3646–3650.
- Baquero F. Evolving resistance patterns of Streptococcus pneumoniae: a link with long-acting macrolide consumption? J Chemother1999; 11( Suppl 1): 35–43.
- García-Rodríguez JA, Fresnadillo Martínez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother2002; 50 ( Suppl S2): 59–73.
- Chan JC, Chu YW, Chu MY, Cheung TK, Lo JY. Epidemiological analysis of Streptococcus pyogenes infections in Hong Kong. Pathology2009; 41: 681–686.
- Chang H, Shen X, Fu Z et al. Antibiotic resistance and molecular analysis of Streptococcus pyogenes isolated from healthy schoolchildren in China. Scand J Infect Dis2010; 42: 84–89.
- Liu X, Shen X, Chang H, Huang G et al. High macrolide resistance in Streptococcus pyogenes strains isolated from children with pharyngitis in China. Pediatr Pulmonol2009; 44: 436–441.
- Logan LK, McAuley JB, Shulman ST. Macrolide treatment failure in streptococcal pharyngitis resulting in acute rheumatic fever. Pediatrics2012; 129: e798–e802.
- Chironna M, Sallustio A, Esposito S et al. Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J Antimicrob Chemother2011; 66: 734–737.
- Xin D, Mi Z, Han X et al. Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob Agents Chemother2009; 53: 2158–2159.
- Perez-Trallero E, Marimón JM, Montes M, Orden B, de Pablos M. Clonal differences among erythromycin-resistant Streptococcus pyogenes in Spain. Emerg Infect Dis1999; 5: 235–240.
- Richter SS, Heilmann KP, Beekmann SE et al. Macrolide-resistant Streptococcus pyogenes in the United States, 2002–2003. Clin Infect Dis2005; 41: 599–608.
- Chiappini E, Regoli M, Bonsignori F et al. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther2011; 33: 48–58.
- Wilson GJ, Talkington DF, Gruber W, Edwards K, Dermody TS. Group A streptococcal necrotizing fasciitis following varicella in children: case reports and review. Clin Infect Dis1995; 20: 1333–1338.
- Imöhl M, van der Linden M, Reinert RR, Ritter K. Invasive group A streptococcal disease and association with varicella in Germany, 1996–2009. FEMS Immunol Med Microbiol2011; 62: 101–109.
- Okamoto S, Kawabata S, Fujitaka H, Uehira T, Okuno Y, Hamada S. Vaccination with formalin-inactivated influenza vaccine protects mice against lethal influenza Streptococcus pyogenes superinfection. Vaccine2004; 22( 21/22): 2887–2893.
- Lee SE, Eick A, Bloom MS, Brundage JF. Influenza immunization and subsequent diagnoses of group A Streptococcus-illnesses among US Army trainees, 2002–2006. Vaccine2008; 26( 27/28): 3383–3386.
- Chaussee MS, Sandbulte HR, Schuneman MJ et al. Inactivated and live, attenuated influenza vaccines protect mice against influenza: Streptococcus pyogenes super-infections. Vaccine2011; 29: 3773–3781.
- Weng TC, Chen CC, Toh HS, Tang HJ. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J Microbiol Immunol Infect2011; 44: 418–423.
- Hamilton SM, Bayer CR, Stevens DL, Lieber RL, Bryant AE. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J Infect Dis2008; 198: 1692–1698.
- Lesko SM, O'Brien KL, Schwartz B, Vezina R, Mitchell AA. Invasive group A streptococcal infection and nonsteroidal antiinflammatory drug use among children with primary varicella. Pediatrics2001; 107: 1108–1115.
- Sharkawy A, Low DE, Saginur R et al.; Ontario Group A Streptococcal Study Group. Severe group a streptococcal soft-tissue infections in Ontario: 1992–1996. Clin Infect Dis 2002; 34: 454–460.
- Aronoff DM, Bloch KC. Assessing the relationship between the use of nonsteroidal antiinflammatory drugs and necrotizing fasciitis caused by group A Streptococcus. Medicine (Baltimore)2003; 82: 225–235.
- Roy S, Kaplan EL, Rodriguez B et al. A family cluster of five cases of group A streptococcal pneumonia. Pediatrics2003; 112( 1 Pt 1): e61–e65.
- Laustrup HK, Justesen US, Pedersen C. Household transmission of invasive group A Streptococcus with necrotizing fasciitis. Scand J Infect Dis2003; 35( 6/7): 414–415.
- Schwartz B, Elliott JA, Butler JC et al. Clusters of invasive group A streptococcal infections in family, hospital, and nursing home settings. Clin Infect Dis1992; 15: 277–284.
- Robinson KA, Rothrock G, Phan Q et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network. Risk for severe group A streptococcal disease among patients' household contacts. Emerg Infect Dis2003; 9: 443–447.
- Kikuta H, Shibata M, Nakata S et al. Efficacy of antibiotic prophylaxis for intrafamilial transmission of group A beta-hemolytic streptococci. Pediatr Infect Dis J2007; 26: 139–141.
- Brundage JF, Gunzenhauser JD, Longfield JN et al. Epidemiology and control of acute respiratory diseases with emphasis on group A beta-hemolytic Streptococcus: a decade of US Army experience. Pediatrics1996; 97 (6 Pt 2): 964–970.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis2002; 35 (8): 950–959. Erratum in: Clin Infect Dis 2003; 36(2): 243.