Abstract
Babesiosis is a tick-borne, zoonotic disease caused by Babesia spp. Two cases of babesiosis were detected by nested polymerase chain reaction (PCR) in Yunnan province, China, and further confirmed by molecular assay. The blood smears showed intraerythrocytic ring form and tetrads typical of small B. microti. In both cases, the rapid diagnostic test (RDT) ruled out the possibility of co-infections with malaria. Neither case was initially diagnosed because of the low Babesia parasitemia. These two cases of babesiosis in areas along the Myanmar–China border pose the question of the emergence of this under recognized infection in countries or areas where malaria is endemic.
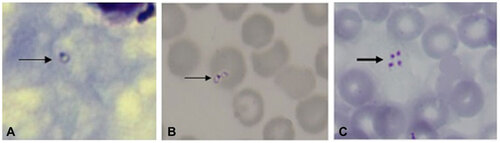
Figure 1 Microscopic evidence of babesiosis in a patient from the China–Myanmar border area. Giemsa stained thick blood smears (A) and thin blood smears (B and C) obtained on the first day of hospitalization for patient show an intraerythrocytic trophozoite (thin arrow). The lacking of hemozoin deposits distinguishes Babesia spp. from Plasmodium spp. The tetrad (thick arrow) is pathognomonic of small Babesia spp. Original magnification, ×1000.
CASE REPORT
Patient 1 was a 60-year-old man who was hospitalized on 5 August 2013, because of two weeks of fever, arthralgias and malaise. The rapid diagnostic test (RDT) for malaria was negative. Furthermore, no Plasmodium parasite was observed on the Giemsa stained blood smears obtained on the first day of hospitalization. The patient had a temperature of 38.5°C, low platelet count (6.9×1010/L) and hematocrit (39.0 %), but other routine blood examinations were normal. He also had increased levels of direct bilirubin (6.10 µmol/L) and alanine aminotransferase (100 U/L). The ultrasonography scan revealed splenomegaly. The patient recalled that he had travelled to Myanmar and back to China frequently, but had never received any blood transfusions or blood products. He also recalled multiple tick bites about 2 months prior to diagnosis, particularly after outdoor activities in the jungle along border areas of China–Myanmar.
Patient 2 was a 30-year-old man who was hospitalized on 9 April 2013, because of two days of fever, diaphoresis, myalgias, progressive dyspnea and fatigue. The patient had a temperature of 38.4°C, a slightly elevated leukocyte count (1.11×1010/L), but normal levels of other routine blood parameters. The RDT for malaria was negative. Originally from Tengchong village, he had travelled to Myanmar 2 months ago, returning home at the beginning of April. He recalled multiple tick bites in the recent past and had also received blood transfusion and blood products for the treatment of renal-malaria caused by infection with Plasmodium falciparum during the summer of 2012.
A nested polymerase chain reaction (PCR) with two sets of Babesia microti specific primers for the small subunit ribosomal RNA (SSU rRNA) was conducted on blood samples.Citation1,Citation2 Positive samples were tested using another set of nested PCR primers to detect the beta-tubulin gene of B. microti.Citation3 To identify the clades to which the isolates belong, a more accurate analysis of the sequence encoding the 18S rRNA gene of the Babesia parasite from the patients was applied with the nested PCR primers Piro1F/rRNA-3′ and BablA/Prio6R as described by Medlin et al.Citation4 Second round PCR products that were full-length cDNAs for the 18S rRNA gene (∼1700 bp) and the beta-tubulin gene (∼590 bp) were sequenced. The sequences were entered in the BLAST database (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). The blood smears showed intraerythrocytic ring form and tetrad typical of small Babesia parasites (Figure ).
Sequences from SSU rRNA and beta-tubulin gene obtained by direct sequencing of PCR products from the two patients were aligned by using the ClustalW method (EMBL-EBI, Hixton, Cambridge, UK). Those sequences were deposited in GenBank with accession NOs KF410825, KJ128385 and KJ128387, respectively. The sequences of our two isolates and other sequences were clustered following the pattern described by Hunfeld et al.Citation5 to identify the group of our isolates.
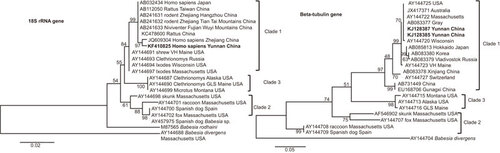
Phylogenetic trees were produced by using the neighbor joining method in MEGA version 5.1 (http://mega.software.informer.com/5.1b/). The case samples (denoted as Yunnan China) clustered with other B. microti isolates. B. divergens and B. rodhaini were set as the outgroups (Figure ).
Figure 2 Phylogenetic trees of the SSU rRNA and beta-tubulin gene sequences for the B. microti isolates obtained from the two patients in the China–Myanmar border area. Phylogenetic analysis produced by the neighbour-joining method using MEGA version 5.1 software. SSU rRNA and beta-tubulin gene sequences of our case study samples were denoted as Yunnan China in bold face. B. divergens and B. rodhaini were set as the outgroups. Scale bar indicates nucleotide substitutions per site.
Infection of human babesiosis has only been reported as a few sporadic cases in China.Citation6 Because malaria RDT was negative for each case,Citation7 and the blood smears failed to find any protozoan parasites, neither patient was treated with any anti-protozoa drugs. Atovaquone, the drug of first choice for treatment of babesiosis is not available in China. However, a combination of penicilin, quinolone and other symptomatic treatments, such as oral paracetamol tablets for the high temperature and supplemental vitamin C and vitamin B1, were administered to these two patients. Both patients had no significant immune deficiency; Babebsia infections were relatively benign since fever and other symptoms resolved within 1 month of therapy.
DISCUSSION AND CONCLUSION
Babesiosis is a tick-borne, zoonotic disease caused by the Babesia protozoa. The severity of human babesiosis depends on the immune status of the host and on the Babesia species causing the infection, ranging from an asymptomatic infection to a severe life threatening disease.Citation6 In some areas, B. microti is known to elicit no symptoms in about half of children and a quarter of adults.Citation8 Cases caused by B. duncani infections have ranged from asymptomatic to fatal.Citation9 Most cases reported on B. divergens infection are severe and most of these severe babesiosis cases occurred in people who lack a spleen.Citation5,Citation10 The first case of babesiosis infection in an immunocompetent person was reported in 1970.Citation11 Babesiosis is now classified as a notifiable disease in the United States and is recognized as an emerging health risk in other parts of the world.Citation6,Citation12,Citation13 In Asia, B. microti-like organisms have caused illness in Japan, Taiwan and Zhejiang province of China.Citation2,Citation14,Citation15,Citation16 Importantly, human babesiosis has sometimes been diagnosed initially as malaria because of the similarity between the two diseases or the two parasites,Citation17 but little is known about the prevalence of Babesia spp. infections in malaria-endemic areas, where misidentification as Plasmodium infection is most probable. We report two cases of ignored babesiosis in China–Myanmar border areas of Yunnan, China, where is endemic area for malaria, suggesting that babesiosis and malaria co-exist in this region. Both cases presented with malaria-like symptoms; babesia infections were confirmed by blood smears, PCR amplification and amplicon sequencing. These two cases were first detected by PCR. Upon review, their blood smears showed intraerythrocytic ring forms and tetrads, the latter forms being an uncommon finding that is considered pathognomonic of small Babesia spp., such as B. microti and B. duncani.Citation18
These two cases reveal that human babesiosis caused by B. microti occurs in areas along the China–Myanmar border that are otherwise known to be highly endemic for malaria. The lessons learnt from this finding are two-fold:
First, diagnosis of babesiasis is usually detected by blood smear, but the ring forms of both P. falciparum and Babesia spp. are difficult to distinguish from each other under clinical microscopy. Previous study showed that at least 300 microscopical fields need to be reviewed to detect low level parasitemia.Citation6 The confirmation of two cases by PCR indicated that human babesiosis can be distinguished from malaria with the assistance of PCR-based diagnostics. B. microti was regarded as a single species before, but then regarded as a genetically diverse species complex. Clade 1 of B. microti contained mostly rodent parasites and also the majority of strains thought to be zoonotic. Clade 2 contained carnivore parasites, and Clade 3 contained rodent parasites that are probably not zoonotic.Citation19 As expected, the B. microti isolates reported herein belong to the clade that contains the majority of zoonotic strains.
Second, babesiosis does not respond to chloroquine which might cause its misidentification as drug-resistant malaria, especially in syndemic areas. Both cases were treated with penicillin and quinolone, although these drugs have not been shown to be effective against babesiosis. It must also be noted that most antimalarial drugs, such as chloroquine and mefloquine have no effect on babesiosis.Citation20,Citation21 The first regimen effective against babesiosis consists of atovaquone and azithromycin whereas the second consists of clindamycin and quinine.Citation6 From the treatment standpoint, the diagnosis and treatment of Babesia infection in malaria syndemic areas deserves more attention. Particularly, artemisinin-resistant P. falciparum malaria has emerged in Cambodia and Thailand–Myanmar border.Citation22 B. microti appears to be responsive to artemisinin derivatives, as per the study on a mouse model.Citation23 But there is still not enough data or studies to confirm that artemisinin derivatives are effective to eliminate Babesia infection in humans.
In conclusion, babesiosis and malaria are caused by pathogens with their similar morphology and overlapping symptoms, but require different treatments. Improved diagnostic approaches would undoubtedly improve case management of these diseases in syndemic areas.
Xia Zhou conceived the study, collected the data and analyzed it, and drafted the manuscript. Sheng-Guo Li, Jia-Zhi Wang, Ji-lei Huang, He-Jun Zhou and Jun-Hu Chen revised the manuscript and provided interpretation of the findings, technical support for data collection and analysis. Xiao-Nong Zhou conceived the study and revised the manuscript. All authors read and approved the final manuscript. Written consent to publish was obtained. We would express our sincere gratitude to Professor Cally Roper from London School of Hygiene and Tropical Medicine for helping to review the manuscript and edit for the language. The research has been partially supported by the Special Fund for Health Research in the Public Interest China (NO 201202019), Strengthen Action Plan for Shanghai Public Health System Construction 2011–2013 (GW-11) and by the National Science & Technology Major Program (NO 2012ZX10004-220).
- Persing DH, Mathiesen D, Marshall WF et al.Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol1992;30: 2097–2103.
- Zhou X, Li SG, Chen SB et al.Co-infections with Babesia microti and Plasmodium parasites along the China–Myanmar border. Infect Dis Poverty2013;2: 24.
- Zamoto A, Tsuji M, Wei Q et al.Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic diversity in the beta-tubulin gene sequences. J Vet Med Sci2004;66: 785–792.
- Medlin L, Elwood HJ, Stickel S, Sogin ML.The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene1988;71: 491–499.
- Hunfeld KP, Hildebrandt A, Gray JS.Babesiosis: recent insights into an ancient disease. Int J Parasitol2008;38: 1219–1237.
- Vannier E, Krause PJ.Human babesiosis. N Engl J Med2012;366: 2397–2407.
- Abba K, Deeks JJ, Olliaro P et al.Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev2011;7 CD008122.
- Krause PJ, McKay K, Gadbaw J et al.Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg2003;68: 431–436.
- Bloch EM, Herwaldt BL, Leiby DA et al.The third described case of transfusion-transmitted Babesia duncani. Transfusion2012;52: 1517–1522.
- Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters TP.Human babesiosis. Ann Trop Med Parasitol1998;92: 489–501.
- Bloch EM, Lee TH, Krause PJ et al.Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion2013;53: 2299–2306.
- Joseph JT, Purtill K, Wong SJ et al.Vertical transmission of Babesia microti, United States. Emerg Infect Dis2012;18: 1318–1321.
- Lobo CA, Cursino-Santos JR, Alhassan A, Rodrigues M.Babesia: an emerging infectious threat in transfusion medicine. PLoS Pathog2013;9: e1003387.
- Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC.Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol1997;35: 450–454.
- Wei Q, Tsuji M, Zamoto A et al.Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol2001;39: 2178–2183.
- Yao LN, Ruan W, Zeng CY et al. [Pathogen identification and clinical diagnosis for one case infected with Babesia.] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi2012;30: 118–121.Chinese.
- Loutan L, Rossier J, Zufferey G et al.Imported babesiosis diagnosed as malaria. Lancet1993;342: 749.
- Homer MJ, Aguilar-Delfin I, Telford SR 3rd, Krause PJ, Persing DH.Babesiosis. Clin Microbiol Rev2000;13: 451–469.
- Goethert HK, Telford SR 3rd.What is Babesia microti? Parasitology2003;127: 301–309.
- Mosqueda J, Olvera-Ramirez A, Aguilar-Tipacamu G, Canto GJ.Current advances in detection and treatment of babesiosis. Curr Med Chem2012;19: 1504–1518.
- Krause PJ.Babesiosis diagnosis and treatment. Vector Borne Zoonotic Dis2003;3: 45–51.
- Phyo AP, Nkhoma S, Stepniewska K et al.Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet2012, 379:1960–1966.
- Goo YK, Terkawi MA, Jia H et al.Artesunate, a potential drug for treatment of Babesia infection. Parasitol Int2010, 59:481–486.
