Dear Editor,
In September 2012, we collected ticks from Missouri with the goal of isolating the novel phlebovirus, Heartland virus (HRTV). HRTV was described in two farmers from northwestern Missouri who presented with thrombocytopenia and severe febrile illness.Citation1 These patients were both bitten by ticks 5–7 days before the onset of their clinical symptoms. Our hypothesis was that we would isolate HRTV from ticks collected in Missouri by inoculation of cell culture and/or by detection of viral RNA on polymerase chain reaction (PCR) assay. Furthermore, we used this field surveillance study as an opportunity to screen for other potential viral and bacterial pathogens in the tick samples we collected.
Information from the published literatureCitation1 was used to identify three geographically relevant collection sites across the central and western region of the state (Supplementary Figure S1A). One thousand two hundred and sixty-nine total ticks were collected from the three locations. Of these, 1191 (93.9%) were Dermacentor albipictus, 74 (5.8%) were Amblyomma americanum and four (0.3%) were Ixodes scapularis (Supplementary Figure S1B). Two nymphs were collected at location 2, but all other ticks collected during this study were larvae.
The speciated ticks were pooled into groups of twenty and screened for tick-borne pathogens (Supplementary Methods and Supplementary Table S1). Using the primers specific for HRTV,Citation1 Powassan virusCitation2 and deer tick virus,Citation3 we were unable to generate any positive PCR amplicons in the viral PCR screening. At location 2, one larval pool and one nymphal pool of ticks generated positive PCR amplicons when screened with the Rickettsia-specific primers for the outer membrane protein A (ompA) and citrate synthase (gltA) genes.Citation4
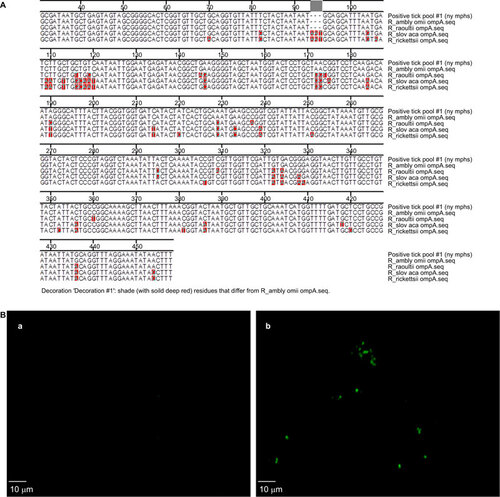
Sequence analysis demonstrated that the two Rickettisa-positive samples aligned with the ompA and gltA genes of Candidatus Rickettsia amblyommii [GenBank: 378930552]. ompA gene sequences for R. amblyommii, R. raoultii, R. slovaca and R. rickettsii were obtained from GenBank. These sequences were trimmed and then underwent ClustalW alignment in the MegAlign program. Specifically, ompA-positive samples from both the larval and nymphal pools shared 100% sequence identity across a 431 bp segment of the ompA gene of Candidatus Rickettsia amblyommii [GenBank: 378930552] (). Additional sequence analysis demonstrated that there was 100% sequence identity between both the larval and nymphal gltA-positive samples and Candidatus Rickettsia amblyommii [GenBank: 378930552] across a 628 bp segment of the gltA gene (Supplementary Figure S2). Furthermore, the presence of Rickettsia in a larval pool of ticks collected at location 2 indicates the occurrence of transovarial transmission of R. amblyommii.
Figure 1 Molecular detection of R. amblyommii. (A) Multiple sequence alignment of the outer membrance protein A (ompA) gene. Sequence ruler applies to R. amblyommii omp A sequence. (B) R. amblyommii polyclonal antibody-stained tick homogenate. Tick homogenates positive or negative for R. amblyommii were further confirmed by immunofluorescence using guinea pig immune serum specific for R. amblyommii. Tick homogenate pools that were not positive for R. amblyommii by molecular detection were used as a control. (a) Negative tick homogenates; (b) positive tick homogenates.
To further confirm our molecular identification of R. amblyommii, we screened the tick samples with Rickettsia-specific primers for the outer membrane protein B (ompB) geneCitation5 and for the 17 kDa gene.Citation6 The tick samples aligned perfectly with the Candidatus Rickettsia amblyommii [GenBank: 378930552] ompB gene sequence (Supplementary Figure S3). Our R. amblyommii-positive samples also completely aligned with the Candidatus Rickettsia amblyommii [GenBank: 378930552] 17 kDa gene sequence (Supplementary Figure S4).
No cytopathic effect was detected in any of the cell lines inoculated with the tick homogenates. However, we used an immunofluorescence assay (Supplementary Methods) to confirm the detection of R. amblyommii antigens in the A. americanum tick homogenates (). Tick mitochondrial 16S rRNA sequence analysis confirmed that the R. amblyommii-positive samples were isolated from A. americanum ticks. The 16S rRNA sequence from our Rickettsia-positive tick pools shared 100% sequence identity with A. americanum 16S rRNA (Supplementary Figure S5).
This field surveillance study was unable to isolate HRTV in any of the ticks we collected from Missouri. The lack of HRTV found in this study may be the result of our small sample sizes; only 5.8% of the total collected ticks were A. americanum. After our field collection of ticks was completed, another group published their findings of detecting HRTV from A. americanum collected in Missouri during 2012.Citation7 This group conducted tick collections ranging from April to early-August 2012. As our collection did not occur until mid-September, the majority of ticks collected in our study were larvae, as would be expected.Citation8
We did confirm the presence of R. amblyommii in two pooled A. americanum tick homogenates. To date, no definitive role has been defined for R. amblyommii in human pathogenesis, but a recent study has shown that A. americanum ticks parasitizing humans are frequently infected with R. amblyommii.Citation9 Two A. americanum ticks collected in Kansas were found to be concurrently infected with R. rickettsii, which causes Rocky Mountain spotted fever, and with R. amblyommii.Citation10 The co-infection of these A. americanum ticks with R. rickettsii and R. amblyommii raises interesting questions about the epidemiology of spotted fever group rickettsiae and Rocky Mountain spotted fever. A recent study in North Carolina screened ticks for spotted fever group rickettsiae and found that there was a high prevalence of A. americanum ticks infected with R. amblyommii.Citation11 As this tick species is relatively aggressive and readily parasitizes humans, the authors suggested that some cases of rickettsiosis diagnosed as Rocky Mountain spotted fever in North Carolina may instead be caused by R. amblyommii. Because other Rickettsia species, such as R. parkeri, were initially thought to be endosymbionts but were later shown to be pathogenic, it is important to continue evaluating the potential public health threat that R. amblyommii-infected A. americanum ticks pose to the humans they parasitize.
supplementary Methods
Download PDF (177.5 KB)supplementary Figure S1
Download PDF (3.8 MB)supplementary Table S1
Download PDF (175.9 KB)supplementary Figure S2
Download PDF (303.1 KB)supplementary Figure S3
Download PDF (447.5 KB)supplementary Figure S4
Download PDF (218.2 KB)supplementary Figure S5
Download PDF (290 KB)Dr Saravanan Thangamani is supported by NIH grants 1UC7AI094660 and 1R21AI097675. Dr Rodrigo I Marques dos Santos is supported by DOD grant #W81XWH-09-2-0053. This research was partially supported by NIH grant 1UC7AI094660 and by the Department of Pathology start-up funds to Dr Saravanan Thangamani. We thank Dr Lucas Blanton (University of Texas Medical Branch, USA) for providing R. amblyommii antibodies.
Note: Supplementary Information for this article can be found on Emerging Microbes and Infections' website (http://www.nature.com/emi/).
- McMullan LK, Folk SM, Kelly AJ et al.A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med2012;367: 834–841.
- Anderson JF, Armstrong PM.Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg2012;87: 754–759.
- Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD.Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg2008;79: 971–973.
- Regnery RL, Spruill CL, Plikaytis BD.Genotypic identification of rickettsiae and estimation of interspecies sequence divergence for portions of two rickettsial genes. J Bacteriol1991;173: 1576–1589.
- Roux V, Raoult D.Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Micr2000;50: 1449–1455.
- Heise SR, Elshahed MS, Little SE.Bacterial diversity in Amblyomma americanum (Acari: Ixodidae) with a focus on members of the genus Rickettsia. J Med Entomol2010;47: 258–268.
- Savage HM, Godsey MS, Lambert A et al.First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg2013;89: 445–452.
- Kollars TM, Oliver JH, Durden LA et al.Host association and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J Parasitol2000;86: 1156–1159.
- Jiang J, Yarina T, Miller MK, Stromdahl EY, Richards AL.Molecular detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. Vector-Borne Zoonot2010;10: 329–340.
- Berrada ZL, Goethert HK, Cunningham J, Telford SR 3rd.Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from Kansas. J Med Entomol2011;48: 461–467.
- Apperson CS, Engber B, Nicholson WL et al.Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector-Borne Zoonot2008;8: 597–606.
