Abstract
In 1988 and 2002, two major phocine distemper virus (PDV) outbreaks occurred in harbour seals (Phoca vitulina) in north-western European coastal waters, causing the death of tens of thousands seals. Here we investigated whether PDV is still circulating among seals of the Dutch coastal waters and whether seals have protective serum-antibodies against PDV. Therefore seal serum samples, collected from 2002 to 2012, were tested for the presence of PDV-neutralizing antibodies. Antibodies were detected in most seals in 2002 and 2003 while after 2003 antibodies were detected only in seals less than two month-old and adult seals that probably had survived the 2002 PDV-epizootic. We estimated the current proportion of seals with antibodies against PDV at 11%. These findings suggest that at present the vast majority of seals are not immune to PDV infection. PDV re-introduction in this area may cause a major epizootic with infection of >80% and mass-mortality of >50% of the population.Emerging Microbes & Infections (2013) 2, e3; doi:10.1038/emi.2013.2
Introduction
Following the introduction of phocine distemper virus (PDV) into seals in north-western Europe in 1988, the virus spread rapidly causing severe morbidity and high mortality rates among mainly harbour seals (Phoca vitulina) with clinical signs similar to those caused by a related morbillivirus, canine distemper virus (CDV).Citation1,Citation2 About 22 000 harbour seals were killed due to infection with the virus and cumulative mortality of the Northern European population was over 50%.Citation3,Citation4 A second epizootic caused by this virus occurred in 2002 causing the death of more than 30 000 seals in north-western Europe.Citation3,Citation4,Citation5 Besides the two outbreaks of PDV among harbour seals, CDV has caused disease outbreaks among Baikal seals (Phoca sibirica) in the Siberian Lake Baikal and among Caspian seals (Phoca caspica) in the Caspian Sea.Citation6,Citation7,Citation8,Citation9
Following the second recorded outbreak of PDV infection in 2002, the population has increased to levels that are at present higher than before the outbreak.Citation10 Based on the occurrence of two PDV epizootics among seals of north-western Europe within a relatively short-time interval and the increase of the population after 2002, another outbreak of PDV epizootic may be expected at some time in the future.Citation11 Since this outbreak will probably again cause high morbidity and mortality rates, it would be important for both conservation and management strategies to estimate when such an epizootic could occur.
Prediction of a novel PDV epizootic is essentially based on estimation of the chance of the re-introduction of PDV into harbour seals of north-western Europe and on the overall susceptibility of the seal population. Since PDV is enzootic in Arctic seals and it has been suggested that grey seals can be immune carriers of the virus,Citation12,Citation13 re-introduction of PDV by migrating seals will probably occur sooner or later. Alternatively, PDV might have continued to circulate among seals of north-western Europe since the last PDV epizootic of 2002, as has been suggested after the epizootic in 1988 for seals of the Dutch coastal waters and for other populations of seals.Citation14,Citation15
However, re-introduction of PDV or introduction of CDV among the population of harbour seals of north-western Europe will not cause a new epizootic in the presence of herd immunity. Since it is unknown how long PDV has been circulating in the population of seals of the Dutch coastal waters after the last outbreak and to what extent the population is susceptible to PDV or CDV infection, we evaluated the presence of antibodies against PDV and CDV in the seal population of the Dutch coastal waters after the last PDV outbreak in 2002. To this end, serum samples were collected from seals that were admitted to the seal research and rehabilitation centre (SRRC) in Pieterburen, the Netherlands for rehabilitation from 2002 to 2012 and were tested for the presence of antibodies against PDV and CDV. A simple mathematical model was built to evaluate the proportion of seals with immunity in the Wadden Sea population, based on annual seal count data. It was used to estimate the size of a novel epizootic should the virus be reintroduced in the population.
MATERIALS AND METHODS
Sample collection
466 serum samples were collected from seals living in the Dutch coastal waters upon admission to the SRRC in Pieterburen from 2002 to 2012. Blood samples had been collected for diagnostic purposes and remaining serum was used for research. Of each year, samples were selected from mainly harbour seals and a number of grey seals of different ages when available, but also from a ringed seal (Phoca hispida) and a hooded seal (Cystophora cristata) (). Seals were divided into three age categories, pups (at the day of blood collection estimated to be less than two months of age), juveniles (at the day of blood collection estimated to be between two and twelve months of age) and (sub)adults (at the day of blood collection estimated to be older than one year of age). During and after the outbreak of PDV in 2002 and 2003, seals that were admitted to the SRRC were vaccinated against PDV using an inactivated CDV vaccine adjuvanted with immune stimulating complexes as described previously.Citation16,Citation17
Table 1 Overview of serum samples of seals used in this study
Preparation of virus stocks
Virus stocks of CDV strain ‘Bussell’ and PDV (collected from seal number 89-34Citation18) were prepared by inoculation of confluent flasks with Vero cells with either strain. When changes were complete, flasks were frozen and thawed once. The supernatant was subsequently cleared by low-speed centrifugation, aliquoted and stored at −70 °C. Titration of virus stocks was performed as described previously.Citation19
Virus neutralization assay
Since both PDV and CDV could cause new outbreaks of disease among seals, serum samples were tested for the presence of antibodies against PDV and CDV virus as described previously.Citation19 In brief, serum samples were heat-inactivated for 30 min at 56 °C and subsequently two-fold serial dilutions of sera were prepared in DMEM (Lonza, Breda, the Netherlands) containing NaHCO3 (0.15%), Hepes (20 mM), penicillin (100 U/mL), streptomycin (100 µg/mL) and L-glutamine (2 mM) in 96 wells flat-bottom plates. Subsequently 100 50% tissue culture infective dose of each virus added and after incubation for 1 h, at 37 °C, in 5% CO2, 104 Vero-cells were added to each well. Following 4 to 6 days incubation at 37 °C, in 5% CO2, all wells were checked for the presence of cytopathic changes. Virus neutralizing antibody titers were calculated as the reciprocal of the highest serum dilution still giving 100% reduction of cytopathic changes. Antibody titers >10 were considered positive. All antibody titers were determined in duplicate and serum samples for which cytopathic changes were not observed due to cytotoxic effects of the serum were excluded from the study. Serum from a seal collected during the 1988 PDV outbreak was used as a positive control.
Mathematical modeling
A simple mathematical model was developed, based on earlier work by Grenfell et al.,Citation20 to evaluate the proportion of individuals immune to PDV in the harbour seal population, and predict the size of an outbreak should the virus be reintroduced. The model was restricted to the Wadden Sea harbour seal population because of the availability of detailed count data for this population.Citation10 Since the harbour seals of the parts of the Wadden Sea of the Netherlands, Germany and Denmark are all part of the Wadden Sea population, we assumed that the results of serology of seals from Netherlands are representative for those from Germany and Denmark. First, a simple model of the harbour seal population dynamics was developed, assuming logistic growth of the population in an environment with a carrying capacity K of 38 000.Citation21
With r, the intrinsic growth rate of the population:
Where a and b are the individual birth rate and death rate, respectively. As previously, we ignored the seasonality of birth and age structure of the population.Citation20 Yearly estimates of the Wadden Sea harbour seal population from 1975 to 2011 were obtained from.Citation10 The model (Equation 1) was fitted to the data using the least square method. The model was fitted separately for the period 1975–1988, 1989–2002 and 2003–2011. Parameter values are indicated in Supplementary Table S1.
We further used the simple disease dynamics model of the Susceptible-Infected-Recovered (SIR)-type proposed by Grenfell et al.Citation20 to model the epizootics of 1988 and 2002.
Susceptible individuals (S) become infected (I) at a rate β. Once infected, they remain infectious for a duration of 1/α. A proportion of (1−δ) recovers, while a proportion of δ dies from infection. Recovered individuals (R) remain immune to re-infection with PDV for the rest of their lives. Since the dynamics of the 2002 epizootic were shown closely similar to those of 1988,Citation22 parameters estimated by Grenfell et al.Citation20 based on the 1988 epizootic were used for both the 1988 and the 2002 epizootics (Supplementary Table S1), Because of the short duration of the epizootics (several weeks), we ignored birth and death processes in the disease dynamics model.Citation20 Here also, we ignored the age structure of the population because of the paucity of data on the forces of PDV transmission among and between different age classes. The model allowed determining the proportion of susceptible and immune (recovered) individuals at the end of the epizootics. These proportions were used in the population model (Equation 1) to follow through time the number of susceptible and immune hosts in the population (with N, the total number of individuals):
In this model, we estimated the number of pups with maternal antibodies separately, using Equation 1. All pups born from immune individuals were considered passively immune to PDV due to maternal antibodies. These individuals entered the susceptible class (S) three months after birth.Citation23,Citation24
The disease dynamics model was then used to estimate the size of future epizootics should PDV be reintroduced in the Wadden Sea harbour seal population.
RESULTS
Antibodies against PDV and CDV in harbour seals
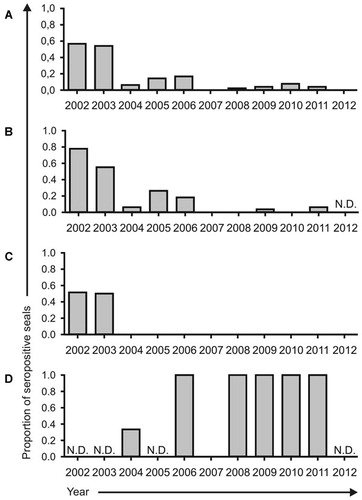
PDV neutralizing antibodies were detected in 70 out of 423 tested serum samples (17%) collected from harbour seals. From all positive samples, 52 had been collected from seals admitted to the SRRC in 2002 and 2003 (74% of all positive samples) during or the year after the PDV epidemic (Figure 1A). After 2003, antibodies were only detected in samples from eleven seal pups and seven (sub)adult seals and not in samples from juvenile seals (Figures 1B, 1C and 1D). Geometric mean (antibody) titer (GMT) of all positive sera was 52 (SD 33). The GMT of positive samples of harbour seals of all ages in 2002 and 2003 was 60 (SD 38). After 2003, lower antibody titers were detected in seal pups (GMT 35, SD 28), but not in adult seals (GMT 96, SD 32). Data of all age groups for all years are indicated in Supplementary Table S2.
Antibodies against CDV were detected in 58 out of 423 serum samples (14%), mainly in 2002 and 2003 (84% of the positive samples). The GMT against CDV of all positive sera was 60 (SD 31). In 15 samples antibodies were detected against PDV, but not against CDV, while in three samples antibodies against CDV were detected but not against PDV. In all other samples, antibodies were detected against both viruses or no morbillivirus-specific antibodies were detected. The presence of antibodies against CDV correlated with the presence of antibodies against PDV and vice versa (Phi correlation coefficient rΦ=0.8), but no correlation was observed between the antibody titer against both viruses in the positive serum samples (Pearson correlation coefficient r=−0.002).
Antibodies against PDV and CDV in other seal species
The presence of antibodies against PDV and CDV was also studied in serum samples collected from 37 grey seals. PDV specific antibodies were detected in four samples (11% of all tested sera); in two serum samples from pups in 2002 (titer of 80 and 20), in serum of a pup in 2005 (titer of 20) and an adult seal in 2006 (titer of 80). Antibodies against CDV were only detected in one of the pups in 2002 (titer of 20) and in the adult seal (titer of 20). No PDV or CDV-specific antibodies were detected in the two serum samples of the ringed seal and hooded seal.
Mathematical modelling
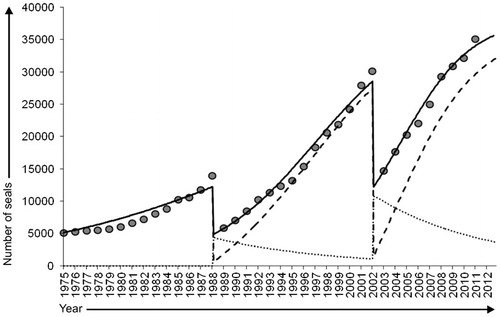
The model of population dynamics of harbour seals in the Wadden Sea was fitted to dataCitation10 for the periods of 1975–1988, 1989–2002 and 2003–2011, and captured well the observed growth of the population (Figure 2). Likewise, the model of disease dynamics using estimated parameters from Grenfell et al.Citation20 captured well the significant drops in the harbour seal population in 1988 and 2002 (Figure 2). At the end of the 1988 epizootic, the model predicted that less than 5% of the population had not been infected by PDV, while 60% had died of the disease, and 35% recovered. At the end of the 2002 epizootic, close to 6% of the population had not been infected with PDV, while 57% died of the disease and 37% recovered. These estimates are in accordance with the mortality rates observed during the 1988 and 2002 epizootics.Citation3,Citation4
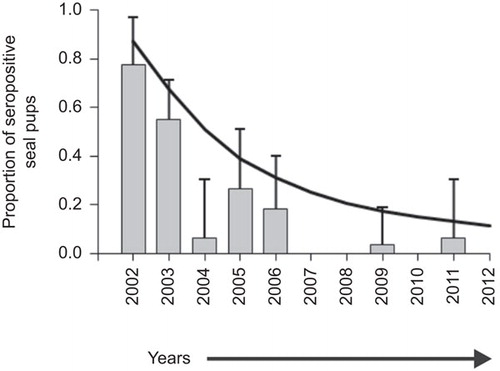
The model predicted that the harbour seal population in the Wadden Sea was constituted of 87% of immune individuals and 13% of susceptible individuals after the 2002 epizootic. These proportions decreased to reach 11% and 89%, respectively, in 2012. Because maternal antibodies are of parental origin, the proportion of pups of the year with antibodies against PDV is a valuable indicator of the proportion of immune individuals in the entire population that were exposed to PDV in previous years. The presence of maternal antibodies reflects the presence of immunity in reproductive females, because pups infected with PDV, orphaned or abandoned during an epidemic will not survive and pregnant females will most likely abort following PDV infection.Citation3,Citation20 As the age structure of the individuals sampled for this study unlikely reflects that of the population, the proportion of pups with maternal antibodies can thus be used to evaluate the model results. The predicted proportion of immune pups from 2002 to 2012 matches well the serological data (Figure 3). The model therefore captures well the proportions of susceptible and immune seals in the population through time, and can be used to predict how these proportions will change in the future. Supplementary Figure S1 shows changes in the number of susceptible and immune hosts in the entire Wadden Sea population over time, from 2003 to 2030. Figure S1 also shows changes in the corresponding size of a new epizootic should PDV be reintroduced into the population. Based on a proportion of immune individuals of 11% in the population in 2012, a new PDV epizootic would result in the death of 52% of the population at this time. As the population continues to grow to carrying capacity levels, the mortality burdens of an epizootic would reach the levels of previous epizootics (>55%) by 2017.
DISCUSSION
In the present manuscript, we evaluated the presence and distribution of virus neutralizing-serum-antibodies against PDV and CDV in seals of the Dutch coastal waters that had been admitted to the SRRC for rehabilitation from 2002 to 2012. Antibodies were detected in the majority of seals admitted to the SRRC in 2002 and 2003. Both the PDV outbreak in spring 2002 and the possible use of vaccines to prevent spread of PDV among seals in the SRRC in 2002 and 2003 account for the high proportion of seals with antibodies against either CDV or PDV in those years. As demonstrated previously, the presence of antibodies against PDV correlated with the presence of antibodies against CDV but the levels of antibodies against PDV and CDV did not correlate as has been observed previously.Citation19 After 2003, antibodies were only detected in seals younger than two months of age and in adult seals. No antibodies were detected in serum samples of juvenile seals, despite the high number of seals of this age tested (n=242).
Although we tested only a small proportion of the seal population and the seals admitted to the SRRC may not be representative of the population of seals of north-western Europe, results of the present study suggest that seals of this area, born after 2003 do not have antibodies against PDV and CDV after two months of age. In addition, results from our study indicate that during the past ten years PDV has not been introduced into the harbour seal population of north-western Europe.
Transfer of maternal antibodies to pups are probably the reason for the presence of antibodies in seal pups since it has been demonstrated that CDV antibodies can be transferred from mother to pup in dogs.Citation23 Maternal antibodies are only present until three months of age, which explains the absence of detectable antibodies in seals between two and twelve months of age. Based on the estimation of the age of adult seals with antibodies, all these animals were born before the outbreak of PDV in 2002. The presence of antibodies in these seals is probably due to infection during the outbreak in 2002 and 2003.
Based on a simple mathematical model, we estimated the percentage of harbour seals in the Wadden Sea that currently has protective immunity against PDV due to previous infection or vaccination to be of the order of 11%. This proportion of immune individuals is not sufficient to provide effective herd immunity against PDV should it be re-introduced in the population. A novel epizootic at this time would affect 82% of the population resulting in the death of more than 18 000 seals in the Wadden Sea. These are high figures, considering that it is only 10 years since the last epizootic. The high growth rate of the seal population since 2002 explains at least in part the predictions of the models, due to high recruitment of susceptible individuals through birth. As the population continues to grow, the burdens of a new epizootic will increase to reach the levels of historical epizootics within the next few years.
The absence of sufficient herd immunity among the population of harbour seals of the Dutch coastal waters indicates that the occurrence of a novel PDV or CDV epizootic depends only on introduction of the virus into this population, e.g. by migrating seals. The increasing number of seals in the Dutch coastal waters might affect the migration patterns of seals. Since the last outbreak of PDV infection in 2002 in Northern Europe, outbreaks of this virus infection have been demonstrated in seals of the east coast of the United Stated and in northern sea otters in the Pacific Ocean (Alaska, United States).Citation25,Citation26,Citation27 Furthermore, it has been demonstrated that this virus is enzootic in arctic seals.Citation12 These studies indicate that PDV is still circulating and although the exact moment of the next PDV epizootic among harbour seals of Northern Europe can not be foreseen, another epizootic among harbour seals of north-western Europe will occur sooner or later, and will likely again result in heavy morbidity and mortality burdens.
Supplementary Figure 1
Download MS Word (14.6 KB)Supplementary Figure 1
Download MS Word (14.6 KB)Supplementary Figure 1
Download PDF (436.9 KB)This work was supported by the European Community's Seventh Framework Programme (FP7/2007–2013) under the project European Management Platform for Emerging and Re-emerging Infectious Disease Entities (EMPERIE; EC grant agreement number 223498) and the Dutch Virgo Consortium. The authors wish to thank SRRC personnel for their assistance in data and samples collection.
- Osterhaus AD, Vedder EJ.Identification of virus causing recent seal deaths. Nature1988;335: 20.
- Osterhaus AD, Groen J, De Vries P, UytdeHaag FG, Klingeborn B, Zarnke R.Canine distemper virus in seals. Nature1988;335: 403–404.
- Harkonen T, Dietz R, Reijnders P et al.The 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Dis Aquat Organ2006;68: 115–130.
- Rijks JM, Van de Bildt MW, Jensen T, Philippa JD, Osterhaus AD, Kuiken T.Phocine distemper outbreak, The Netherlands, 2002. Emerg Infect Dis2005;11: 1945–1948.
- Jensen T, van de Bildt M, Dietz HH et al.Another phocine distemper outbreak in Europe. Science2002;297: 209.
- Likhoshway Ye V, Grachev MA, Kumarev VP et al.Baikal seal virus. Nature1989;339: 266.
- Barrett T, Blixenkrone-Moller M, Di Guardo G et al.Morbilliviruses in aquatic mammals: report on round table discussion. Vet Microbiol1995;44: 261–265.
- Osterhause AD, Groen J, Uytdehaag FG et al.Distemper virus in Baikal seals. Nature1989;338: 209–210.
- Kennedy S, Kuiken T, Jepson PD et al.Mass die-Off of Caspian seals caused by canine distemper virus. Emerg Infect Dis2000;6: 637–639.
- Trilateral Seal Expert Group (TSEG). Aerial surveys of Harbour Seals in the Wadden Sea in 2011.Wilhelmshaven: Common Wadden Sea Secretariat, 2011.Available at http://www.waddensea-secretariat.org/news/news/Seals/Annual-reports/Trilateral%20Seal%20Counts%20Report_2011.pdf. (accessed 22 October 2012).
- Rijks JM.Phocine Distemper Revisited: Multidisciplinary Analysis of the 2002 Phocine Distemper Virus Epidemic in the Netherlands. PhD thesis, Erasmus Medical Centre, Rotterdam, 2008.
- Duignan PJ, Nielsen O, House C et al.Epizootiology of morbillivirus infection in harp, hooded, and ringed seals from the Canadian Arctic and western Atlantic. J Wildl Dis1997;33: 7–19.
- Hammond JA, Pomeroy PP, Hall AJ, Smith VJ.Identification and real-time PCR quantification of Phocine distemper virus from two colonies of Scottish grey seals in 2002. J Gen Virol2005;86: 2563–2567.
- Fujii K, Sato H, Kakumoto C et al.Seroepidemiological survey of morbillivirus infection in Kuril harbor seals (Phoca vitulina stejnegeri) of Hokkaido, Japan. Jpn J Vet Res2006;54: 109–117.
- Visser IK, Vedder EJ, Vos HW, van de Bildt MW, Osterhaus AD.Continued presence of phocine distemper virus in the Dutch Wadden Sea seal population. Vet Rec1993;133: 320–322.
- Visser IK, vd Bildt MW, Brugge HN et al.Vaccination of harbour seals (Phoca vitulina) against phocid distemper with two different inactivated canine distemper virus (CDV) vaccines. Vaccine1989;7: 521–526.
- Osterhaus AD, Uytdehaag FG, Visser IK et al.Seal vaccination success. Nature1989;337: 21.
- Visser IK, Vedder EJ, van de Bildt MW, Orvell C, Barrett T, Osterhaus AD.Canine distemper virus ISCOMs induce protection in harbour seals (Phoca vitulina) against phocid distemper but still allow subsequent infection with phocid distemper virus-1. Vaccine1992;10: 435–438.
- Visser IK, Kumarev VP, Orvell C et al.Comparison of two morbilliviruses isolated from seals during outbreaks of distemper in north west Europe and Siberia. Arch Virol1990;111: 149–164.
- Grenfell BT, Lonergan ME, Harwood J.Quantitative investigations of the epidemiology of phocine distemper virus (PDV) in European common seal populations. Sci Total Environ1992;115: 15–29.
- Reijnders PJH, Ries EH, Tougaard S et al.Population development of harbour seals Phoca vitulina in the Wadden Sea after the 1988 virus epizootic. J Sea Res1997;38: 161–168.
- Harding KC, Harkonen T, Caswell H.The 2002 European seal plague: epidemiology and population consequences. Ecol Lett2002;5: 727–732.
- Winters WD.Time dependent decreases of maternal canine virus antibodies in newborn pups. Vet Rec1981;108: 295–299.
- Ross PS, de Swart RL, Visser IK et al.Relative immunocompetence of the newborn harbour seal, Phoca vitulina. Vet Immunol Immunopathol1994;42: 331–348.
- Philip Earle JA, Melia MM, Doherty NV, Nielsen O, Cosby SL.Phocine distemper virus in seals, east coast, United States, 2006. Emerg Infect Dis2011;17: 215–220.
- Goldstein T, Mazet JA, Gill VA, Doroff AM, Burek KA, Hammond JA.Phocine distemper virus in northern sea otters in the Pacific Ocean, Alaska, USA. Emerg Infect Dis2009;15: 925–927.
- Goldstein T, Gill VA, Tuomi P et al.Assessment of clinical pathology and pathogen exposure in sea otters (Enhydra lutris) bordering the threatened population in Alaska. J Wildl Dis2011;47: 579–592.