Abstract
Enterovirus 71 (EV71) is one of the major causative agents of hand, foot and mouth disease (HFMD). Occasionally, EV71 infection is associated with severe neurological diseases, such as acute encephalitis, acute flaccid paralysis and cardiopulmonary failure. Several molecules act as cell surface receptors that stimulate EV71 infection, including scavenger receptor B2 (SCARB2), P-selectin glycoprotein ligand-1 (PSGL-1), sialylated glycan, heparan sulfate and annexin II (Anx2). SCARB2 plays critical roles in attachment, viral entry and uncoating, and it can facilitate efficient EV71 infection. The three-dimensional structures of the mature EV71 virion, procapsid and empty capsid, as well as the exofacial domain of SCARB2, have been elucidated. This structural information has greatly increased our understanding of the early steps of EV71 infection. Furthermore, SCARB2 plays essential roles in the development of EV71 neurological disease in vivo. Adult mice are not susceptible to infection by EV71, but transgenic mice that express human SCARB2 become susceptible to EV71 infection and develop similar neurological diseases to those found in humans. This mouse model facilitates the in vivo investigation of many issues related to EV71. PSGL-1, sialylated glycan, heparan sulfate and Anx2 are attachment receptors, which enhance viral infection by retaining the virus on the cell surface. These molecules also contribute to viral infection in vitro either by interacting with SCARB2 or independently of SCARB2. However, the cooperative effects of these receptors, and their contribution to EV71 pathogenicity in vivo, remain to be elucidated.
Introduction
Human enteroviruses (HEVs) comprise a large family of human pathogens, which belong to the genus Enterovirus within the family Picornaviridae. HEVs are classified into four groups: species A (HEV-A) to species D (HEV-D). HEV-A includes at least 16 members with different serotypes: coxsackievirus (CV) A2, CVA3, CVA4, CVA5, CVA6, CVA7, CVA8, CVA10, CVA12, CVA14, CVA16, enterovirus 71 (EV71), EV76, EV89, EV90 and EV91.Citation1,Citation2 HEV-A cause several diseases including hand, foot and mouth disease (HFMD), herpangina, meningitis, polio-like flaccid paralysis, and respiratory disease.Citation3 In particular, EV71 and CVA16 are the major causative agents of HFMD. HFMD is normally a mild disease; however, HFMD caused by EV71 is sometimes associated with severe neurological diseases, such as acute fatal encephalitis, polio-like acute flaccid paralysis and neurogenic pulmonary edema.Citation4,Citation5,Citation6,Citation7,Citation8,Citation9 Recent repeated outbreaks of EV71 associated with severe neurological diseases have occurred in the Asia-Pacific regionCitation4,Citation9,Citation10,Citation11,Citation12,Citation13,Citation14,Citation15,Citation16,Citation17 and EV71 has become a serious public health concern.Citation18
The mature virions of enteroviruses have an icosahedral shell with T=1 (quasi-T=3) symmetry; 60 copies each of VP1, VP2, VP3 and VP4; and a single-stranded RNA genome of approximately 7500 bases. VP1, VP2 and VP3 comprise wedge-shaped eight-stranded β-barrels.Citation19 An overview of enterovirus infection is presented in Figure 1. Virus infection is initiated by attachment to a cellular receptor on the surface of a susceptible cell, followed by internalization and uncoating.Citation20 A variety of enterovirus receptors play critical roles in these steps, such as the poliovirus receptor (PVR), coxsackie-adenovirus receptor and intercellular adhesion molecule-1 that is major group human rhinovirus receptor. These receptors all possess an immunoglobulin (Ig)-like fold. The Ig-like domains of these receptors bind to the depression around the five-fold axis, which is called a canyon. These receptors also mediate the internalization of the virus–receptor complex. Finally, the binding of the virus to the receptor triggers a conformational change in the native virion, resulting in an altered (A)-particle, which sediments at ca 135S by sucrose gradient centrifugation, lacks VP4, and harbors an externalized VP1 N-terminus. The externalized N-termini of VP1 anchors to the cell membrane and the extruded VP4s associate to form a channel through the membrane.Citation21,Citation22,Citation23,Citation24 The viral RNA is then released from a hole near the two-fold axis.Citation25,Citation26 The virion then becomes an ‘empty capsid’, which sediments at ca 80S. Minor group human rhinoviruses bind to receptors that belong to the low-density lipoprotein receptor family:Citation27,Citation28,Citation29 the low-density lipoprotein receptor binds to a star-shaped mesa on the five-fold axes, and does not induce a conformational change. After internalization of the virus, a conformational change is induced by the low endosomal pH.Citation30 The early events during infection by other picornaviruses have been clarified, whereas those related to EV71 remain poorly understood. Recently, the three-dimensional structure of the EV71 virion was reported,Citation31,Citation32,Citation33,Citation34 and several research groups have identified molecules that enhance either the attachment of EV71 to cells or the establishment of infection,Citation35,Citation36,Citation37,Citation38,Citation39 including sialylated glycans, heparan sulfate, annexin II (Anx2), P-selectin glycoprotein ligand-1 (PSGL-1) and scavenger receptor B2 (SCARB2) ().
Figure 1 Schematic showing the process of enterovirus uncoating. The mature virion (left) comprises 60 copies each of VP1, VP2, VP3 and VP4, and agenomic RNA. The virion is captured by its cognate receptor on the target cell surface and then internalized. The Ig-like domain in PVR, CAR and ICAM-1 binds to the canyon of the virus and induces a conformational change. The A-particle (middle) comprises 60 copies each of VP1, VP2 and VP3, together with the genomic RNA. The A-particle increases in diameter by approximately 4% and has a large hole near the two- and three-fold axes. The N-terminus of VP1 is externalized and anchors the virus to the membrane, where extruded VP4s associate to form a channel through the membrane. The viral RNA is then released from the hole close to the two-fold axis and enters the cell cytoplasm. Minor group human rhinoviruses bind to LDLR family members and the conformational change of the virionis induced by the low endosomal pH. The resulting empty capsid (right) comprises 60 copies each of VP1, VP2 and VP3. CAR, coxsackie-adenovirus receptor; ICAM-1, intercellular adhesion molecule-1; LDLR, low-density lipoprotein receptor.
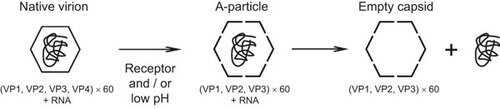
Table 1 EV71 receptors and their functions
Enterovirus receptors are the primary determinants of species and tissue tropism.Citation20 For example, mice are not susceptible to poliovirus infection, but they become susceptible after the transgenic expression of human PVR.Citation40,Citation41 PVR transgenic mice develop neurological diseases that are similar to those found in infected humans and monkeys. In both humans and PVR transgenic mice, PVR is expressed in a wide variety of tissues, including the central nervous system (where poliovirus replicates efficiently), as well as in other tissues that are not targets of poliovirus replication. Therefore, viral receptors are required to establish in vivo infections, although other factors also contribute to susceptibility.Citation42,Citation43
In this review, we summarize recent advances in EV71 research, including the structure of the EV71 virion, the identification and characterization of EV71 receptors, the mechanism of infection and the in vivo roles of receptors involved in EV71 infection.
Structure Of The Ev71 Virion
Two types of virus particles are produced when EV71 is grown in cultured cells.Citation44 The first type is the mature virion, which comprises 60 copies of the four capsid proteins and contains genomic RNA. The second type is the non-infectious procapsid, which comprises VP0 (VP4+VP2), VP1 and VP3, and does not contain genomic RNA. Recently, the high-resolution three-dimensional structures of the mature virions of EV71 strains were reported (Fuyang, MY104, and 1095, which belong to C4, B3 and C2 genogroups, respectively).Citation31,Citation32,Citation33 The mature EV71 virion possesses the common features of enteroviruses. It has a canyon around the five-fold axes, which is likely to serve as a binding site for an EV71 receptor. Chen et al.Citation45 showed that mutations in Gln172 and the surrounding amino acids in VP1, which form the canyon, impaired the binding to SCARB2. The canyon of EV71 is shallower than those found in poliovirus and human rhinoviruses, which suggests that the EV71 canyon is not sufficiently large to bind an Ig fold. VP1 in the mature virion harbors a hydrophobic pocket (which binds natural lipids) in the floor of the canyon. Based on molecular modeling, Wang et al.Citation32 suggest that the ligand is sphingosine, whereas Plevka et al.Citation31 identify it as lauric acid. The presence of the ‘pocket factor’ is thought to contribute to the stabilization of the virion.
In addition to the mature native EV71 virion, Wang et al.Citation32 determined the three-dimensional structure of the procapsid. Shingler et al.Citation34 determined the structure of the A-particle that was generated by heating purified mature virions at 56°C for 12 min. The procapsid and A-particle are expanded by approximately 4% compared with the mature virion. The structures of these particles were very similar to those of the A-particles and 80S empty capsids of poliovirus and human rhinoviruses.Citation25,Citation46,Citation47,Citation48 The GH loop of VP1 was shifted in the expanded particles, which meant that the hydrophobic pocket collapsed and the pocket could no longer accommodate the lipid. Large holes were present at the two-fold axes and in the base of the canyon, which were thought to allow the exit of the genomic RNA and the extrusion of the VP1 N-terminus. Based on these observations, Wang et al.Citation32 suggested that enteroviruses may adopt two fundamental configurations: the rigid configuration of the mature virion or the expanded configuration of the A-particle, procapsid and empty capsid. They also proposed a ‘sensor–adaptor mechanism’ for uncoating, which may be applicable to all enterovirus uncoating mechanisms. First, receptor binding to the canyon of the native virion induces structural rearrangements in the GH loop of VP1. This triggers the expulsion of the pocket factor, the coordinated dislocation of capsid proteins, the generation of holes near the two-fold axes and at the base of the canyon, extrusion of the N-terminus of VP1, VP4 release and, finally, the release of genomic RNA.
Identification Of Scarb2 As A Receptor For Ev71
Human RD cells and monkey Vero cells are susceptible to infection by EV71 strains. By contrast, mouse cells, such as L929 cells, are not susceptible to EV71 infection because they lack the cellular receptor. Yamayoshi et al.Citation37 transfected human genomic DNA into mouse L929 cells and successfully established cell lines that became susceptible to EV71. The transformant cell line, Ltr051, was highly susceptible to EV71, with an infection efficiency similar to that of RD cells. A microarray analysis of the RNAs expressed by the transformant cells showed that the Ltr051 cells carried the gene for human SCARB2. Thus, L-SCARB2 cells, which stably expressed SCARB2 cDNA in mouse L929 cells, were susceptible to EV71.
SCARB2 (also known as lysosomal integral membrane protein II, LGP85, or CD36b like-2) belongs to the CD36 family, which includes CD36 and scavenger receptor B, member 1 (SR-BI and its splice variant SR-BII).Citation49 SCARB2 is a type III double-transmembrane protein, which comprises 478 amino acids, a large exofacial domain (an extracellular domain when SCARB2 is presented at the cell surface or a luminal domain when presented in the endosomal compartment), and short cytoplasmic domains at the amino- and carboxy-termini (Figure 2).Citation49 SCARB2 participates in membrane transportation and in the reorganization of the endosomal/lysosomal compartment.Citation50,Citation51,Citation52 The best-known physiological function of SCARB2 is mediation of β-glucocerebrosidase delivery from the endoplasmic reticulum to lysosomes.Citation53 Thus, the majority of SCARB2 is localized in the lysosomal membrane. Yamayoshi et al.Citation37,Citation54 showed that SCARB2 on the cell surface binds to EV71; the EV71 is then internalized, possibly as a SCARB2–EV71 complex. These observations suggest that (at least) a small proportion of SCARB2 molecules shuttle between the endosomal/lysosomal compartments and the plasma membrane, where they act as EV71 receptors (Figure 2).
Figure 2 EV71 infection mediated by SCARB2 and other receptors. SCARB2 delivers β-GC from the ER to the lysosomes under physiological conditions. SCARB2 is abundant in the lysosomal and endosomal compartments, and it also shuttles to the plasma membrane where it encounters EV71. After binding the virus on the cell surface, the virus–receptor complex is internalized via the clathrin-mediated endocytosis pathway. In the endosome or lysosome, where the pH is low, the virus initiates a conformational change that leads to uncoating. PSGL-1 can bind EV71 and internalize via caveolin-mediated endocytosis, but PSGL-1 cannot initiate uncoating. Anx2, heparan sulfate, and sialylated glycans can also bind EV71. However, the mechanism of internalization and uncoating is unknown. They may deliver EV71 to SCARB2 or they may establish infections via their own mechanisms. β-GC, β-glucocerebrosidase; ER, endoplasmic reticulum.

Scarb2 Structure Required For Ev71 Binding
Recently, the crystal structure of the SCARB2 ectodomain was elucidated.Citation55 In contrast to PVR and coxsackie-adenovirus receptor, SCARB2 lacks an Ig-like fold, but comprises an anti-parallel β-barrel with many short α-helical segments. There are two α-helices at the bottom of the β-barrel fold, i.e., α1 and α15, which are connected to the N-terminal and C-terminal transmembrane regions, respectively (Figure 3). The head region at the top comprises a three α-helix bundle (which is formed by α-helices 4, 5 and 7), two other short helices (α2 and α14) and the β7 strand. Nine N-glycosylation sites are present in SCARB2, but the carbohydrate chains are localized in the middle or lower regions (the head region is free of carbohydrate chains). The binding site of β-glucocerebrosidase, the natural ligand of SCARB2, has been mapped to this head region. Mutagenesis and model reconstitution studies using other members of the CD36 family suggest that this apical face also acts as the binding site for their respective ligands.
Figure 3 Crystal structure of SCARB2. The crystal structure of the SCARB2 ectodomain (PDB: 4F7B) was determined by X-ray diffraction. The important amino acid region responsible for binding to EV71 (amino acids 142–204)Citation56 is shown in yellow. Arrows represent β-strands and tubes represent α-helices. Black, red and blue sticks and balls represent the carbohydrate chains.
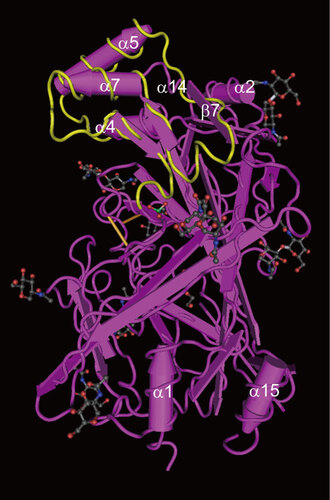
The amino acids in SCARB2 that are required for EV71 binding and infection were mapped using chimeric mutants comprising human and mouse SCARB2. Mouse SCARB2 shares 85.8% amino-acid identity with human SCARB2.Citation56 Chimeras that possessed amino acids 142–204 from the human sequence were able to act as functional receptors for EV71, whereas chimeras possessing the mouse sequence in this region were not. This region of the SCARB2 protein shows 76.2% identity at the amino-acid level between human and mouse sequences, and this region also contains the head region in the crystal structure, suggesting that EV71 binds to the head region of SCARB2. Removal of the carbohydrate moiety from the recombinant soluble SCARB2 protein using PNGase F did not abolish virus binding to the receptor, which is consistent with the structural data. Chen et al.Citation45 also identified the residues that are critical for human SCARB2 binding to EV71, i.e., residues 144–151 within a region that is highly variable among species. This region corresponds to the α5 helix within the head region. It is likely that head region of SCARB2 binds to the canyon of the EV71 virion, but the precise footprint of SCARB2 on the virion has not yet been determined by cryoelectron microscopy.
Mechanism Of Ev71 Internalization Via Scarb2
EV71 infection of RD cells occurs in a SCARB2-dependent manner. Hussain et al.Citation57 investigated the host factors required for EV71 entry into RD cells using a small interfering RNA (siRNA) library and found that knockdown of proteins associated with clathrin-mediated endocytosis, such as adaptor-related protein complex 2, alpha 1 subunit, arrestin, beta 1, clathrin, heavy chain, clathrin, heavy chain-like 1, synaptojanin 1, actin-related protein 2/3 complex, subunit 5, p21 protein (Cdc42/Rac)-activated kinase 1, Rho-associated, coiled-coil containing protein kinase 1 and WAS protein family, member 1, inhibited EV71 infection significantly. They observed the colocalization of EV71 with clathrin in an immunofluorescence assay, and visualized the virions in clathrin-coated pits by electron microscopy. The entry of EV71 into cells was inhibited when a dominant-negative mutant of epidermal growth factor receptor pathway substrate 15, which bound to AP-2, was expressed, and when cells were treated with drugs that selectively inhibited clathrin-dependent endocytosis (chlorpromazine and cytochalasin B). Viral entry was not impaired by inhibitors of caveolae-dependent endocytosis and macropinocytosis. Hussain et al.Citation57 also showed that EV71 infection was markedly inhibited when cells were treated with drugs that prevented the acidification of the endosome (bafilomycin A1 and concanamycin A). Overall, these results suggest that EV71 entry into RD cells is dependent on a clathrin-mediated pathway and that endosomal acidification is required for the establishment of infection. In addition, Lin et al.Citation58 showed that EV71 entry is dependent on clathrin and dynamin by transfecting siRNAs for clathrin light chain B and dynamin-2, respectively, into NIH3T3 cells that stably expressed human SCARB2. They also showed that chlorpromazine inhibited EV71 infection, whereas caveolar endocytosis inhibitors such as genistein and flipin did not.
Scarb2 Is The Ev71 Uncoating Receptor
PVR and intercellular adhesion molecule-1 bind poliovirus and the major group rhinoviruses, respectively, which induce conformational changes that lead to uncoating of the viral genome. Similarly, SCARB2 induces a conformational change that leads to uncoating of the EV71 virion. Yamayoshi et al.Citation54 demonstrated that the incubation of 35S-labeled EV71 with L-SCARB2 cells or soluble SCARB2 molecules induced a conformational change. An 80S particle was detected by sucrose density gradient centrifugation, which was an empty capsid that comprised VP1, VP2, and VP3 without genomic RNA. This reaction occurred at an acidic pH (below 6.0). These results suggest that both SCARB2 and acidic pH are required for EV71 uncoating. Chen et al.Citation45 reported that SCARB2, but not PSGL-1, induced 160S virions (in which the genomic RNA was quantified by real time reverse transcription-polymerase chain reaction) to undergo a conformational change to yield slowly sedimenting particles on sucrose density gradient centrifugation, and that this change was enhanced in an acidic environment (pH 5.6). The slowly sedimenting particle was thought to be the A-particle, although the composition of the particle was not determined. The uncoating products reported in the two previous studies were not identical, but both results strongly suggest that SCARB2 can initiate a conformational change that leads to the uncoating of the virion at acidic pH. In contrast to poliovirus and group B coxsackieviruses, EV71 uncoating requires a low pH. Thus, this uncoating may occur after the virus-receptor complex reaches the endosome or lysosome.
in vivo IMPORTANCE OF SCARB2
SCARB2 is expressed in a variety of human tissues.Citation59 In particular, high expression of SCARB2 is observed in neurons in the central nervous system, lung pneumocytes, hepatocytes, renal tubular epithelium, splenic germinal centers and intestinal epithelium. SCARB2, but not PSGL-1, expression was observed in EV71 antigen-positive neurons and in epithelial cells in the crypts of palatine tonsils from patients that died of acute neurological disease.Citation60 Thus, it is suggested that SCARB2 plays an essential role in establishing EV71 infection and in the development of neurological disease in humans. This hypothesis is supported by the transgenic expression of human SCARB2 in mice. Some EV71 strains are able to infect suckling mice, but none can infect and cause disease in adult mice. Fujii et al.Citation59 generated transgenic mice that expressed human SCARB2 using a human bacterial artificial chromosome clone that encoded the entire SCARB2 gene in which SCARB2 gene expression was driven by its own promoter. As expected, the expression profile of human SCARB2 in mice was quite similar to that in humans. When EV71 was used to inoculate adult transgenic mice via the intracerebral, intravenous and intraperitoneal routes, the mice exhibited paralytic diseases similar to those observed in humans infected with EV71. EV71 antigens were detected in neurons in the brainstem, the cerebellar nuclei and the spinal cord, suggesting that SCARB2 expression alone is sufficient to cause neurological disease in infected mice. Lin et al.Citation61 generated another transgenic mouse model that expressed human SCARB2 via a ubiquitous promoter. Suckling transgenic mice were susceptible to EV71, and the main EV71 replication site was skeletal muscle; however, susceptibility was lost when the mice reached 3 weeks of age. These results suggest that expression of human SCARB2 is required to confer EV71 susceptibility in mice. However, the expression of SCARB2 at appropriate sites is important if it is to cause diseases similar to those found in humans. It should be noted that SCARB2 expression is observed widely among tissues, and that not all cells expressing SCARB2 allow efficient viral replication. Thus, it is possible that SCARB2 expression is necessary for viral replication, although other mechanisms may also contribute to EV71 infection susceptibility or permissiveness.
Scarb2 Is A Receptor For Other Members Of The Hev-A
Most members of the HEV-A cannot infect mouse L929 cells. Yamayoshi et al.Citation62 used L-SCARB2 cells to test whether HEV-A members could infect via SCARB2. They found that all clinical isolates of EV71, CAV14 and CVA16 were capable of infecting L-SCARB2 cells, whereas CVA2, CVA3, CVA4, CVA5, CVA6, CVA8, CVA10 and CVA12 were not. However, CVA7 could infect the parental L929 cells; therefore, its SCARB2-dependency was not confirmed in this experiment. SCARB2-dependent infection of EV71, CVA7, CVA14 and CVA16 was also confirmed using other methods. The infection of RD cells by EV71, CVA14, CVA16 and CVA7 was severely inhibited when SCARB2 expression was knocked down using a siRNA targeting SCARB2. In addition, EV71, CVA7, CVA14 and CVA16 were coprecipitated with a soluble SCARB2 protein. These results suggest that CVA7, CVA14 and CVA16, which are most closely related to EV71 based on a phylogenetic analysis of the capsid sequences, can utilize SCARB2 as a receptor. Both EV71 and CVA16 are major causative agents of HFMD. Thus, it is reasonable to assume that they use the same receptor. However, EV71 sometimes causes severe neurological disease, whereas this is seldom the case with CVA16. This suggests that the differences in the neuropathogenicity of these two viruses cannot be attributed simply to receptor usage.
PSGL-1
PSGL-1 is a sialomucin leukocyte membrane protein, which can bind to three different selectins.Citation63,Citation64,Citation65 PSGL-1 plays critical roles in the tethering and rolling of leukocytes during the recruitment of cells from blood vessels to the sites of acute inflammation after stimulation by infection. Nishimura et al.Citation36 found that Jurkat T cells were susceptible to infection by the EV71 1095 strain. They prepared a cDNA library from Jurkat cells and expressed it in P3X63Ag8U.1 cells via a retrovirus vector. They then enriched the cells that could bind to EV71 1095 strain immobilized on plates. Finally, they showed that human PSGL-1 could bind EV71, and that sulfation of three tyrosine residues (Tyr 46, Tyr 48 and Tyr 51) near the N-terminus was required.Citation66 However, PSGL-1 is not a receptor for all EV71 strains. Thus, EV71 can be classified into two groups: PSGL-1-binding strains (PB) and PSGL-1 non-binding strains (non-PB). The molecular mechanism that underlies the PB and non-PB phenotypes has been clarified: Gly/Glu 145 of VP1 acts as a molecular switch that determines the PB and non-PB phenotype.Citation67 The three-dimensional structure and mutagenesis of EV71 suggest that PSGL-1 binds to positively charged amino acids located near the five-fold vertex via an electrostatic interaction. In PB strains (VP1 Gly 145), Lys residues located at positions 242 and 244 near the five-fold axis vertex are exposed on the virion surface, whereas these amino acids are less exposed in the non-PB (VP1 Glu 145) strains. PSGL-1 binds to these positively charged amino acids. According to the available sequence data, approximately 80% of EV71 isolates are non-PB strains.
The infection efficiency mediated by PSGL-1 is much lower than that mediated by SCARB2. The appearance of the cytopathic effect mediated by PSGL-1 required a few days, and EV71 infection of L929 cells expressing PSGL-1 (L-PSGL-1 cells) was successful in only a small subset of EV71 strains.Citation68 In addition, four out of five PB strains replicated poorly in L-PSGL-1 cells. An additional mutation in the capsid region (VP2 Lys149Ile/Met) was required for better replication. The affinity of PSGL-1 for PB strains is much higher than that of SCARB2 for EV71 strains. The PSGL-1–EV71 complex is able to enter the cell via a caveolin-dependent pathway, and disturbing caveolar endocytosis using specific inhibitors (genistein and flipin) or the use of caveolin-1 siRNA in Jurkat and L-PSGL-1 cells significantly inhibits EV71 infection.Citation69 However, no uncoating products of EV71 was observed in infected cells, or when EV71 was mixed with PSGL-1 in vitro under any of the conditions examined.Citation54 This suggests that PSGL-1 can bind PB strains and internalize the virus, whereas it cannot initiate conformational change. Thus, another molecule that stimulates either the uncoating of the PSGL-1-captured virion or thermal degradation of the virion may be required to establish an infection.
The tissue distribution of PSGL-1 is restricted to myeloid, lymphoid, dendritic lineages and platelets. PSGL-1 is also expressed on dendritic cells in the lymph nodes and macrophages in the intestinal mucosa.Citation64 However, PSGL-1 expression has not been reported in the neurons of the central nervous system and the epithelial cells in the crypt of palatine tonsil.Citation60 No reports suggest that PB and non-PB strains have different pathological outcomes. Furthermore, the transgenic expression of PSGL-1 is not sufficient to cause disease.Citation70 Taken together, these results suggest that PSGL-1 may enhance EV71 infection by PB strains but may not play a critical role in EV71 pathogenesis.
Anx2
Anx2 is a member of the annexin family. Anx2 is a calcium- and phospholipid-binding protein, which serves as a profibrinolytic coreceptor for tissue plasminogen activator and plasminogen on endothelial cells.Citation71 Yang et al.Citation38 captured proteins from RD cells lysates using a recombinant EV71 VP1 protein fused to a calmodulin-binding protein tag. The VP1-captured proteins were subjected to a virus overlay protein-binding assay, which detected a 36 KDa protein. This protein was identified as Anx2 by mass spectrometry analysis. The direct binding of Anx2 and EV71 by five strains of EV71 with different genotypes was confirmed in a pull-down assay; CVA16 did not bind Anx2. Pretreatment of EV71 with soluble recombinant Anx2 or pretreatment of host cells with an anti-Anx2 antibody decreased viral attachment to the cell surface and subsequently reduced the virus yield in vitro. HepG2 cells, which do not express Anx2, were susceptible to EV71. However, HepG2 cells that stably expressed Anx2 yielded significantly higher titers than the parental HepG2 cells. Yeast two-hybrid analysis mapped the Anx2-interacting domain to VP1 amino acids 40–100, which comprise β-sheet B and the partial BC loop, i.e., not the EV71 canyon region. Viral entry and uncoating via Anx2 have not been reported, which suggests that Anx2 may be an attachment receptor.
Sialylated Glycans
In general, sialic acid (SA) is present in terminal monosaccharides expressed on the glycan chains of glycolipids and glycoproteins.Citation72 In particular, gastrointestinal and respiratory epithelial cells express an abundance of SA-containing glycoproteins and SA-containing glycolipids. Yang et al.Citation35 postulated that EV71 might use the SA-linked glycan on intestinal epithelial cells as a receptor, and that natural SA-linked glycans may protect human intestinal cells from EV71 infection. DLD-1 intestinal cells are susceptible to EV71 infection; however, the depletion of O-linked glycans or glycolipids, but not N-linked glycans, using O- or N-linked glycan synthesis inhibitors significantly reduces the incidence of EV71 infection. Pretreatment with sialidase also reduces EV71 replication in DLD-1 cells. Furthermore, the addition of purified SA-α2,3Gal and SA-α2,6Gal from human milk to cell cultures significantly inhibits EV71 infection of DLD-1 cells. These results suggest that SA-linked glycans are EV71 receptors on DLD-1 cells, although no evidence of a direct interaction between sialylated glycans and EV71 has been reported.
Heparan Sulfate
Tan et al.Citation39 reported that heparan sulfate contributes to the binding of EV71 to the cell surface. Preincubation of EV71 with heparin, polysulfated dextran sulfate and suramin significantly inhibited EV71 infection of RD cells. They also demonstrated that preincubation of RD cells with poly-D-lysine neutralized the negative charges on the cell surface and inhibited EV71 infection. Blocking of heparan sulfate biosynthesis by sodium chlorate, or knockdown of N-deacetylases/N-sulfotransferase-1 and exostosin-1 also reduced EV71 infection in RD cells. Heparinase I/II/III treatment also reduced the amount of EV71 that bound to the surface of RD cells, while EV71 particles bound to heparin-Sepharose columns at physiological salt concentrations.
Conclusions
Recent studies suggest that SCARB2 is a pivotal receptor that mediates the attachment of EV71 and closely related HEVs. SCARB2 can bind EV71, internalize the bound virus, and induce conformational changes at low pH (Figure 2). It is likely that SCARB2 binds to the canyon of the EV71 virion, but the precise binding site needs to be confirmed. Other molecules, which might be attachment receptors, have also been reported as EV71 receptors. Since these molecules cannot induce uncoating of the virion, their function may be to capture EV71 on the cell surface and deliver it to SCARB2 expressed either on the cell surface or in endosomes (Figure 2). Alternatively, they may facilitate EV71 infection via their own mechanisms independently of SCARB2 (Figure 2). The contribution(s) of these receptors to EV71 infection, and/or the cooperative interactions between them, require further study. SCARB2 also plays an essential role in EV71 infection in vivo. Using a transgenic mouse model that expresses SCARB2, it will be possible to evaluate the virulence of EV71 strains and identify the genetic information that determines the neurovirulent phenotype of this virus. It will also be possible to establish an animal model to evaluate the efficacy of a vaccine and anti-EV71 drugs.
- Oberste MS, Jiang X, Maher K, Nix WA, Jiang B.The complete genome sequences for three simian enteroviruses isolated from captive primates. Arch Virol2008;153: 2117–2122.
- Oberste MS, Maher K, Pallansch MA.Complete genome sequences for nine simian enteroviruses. J Gen Virol2007;88: 3360–3372.
- Pallansch M, Roos R.Enteroviruses. polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses.In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed.) Fields Virology.5th ed.Philadelphia, PA: Lippincott Williams & Wilkins, 2007: 839–893.
- Ho M, Chen ER, Hsu KH et al.An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med1999;341: 929–935.
- Schmidt NJ, Lennette EH, Ho HH.An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis1974;129: 304–309.
- Chumakov KM, Lavrova IK, Martianova LI, Korolev MB, Bashkirtsev VN, Voroshilova MK.Investigation of physicochemical properties of Bulgarian strain 258 of enterovirus type 71. Brief report. Arch Virol1979;60: 359–362.
- Chumakov M, Voroshilova M, Shindarov L et al.Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol1979;60: 329–340.
- Melnick JL.Enterovirus type 71 infections: a varied clinical pattern sometimes mimicking paralytic poliomyelitis. Rev Infect Dis1984;6( Suppl 2): S387–S390.
- Chan LG, Parashar UD, Lye MS et al.Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis2000;31: 678–683.
- McMinn P, Lindsay K, Perera D, Chan HM, Chan KP, Cardosa MJ.Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J Virol2001;75: 7732–7738.
- McMinn P, Stratov I, Nagarajan L, Davis S.Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis2001;32: 236–242.
- Komatsu H, Shimizu Y, Takeuchi Y, Ishiko H, Takada H.Outbreak of severe neurologic involvement associated with Enterovirus 71 infection. Pediatr Neurol1999;20: 17–23.
- Fujimoto T, Chikahira M, Yoshida S et al.Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol2002;46: 621–627.
- Wang JR, Tuan YC, Tsai HP, Yan JJ, Liu CC, Su IJ.Change of major genotype of enterovirus 71 in outbreaks of hand-foot-and-mouth disease in Taiwan between 1998 and 2000. J Clin Microbiol2002;40: 10–15.
- Ahmad K.Hand, foot, and mouth disease outbreak reported in Singapore. Lancet2000;356: 1338.
- De W, Changwen K, Wei L et al.A large outbreak of hand, foot, and mouth disease caused by EV71 and CAV16 in Guangdong, China, 2009. Arch Virol2011;156: 945–953.
- Yip CC, Lau SK, Woo PC, Yuen KY.Human enterovirus 71 epidemics: what’s next? Emerg Health Threats J2013;6: 19780.
- Qiu J.Enterovirus 71 infection: a new threat to global public health? Lancet Neurol2008;7: 868–869.
- Tuthill TJ, Groppelli E, Hogle JM, Rowlands DJ.Picornaviruses. Curr Top Microbiol Immunol2010;343: 43–89.
- Bergelson JM.Receptors.In: Ehrenfeld E, Domingo E, Roos RP (ed.) The Picornaviruses.Washington, DC: ASM Press, 2010: 73–86.
- Fricks CE, Hogle JM.Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol1990;64: 1934–1945.
- Tuthill TJ, Bubeck D, Rowlands DJ, Hogle JM.Characterization of early steps in the poliovirus infection process: receptor-decorated liposomes induce conversion of the virus to membrane-anchored entry-intermediate particles. J Virol2006;80: 172–180.
- Danthi P, Tosteson M, Li QH, Chow M.Genome delivery and ion channel properties are altered in VP4 mutants of poliovirus. J Virol2003;77: 5266–5274.
- Tosteson MT, Wang H, Naumov A, Chow M.Poliovirus binding to its receptor in lipid bilayers results in particle-specific, temperature-sensitive channels. J Gen Virol2004;85: 1581–1589.
- Levy HC, Bostina M, Filman DJ, Hogle JM.Catching a virus in the act of RNA release: a novel poliovirus uncoating intermediate characterized by cryo-electron microscopy. J Virol2010;84: 4426–4441.
- Bostina M, Levy H, Filman DJ, Hogle JM.Poliovirus RNA is released from the capsid near a twofold symmetry axis. J Virol2011;85: 776–783.
- Neumann E, Moser R, Snyers L, Blaas D, Hewat EA.A cellular receptor of human rhinovirus type 2, the very-low-density lipoprotein receptor, binds to two neighboring proteins of the viral capsid. J Virol2003;77: 8504–8511.
- Hewat EA, Neumann E, Conway JF et al.The cellular receptor to human rhinovirus 2 binds around the 5-fold axis and not in the canyon: a structural view. EMBO J2000;19: 6317–6325.
- Verdaguer N, Fita I, Reithmayer M, Moser R, Blaas D.X-ray structure of a minor group human rhinovirus bound to a fragment of its cellular receptor protein. Nat Struct Mol Biol2004;11: 429–434.
- Prchla E, Kuechler E, Blaas D, Fuchs R.Uncoating of human rhinovirus serotype 2 from late endosomes. J Virol1994;68: 3713–3723.
- Plevka P, Perera R, Cardosa J, Kuhn RJ, Rossmann MG.Crystal structure of human enterovirus 71. Science2012;336: 1274.
- Wang X, Peng W, Ren J et al.A sensor–adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol2012;19: 424–429.
- Cifuente JO, Lee H, Yoder JD et al.Structures of the procapsid and mature virion of enterovirus 71 strain 1095. J Virol2013;87: 7637–7645.
- Shingler KL, Yoder JL, Carnegie MS et al.The enterovirus 71 A-particle forms a gateway to allow genome release: a cryoEM study of picornavirus uncoating. PLoS Pathog2013;9: e1003240.
- Yang B, Chuang H, Yang KD.Sialylated glycans as receptor and inhibitor of enterovirus 71 infection to DLD-1 intestinal cells. Viral J2009;6: 141.
- Nishimura Y, Shimojima M, Tano Y, Miyamura T, Wakita T, Shimizu H.Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med2009;15: 794–797.
- Yamayoshi S, Yamashita Y, Li J et al.Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med2009;15: 798–801.
- Yang SL, Chou YT, Wu CN, Ho MS.Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J Virol2011;85: 11809–11820.
- Tan CW, Poh CL, Sam IC, Chan YF.Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J Virol2013;87: 611–620.
- Koike S, Taya C, Kurata T et al.Transgenic mice susceptible to poliovirus. Proc Natl Acad Sci USA1991;88: 951–955.
- Ren RB, Costantini F, Gorgacz EJ, Lee JJ, Racaniello VR.Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell1990;63: 353–362.
- Ida-Hosonuma M, Iwasaki T, Yoshikawa T et al.The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J Virol2005;79: 4460–4469.
- Abe Y, Fujii K, Nagata N et al.The Toll-like receptor 3-mediated antiviral response is important for protection against poliovirus infection in poliovirus receptor transgenic mice. J Virol2012;86: 185–194.
- Liu CC, Guo MS, Lin FH et al.Purification and characterization of enterovirus 71 viral particles produced from Vero cells grown in a serum-free microcarrier bioreactor system. PloS ONE2011;6: e20005.
- Chen P, Song Z, Qi Y et al.Molecular determinants of enterovirus 71 viral entry: cleft around GLN-172 on VP1 protein interacts with variable region on scavenge receptor B 2. J Biol Chem2012;287: 6406–6420.
- Hewat EA, Blaas D.Cryoelectron microscopy analysis of the structural changes associated with human rhinovirus type 14 uncoating. J Virol2004;78: 2935–2942.
- Hewat EA, Neumann E, Blaas D.The concerted conformational changes during human rhinovirus 2 uncoating. Mol Cell2002;10: 317–326.
- Garriga D, Pickl-Herk A, Luque D et al.Insights into minor group rhinovirus uncoating: the X-ray structure of the HRV2 empty capsid. PLoS Pathog2012;8: e1002473.
- Calvo D, Dopazo J, Vega MA.The CD36, CLA-1 (CD36L1), and LIMPII (CD36L2) gene family: cellular distribution, chromosomal location, and genetic evolution. Genomics1995;25: 100–106.
- Kuronita T, Eskelinen EL, Fujita H, Saftig P, Himeno M, Tanaka Y.A role for the lysosomal membrane protein LGP85 in the biogenesis and maintenance of endosomal and lysosomal morphology. J Cell Sci2002;115: 4117–4131.
- Blanz J, Groth J, Zachos C, Wehling C, Saftig P, Schwake M.Disease-causing mutations within the lysosomal integral membrane protein type 2 (LIMP-2) reveal the nature of binding to its ligand beta-glucocerebrosidase. Hum Mol Genet2010;19: 563–572.
- Eskelinen EL, Tanaka Y, Saftig P.At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol2003;13: 137–145.
- Reczek D, Schwake M, Schroder J et al.LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell2007;131: 770–783.
- Yamayoshi S, Ohka S, Fujii K, Koike S.Functional comparison of SCARB2 and PSGL1 as receptors for enterovirus 71. J Virol2013;87: 3335–3347.
- Neculai D, Schwake M, Ravichandran M et al.Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature2013;504: 172–176.
- Yamayoshi S, Koike S.Identification of a human SCARB2 region that is important for enterovirus 71 binding and infection. J Virol2011;85: 4937–4946.
- Hussain KM, Leong KL, Ng MM, Chu JJ.The essential role of clathrin-mediated endocytosis in the infectious entry of human enterovirus 71. J Biol Chem2011;286: 309–321.
- Lin YW, Lin HY, Tsou YL et al.Human SCARB2-mediated entry and endocytosis of EV71. PloS ONE2012;7: e30507.
- Fujii K, Nagata N, Sato Y, Ong KC, Wong KT, Yamayoshi S et al.Transgenic mouse model for the study of entero virus 71 neuropathogenesis. Proc Natl Acad Sci USA2013;110: 14753–14758.
- He Y, Ong KC, Gao Z et al.Tonsillar crypt epithelium is an important extra-central nervous system site for viral replication in EV71 encephalomyelitis. Am J Pathol2014;184: 714–720.
- Lin YW, Yu SL, Shao HY et al.Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PloS ONE2013;8: e57591.
- Yamayoshi S, Iizuka S, Yamashita T et al.Human SCARB2-dependent infection by coxsackievirus A7, A14, and A16 and enterovirus 71. J Virol2012;86: 5686–5696.
- Sako D, Chang XJ, Barone KM et al.Expression cloning of a functional glycoprotein ligand for P-selectin. Cell1993;75: 1179–1186.
- Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL.P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood1996;88: 3010–3021.
- Somers WS, Tang J, Shaw GD, Camphausen RT.Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell2000;103: 467–479.
- Nishimura Y, Wakita T, Shimizu H.Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS Pathog2010;6: e1001174.
- Nishimura Y, Lee H, Hafenstein S et al.Enterovirus 71 binding to PSGL-1 on leukocytes: VP1-145 acts as a molecular switch to control receptor interaction. PLoS Pathog2013;9: e1003511.
- Miyamura K, Nishimura Y, Abo M, Wakita T, Shimizu H.Adaptive mutations in the genomes of enterovirus 71 strains following infection of mouse cells expressing human P-selectin glycoprotein ligand-1. J Gen Virol2011;92: 287–291.
- Lin HY, Yang YT, Yu SL et al.Caveolar endocytosis is required for human PSGL-1-mediated enterovirus 71 infection. J Virol2013;87: 9064–9076.
- Liu J, Dong W, Quan X, Ma C, Qin C, Zhang L.Transgenic expression of human P-selectin glycoprotein ligand-1 is not sufficient for enterovirus 71 infection in mice. Arch Virol2012;157: 539–543.
- Hajjar KA, Acharya SS.Annexin II and regulation of cell surface fibrinolysis. Ann NY Acad Sci2000;902: 265–271.
- Varki NM, Varki A.Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest2007;87: 851–857.
