Abstract
Epstein-Barr virus (EBV) has been used as a paradigm for studying host–virus interactions, not only because of its importance as a human oncogenic virus associated with several malignancies including nasopharyngeal carcinoma (NPC) but also owing to its sophisticated strategies to subvert the host antiviral responses. An understanding of the interplay between EBV and NPC is critical for the development of EBV-targeted immunotherapy. Here, we summarize the current knowledge regarding the host immune responses and EBV immune evasion mechanisms in the context of NPC.
Introduction
Epstein-Barr virus (EBV/HHV-4), which latently infects more than 90% of the world's adult human population, is associated with nasopharyngeal carcinoma (NPC).
In NPC patients, EBV typically exists in a type II latency program (particularly the undifferentiated or poorly differentiated types). Type II latency is characterized by the expression of a subset of latent genes, including EBV-determined nuclear antigen 1 (EBNA1), latent membrane proteins (LMP1, LMP2A, and LMP2B), and several EBV non-coding RNAs (primarily EBER1 and EBER2).Citation1,Citation2,Citation3 In addition, BamHI-A rightward transcripts (BARTs) and BamHI-A rightward frame 1 (BARF1) of EBV are expressed abundantly and detected consistently in NPC.Citation4,Citation5,Citation6
The detection of EBV in NPC and the prominent role of EBV in promoting tumor development support EBV as a potential therapeutic target for NPC. In fact, with the accumulation of knowledge regarding EBV oncogenicity and interactions between EBV and the host immune responses, immunological approaches, such as adoptive T-cell immunotherapy and vaccine-based strategies to induce EBV-specific T-cell responses, are emerging. In this review, we summarize the current understanding of how EBV stimulates the host immunity and the mechanisms exploited by EBV to circumvent immune responses in the context of NPC.
EVIDENCE FOR EBV CONTRIBUTING TO NPC
EBV factors detected in NPC patients
In the 1960s, antibodies against the EBV antigen were first identified in NPC patients,Citation7 and subsequent studies reported higher levels of anti-EBV antibodies in NPC patients than in healthy controls.Citation8,Citation9 More direct and stronger evidence has been obtained regarding the detection of EBV DNA,Citation10,Citation11 protein antigens,Citation2 and miRNA productsCitation1 in NPC patients. Viral DNA is considered a specific prognostic marker for both pre- and post-treatment NPC patients,Citation11,Citation12,Citation13,Citation14,Citation15,Citation16,Citation17 regardless of prevalence in the region studied.Citation18 Recent comprehensive profiles with methods that are more sensitive and specific (e.g., multiplexed stem-loop reverse transcription polymerase chain reactionCitation19 and miRNA microarrayCitation20) identified panels of upregulated viral miRNAs in both NPC lesions and sera, some of which were shown to function as potential biomarkers for the diagnosis and prognosis of NPC.Citation21
Mechanisms exploited by EBV products to promote NPC
A set of EBV latent genes have been identified that play an important role in NPC development, and multiple mechanisms including the restriction of cell homeostasis, the enhancement of cell mobility, and the induction of stem-like cancer cells were proposed.
EBNA1, which is expressed in all EBV-related tumors, is believed to be one of the most important viral proteins that promote NPC and is required for maintaining the viral latency in NPC.Citation22,Citation23 The introduction of EBNA1 enables EBV-negative NPC cells to grow more rapidly and to achieve increased metastasis in immunodeficiency mice.Citation24 The potential mechanisms of EBNA1 function involve upregulation of tumor angiogenesis cytokines;Citation25 degradation of promyelocytic leukemia (PML) protein, which is associated with p53 activation, DNA repair and cell apoptosis;Citation26 inhibition of the anti-oncogenesis canonical p65 nuclear factor-κB (NF-κB) pathway;Citation27 and induction of metastatic potential proteinsCitation28 as well as epithelial–mesenchymal transition (EMT).Citation29
LMP1, another major viral oncoprotein, is closely associated with epithelial transformationCitation30,Citation31 and angiogenesis.Citation32,Citation33,Citation34 LMP1 is detected primarily in preinvasive lesions, including dysplasia and carcinoma in situ, but not in late stage, suggesting that its expression may be an early, initiating event for NPC.Citation35 LMP1 has been shown to promote tumor invasion and metastasis via remodeling actin filaments,Citation36,Citation37,Citation38 inducing EMTCitation39 and upregulating the expression of various matrix metalloproteinases (MMPs).Citation40,Citation41,Citation42 In addition, LPM1 inhibits apoptosisCitation43,Citation44,Citation45 and induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines.Citation46,Citation47
LMP2A and LMP2B are also expressed in NPC.Citation2,Citation48,Citation49 LMP2B negatively regulates LMP2A activity by binding to this protein, preventing its phosphorylation without altering its cellular localization.Citation50 LMP2A possesses the ability to induce stem-like cancer cells,Citation47,Citation51 EMT,Citation51 and MMP expressionCitation52 in NPC. No direct evidence has been found for the role of LMP2B in NPC, although LMP2B itself may facilitate the spread and motility of epithelial cells.Citation53
Emerging evidence has revealed that EBERs, BARTs and BARF1 also contribute directly to NPC development. EBERs accelerate the growth of NPC cellsCitation54 and confer resistance against apoptotic stress.Citation55 BARF1 is not expressed during EBV infection of the NPC-derived EBV-negative cell lines HONE-1 and CNE-1; however, when infected by a recombinant EBV carrying the BARF1 gene under the control of the SV40 promoter, the infected NPC cells grew faster and were more resistant to apoptosis compared with wild-type EBV-infected cells.Citation56 BARTs are very abundant EBV transcripts in NPC, contain several open reading frames, and are precursors for 22 miRNAs. Their roles in NPC (for instance, miR-BART1Citation57 and 3Citation58 in cell transformation, miR-BART1Citation59 and 5Citation60 in anti-apoptotic activity, and miR-BART7Citation61,Citation62 and 9Citation63 in EMT) were recently reviewedCitation64 ().
Table 1 A brief summary of mechanisms exploited by EBV latent products to promote NPC formation and development
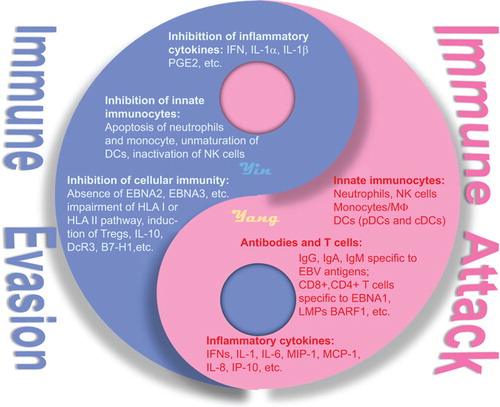
THE INTERPLAY BETWEEN HOST INNATE IMMUNITY AND EBV
EBV mounts innate responses
One major characteristic of NPC is the presence of abundant infiltrating leukocytes in tumor stroma where various cell types, including neutrophils,Citation65 natural killer (NK) cells,Citation66,Citation67 monocytes/macrophages, and dendritic cells (DCs),Citation68,Citation69,Citation70 are detected and represent the first defense line for EBV infection. Nevertheless, the interaction between EBV and the host innate immunity system is not fully understood.
Based on flow cytometry, EBV was shown to bind to the neutrophil surface with its major envelope glycoprotein gp350 and subsequently stimulate the production of antiviral cytokines, including interleukin 1α (IL-1α), IL-1β,Citation71 chemokines IL-8, and macrophage inflammatory protein (MIP)-1.Citation72
Conventional DCs (cDCs) and plasmacytoid DCs (pDCs), the two major human DC subsets, sense EBV products through Toll-like receptors. When challenged with either live EBV virions or unmethylated EBV DNA, pDCs were found to produce interferon-α (IFN-α).Citation73 In addition, treatment of cDCs with EBERs induces the production of IFN-β, IFN-γ, and tumor necrosis factors (TNFs).Citation74 EBV-stimulated cDCs and pDCs can promote the cytotoxicity of NK cells through type I IFNs.Citation75 NK cells are potential targets for EBV infection because the gp85-gp25-gp42 complex of EBV can directly combine with human leukocyte antigen (HLA) class II molecules on NK cells.Citation76
EBV can activate monocytesCitation77 and macrophages.Citation78 dUTPase of EBV induces macrophages to express and secrete TNF-α, IL-1β, and IL-6 via the MyD88-dependent activation of NF-κB.Citation78,Citation79 For monocytes, in addition to the inflammatory cytokines that are also produced by activated macrophages,Citation80 EBV also stimulates production of several chemokines, including IFN-inducible protein-10 (IP-10), MIP-1, monocyte chemotactic protein-1 (MCP-1), and IL-8 at the mRNA level.Citation77
Evasion of innate immune responses
The establishment of life-long persistence in more than 90% of the worldwide adult human population clearly indicates that EBV has delicately evolved to evade the innate immune response. In addition to the above-mentioned latent genes, a portion of viral lytic antigens are frequently detected in NPC, probably due to EBV reactivation upon some poorly defined triggers.Citation81,Citation82 Recently, the mechanisms by which individual EBV products (including both lytic and latent genes) evade the innate immune response were reviewed.Citation83 Here, we focus on summarizing two common and efficient strategies to circumvent the innate immune response in the NPC-induced modulation of phagocyte function and blockade of antiviral cytokines.
Modulation of phagocyte apoptosis and maturation
Subsequent to the finding that the binding of EBV to the surface of neutrophils induces inflammatory cytokine expression,Citation71 Gosselin J et al. found that EBV penetrates neutrophils and localizes to their nuclei. After infecting neutrophils, EBV launches apoptosis by modulating the Fas/Fas ligand (L) pathway,Citation84 as indicated by a significant increase in both membrane-bound Fas/Fas-L and soluble Fas-L. This study was the first to explain why EBV cannot establish robust infection in neutrophils. EBV also impairs the phagocytic activity of primary monocytes by inhibiting protein kinase C (PKC) activity.Citation85,Citation86 Monocyte apoptosis caused by EBV contact during DC development results in a reduction in mature DCs.Citation87 This reduction may provide EBV with a time window for productive replication by temporarily delaying the onset of immune responses. In addition to the decrease in the number of pDCs during EBV infection, the maturation of pDCs is also compromised, as indicated by reduced secretion of TNF-α, which could partly facilitate pDC development.Citation88 pDCs have a dual role in defending viral infection, by secreting a high level of type I IFNs to inhibit viral replication directly and by initiating and tuning the specific adaptive immunity. EBV infection undermines the ability of pDCs to mature, thereby preventing these cells from mounting antiviral T-cell responses.Citation88
Blockade of antiviral cytokines
The apoptosis of innate effector cells results in a significant reduction in IFN production. In addition, certain EBV proteins and transcripts, such as EBERs and LMP2, can inhibit the type I IFN responses by disrupting IFN-stimulated transcriptionCitation89,Citation90 and by targeting IFN receptors for degradation.Citation91
Inducing the innate immune cells to produce antagonistic factors to block the function of those antiviral cytokines demonstrates another strategy by which EBV eludes the immune responses. For example, in addition to IL-1α and IL-1β, EBV also initiates the production of their natural inhibitor IL-1 receptor antagonist (IL-1Ra).Citation71,Citation91,Citation92 IL-1Ra competitively inhibits the binding of IL-1α and IL-1β to their receptors.Citation93 Moreover, IL-Ra is secreted approximately 3200 and 610 times more than IL-1α and IL-1β, respectively, from EBV-stimulated neutrophils,Citation92 indicating another effective mechanism by which EBV counteracts the host innate immune response.
In addition, EBV prevents the production of prostaglandin E2 (PGE2) by monocytes by inhibiting the expression of inducible cyclooxygenase 2 (COX-2), a critical enzyme in the PGE2 biosynthesis pathway. This inhibition of COX-2 may be a result of EBV interfering with the activation of the NF-κB pathway, which plays an important role in COX-2 induction in monocytes.Citation94 NF-κB is also critical for TNF-α induction, and consequently, EBV suppresses TNF-α secretion from lipopolysaccharide-treated monocytes by 70%–90%.Citation95 Because simple contact between EBV and monocytes upregulates TNF-α,Citation80,Citation96 inhibition of the NF-κB pathway after EBV replication in monocytes may be a mechanism by which the virus shuts down further TNF-α production. Additional evidence of this mechanism may be needed. First, TNF-α suppression by EBV was not observed at a basal expression level, and second, the exact mechanism of this suppression may be largely attributable to monocyte apoptosis upon EBV penetration.
THE INTERPLAY BETWEEN HOST ADAPTIVE IMMUNITY AND EBV
Antibodies detected during EBV infection
EBV-specific antibodies, primarily immunoglobulin G (IgG) and IgA, are detected in the sera of NPC patients. These antibodies recognize various EBV targets, including EBV structural antigens (e.g., viral capsid antigen-proteins VCA-p18 and VCA-p40,Citation97 glycoproteins gp350/220,Citation98 and gp78Citation99), lytic antigens (e.g., Bam HI rightward reading frame 1 (BRLF1),Citation82 Bam HI leftward reading frame 1 (BZLF1),Citation100 and EBV-DNaseCitation101), and latent antigens (e.g., EBNA1 and LMPsCitation102). One recent study that enrolled a relatively larger number of samples studied the humoral immune response to EBV-encoded tumor-associated proteins in NPC patients. The results indicated that there exists a stronger IgG antibody response to EBNA1 than that of LMP1, LMP2, and BARF1. Except for EBNA1, only low IgA titers against LMP1, LMP2, and BARF1 were present.Citation102 The marginal immunogenicity of LMPs and BARF1 to humoral immune responses may be due to their intrinsic properties (for example, rapid and complete secretion of BARF1 leaves little protein within or on the surfaces of cells for detectionCitation103) and to their limited expression on the plasma membrane.
The presence of high titers of antibodies against EBV structural and early lytic antigensCitation97,Citation98,Citation100,Citation101,Citation104 indicates the status of either sporadic reactivation from latency in malignant cells or new infection of naive cells within/surrounding the NPC tumor. However, antibodies may not be able to effectively block EBV infection because EBV has the capacity of spreading through cell–cell contact, which is an efficient mode of infecting the epithelium from reactivating B cells without releasing cell-free virions.Citation105,Citation106
Cellular responses to EBV infection
Cellular immunity is essential for controlling EBV during both primary and persistent phases. The complete view of EBV-specific cellular immunity in NPC patients remains to be elucidated, despite the fact that many novel technical approaches have been introduced to assess CD8+ T and CD4+ T-cell responses to EBV.Citation107,Citation108,Citation109
Circulating EBV-specific cytotoxic lymphocytes (CTLs) can be detected in NPC patients,Citation110,Citation111 and EBV-specific memory CTL responses can be reactivated in vitro after those cells were extracted from blood.Citation112 Nevertheless, the antigen-specific CD8+ CTLs against several consistently expressed viral lytic genes, including BZLF1, BRLF1, BamHI-M leftward frame 1, BamHI-M rightward frame 1, and BamHI-A leftward frame 2, are rarely found in NPC tumor lesions.Citation113,Citation114 In regard to latent antigens, Fogg MH et al. found that CTLs targeting the EBNA1 significantly decrease in EBV-associated NPC patients.Citation115 It is possible that presentation of EBNA1 by major histocompatibility complex (MHC) I molecules is diminished in tumors; however, this interesting finding requires further validation. For the subdominant latent antigens (LMP1, LMP2, and BARF1), CTLs specific to these proteins can be detected in most of NPC patients.Citation111,Citation116,Citation117,Citation118
CD4+ T cells play a pivotal role in supporting the production of high affinity antibodies, maintaining the number and biological function of CTLs, and possessing cytotoxic activities.Citation119 However, the understanding of CD4+ T-cell responses to EBV is less clear due to the small size of the CD4+ compartment because of a lack of detectable CD4+ T-cell expansion during EBV infection.Citation120 Most knowledge concerning CD4+ responses to EBV has been built on observations from either healthy EBV carriers or in vitro experiments. For example, specific CD4+ T-cell clones or T-cell lines against EBV were evaluated by co-culture with autologous B-lymphoblastoid cell lines or DCs infected with recombinant vaccinia virus encoding individual lytic or latent proteins.Citation109 Similar to the CD8+ T-cell response, a hierarchy of immunodominance of EBV antigens has been classified. EBNA1 and EBNA3 are the dominant targets, and LMPs and BARF1 are the subdominant targets.Citation117,Citation121,Citation122 CD4+ T cells specific for EBNA1, LMPs, and BARF1 can be detected in NPC patients, albeit at low levels.Citation111,Citation117
Evasion of adaptive immune responses
Switching off immunodominant viral antigen expression
EBV has developed multiple strategies to evade cellular immune responses during its long-term co-evolution with the host. Like all other herpesviruses, the major strategy EBV uses for establishing and maintaining latency in the face of the cellular immunity, particularly the CD8+ T-cell response, is to switch off the expression of most viral genes, particularly the viral genes with strong immunogenicities or that present a “non-immunogenic” phenotype that makes them invisible to the immune system. For example, several vital latent factors with high immunodominance, such as the EBNA3 family and EBNA2,Citation123 are consistently absent in NPC patients. Nevertheless, when co-cultured in vitro with autologous EBV-transformed lymphoblastoid cell lines, the virus-specific CTLs extracted from NPC patients sufficiently recognize antigens from the EBNA3 family.Citation110
Impairment of the antigen-presenting HLA I or HLA II pathway
NPC cells are positive for both HLA class I and II molecules; thus, these cells may present viral peptides to be recognized by both CD8+ and CD4+ T cells. However, EBV impairs both HLA I and HLA II antigen presentation pathways to circumvent T-cell surveillance. Notably, NPC cells retain their antigen presentation capacity when they are cultured in vitro.Citation110,Citation124
EBNA1 is the primary target for the CD4+-, but not the CD8+-, T-cell response because ENBA1 is highly resistant to proteasomal digestion and thus is protected from being presented by MHC I molecules endogenously.Citation125,Citation126 This strategy is also utilized by latency-associated nuclear antigen 1, a homolog of EBNA1 in Kaposi sarcoma-associated herpes virus, to avoid being presented through the MHC class I pathway.Citation127 Exogenously supplied EBNA1 can be presented by MHC class I molecules through a transporter associated with Ag processing (TAP)-independent pathway, whereas endogenously expressed EBNA1 can only be presented when the glycine-alanine repeat (GAr) domain of EBNA1 is deleted.Citation128,Citation129 Therefore, the GAr domain of EBNA1 is thought to control the presentation of endogenous EBNA1. However, further results have indicated that the GAr domain itself does not completely protect EBNA1 from presentation to CD8+ T cells.Citation130,Citation131,Citation132
The expression of LMP1 in human cells dramatically enhances HLA I processing;Citation133,Citation134 however, LMP1 is a poor CD8+ T-cell target in vivo. Additionally, overall downregulation of HLA class I antigen presentation machinery (APM) was observed in NPC biopsies.Citation135 This discrepancy may be explained by the finding that LMP1 induces c-myelocytomatosis (c-Myc), which has been shown to downregulate HLA class I APM, subsequently counteracting the stimulatory effect of LMP1.Citation135 In addition, the first transmembrane domain of LMP1 is able to mediate self-aggregation to severely impair the cis-presentation of an LMP1-derived epitope,Citation136 demonstrating another novel mechanism of immune evasion.
Among the detectable EBV lytic antigens in NPC patients, BZLF1, BamHI-G leftward frame 5 (BGLF5), and BamHI-N leftward frame 2a (BNLF2a) are able to dysregulate the cellular immune response via various mechanisms. BGLF5, a DNase/alkaline exonuclease (AE) gene, exerts a host shutoff function to block the synthesis of host HLA I, thereby limiting CD8+ T-cell recognition.Citation137 In addition, this shutoff function of BGLF5 is also involved in repressing DNA repair, inducing genomic instability in human epithelial cells.Citation138 BZLF1 inhibits MHC class II expression by suppressing the transcription of the transactivator class II, MHC, transactivator (CIITA),Citation139 a critical transcriptional coactivator of MHC class II expression. BNLF2a specifically affects the presentation of immediate early and early proteins to HLA I molecules by inhibiting TAP and surface HLA I expression.Citation140,Citation141
Regulation of immuno-inhibitory biomolecules
IL-10 is a well-known cytokine with immune-suppressive function. An association between increased IL-10 secretion and a significantly decreased number of cytotoxic T cells was observed in EBV-positive NPCs.Citation142 Both EBV structural proteins and EBV-encoded miRNAs are involved in IL-10 induction. LMP1 was the first identified viral protein responsible for IL-10 induction via the activation of p38/stress-activated protein kinase 2 (SAPK2).Citation143 In addition, EBER1 and EBER2 were shown to be associated with enhanced IL-10 expression at the transcription level through a novel signaling pathway independent of an IFN-inducible protein kinase R (PKR).Citation144
Decoy receptor 3 (DcR3), a recently identified molecule with immune inhibitory function, has the capacity to induce DC apoptosis via the formation of the death domain-containing receptor/death-inducing signaling complex.Citation145 DcR3 also reduces MHC class II expression in tumor-associated macrophages.Citation146 LMP1 was found to upregulate DcR3 expression via the NF-κB and phosphatidyl inositol 3-kinase (PI3K) signaling pathways.Citation147 Because NPC-associated macrophages are positive for EBV,Citation148 DcR3 may also be involved in immune evasion by EBV.
In addition, B7 homolog 1 (B7-H1), a T-cell inhibitory molecule, was upregulated during EBV infection of pDCsCitation88 and NPC cell lines,Citation149 and further studies are required to explore the role of B7-H1 in EBV immune evasion in NPCs.
Induction of T regulatory cell activation and T-cell anergy
T regulatory cells (Tregs), a subset of T cells with immune inhibitory functions, work in a cell-to-cell contact manner and secrete granzyme or cytokines such as IL-10 and transforming growth factor β (TGF-β).Citation150,Citation151,Citation152 Tregs are consistently detected in the circulation and tumor microenvironment in EBV-positive NPC, where approximately 12% of tumor-infiltrating leucocytes (TILs) in NPC harbor a Treg phenotype (CD4+ CD25high forkhead box P3+).Citation153 LMP1 dominantly induces Tregs to secrete IL-10, which suppresses the proliferation of mitogen or the withdrawal of Ag-stimulated T-effector cells and their release of IFN-γ.Citation154 LALLFWL peptides of LMP1 show strong and direct inhibition of T-cell proliferation and NK cytotoxicity. This T-cell anergy is most likely attributable to the enhanced expression of IL-10 and TGF-β, resembling Treg responses.Citation155 Tregs are also involved in the immune evasion of EBNA1 and LMP2 because Treg depletion restores EBNA1- and LMP2-specific CD8+ T-cell responses, as well as the immune control of EBV-infected cells in vitroCitation156 ().
Table 2 Strategies of cellular response evasion exploited by individual EBV antigens detected in NPC
CONCLUDING REMARKS
NPC patients maintain efficient immune functions, including innate and adaptive immunities, to address EBV infection. However, this ancient virus has evolved multiple elaborate strategies to counteract and evade the host immunity, leading to its high prevalence among the human population. Seemingly, symbiosis is established between EBV and NPC that EBV facilitates NPC development by promoting the growth of EBV-infected cells and by preventing apoptosis.Citation157,Citation158 EBV also counteracts the host immunity by modulating numerous cellular signaling pathways,Citation159 and an increased number of cancer cells provides more potential neo-hosts for EBV ().
The limited knowledge regarding the virus–host interaction in the NPC environment and in systemic immune responses contributes to the failure or low efficacy of most EBV-targeted immunotherapies. More importantly, selection pressure-driven evolution constantly stimulates the emergence of EBV variants,Citation160,Citation161 which may be more oncogenic and less immunogenic than the parental strain. For instance, a recent study identified an EBV variant from NPC with unusually high tropism for epithelial cells but low tropism for B cells,Citation162 suggesting the existence of EBV variants with increased NPC risk.
To date, the induction of an EBV antigen-specific T-cell response (primarily CD8+ T cells) in patients with vaccines and adoptive T-cell therapy are the two most common strategies for the immunological treatment of EBV-associated cancers. Because targeting only one specific antigen led to limited tumor regression in NPC patients,Citation163,Citation164,Citation165,Citation166 vaccines composed of multiple EBV antigens to activate T-cell responses that are more potent has emerged as a novel strategy. In this respect, two different teams constructed two recombinant viruses. The recombinant virus called Ad-SAVINE incorporates peptide sets from EBNA1, LMP1, and LMP2,Citation167 and the other recombinant virus, called MVA-EL, contains an EBNA1/LMP2 fusion protein.Citation168 Phase I trials in NPC patients showed that both of these vaccinia viruses activate CD4+ and CD8+ T-cell responses; encouraging clinical progress with full tolerance has been made.Citation168,Citation169,Citation170
However, many questions regarding host immunity and EBV remain to be addressed for the development of EBV-targeted therapy. For instance, the immunodominance hierarchy of individual viral antigens (particularly for EBV-encoding RNAs) and the crosstalk among multiple signaling pathways activated by EBV should be addressed. New technologies (for example, a molecular-based tag linkage method our lab developed that enables haplotype phasing greater accuracy and sensitivity for viral quasispecies determinationCitation171) with higher sensitivity and precision to examine viral quasispecies in the NPC environment are required to monitor viral evolution. Exploring novel cellular factors or chemical substances that can reactivate EBV from latency will provide a promising strategy for treating EBV-related tumors by inducing cell lysis through viral reactivation. Greater attention should be given to the local suppression of EBV-specific immunity because immunosuppression contributes greatly to NPC development.
This work was supported by grants from National Key Basic Research Program of China (2011CB504803 to Ren Sun, 2011CB504305 to Tingting Wu), the National Natural Science Foundation of China (81270063 to Jing Qian), the Natural Science Foundation of Zhejiang Province (LY12H19008 to Jing Qian), the Qianjiang Talent Project of Zhejiang Province (2013R10034 to Jing Qian).
- Young LS, Dawson CW, Clark D et al.Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol1988;69 (Pt 5): 1051–1065.
- Brooks L, Yao QY, Rickinson AB et al.Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol1992;66: 2689–2697.
- Sam CK, Brooks LA, Niedobitek G et al.Analysis of Epstein-Barr virus infection in nasopharyngeal biopsies from a group at high risk of nasopharyngeal carcinoma. Int J Cancer1993;53: 957–962.
- Gilligan KJ, Rajadurai P, Lin JC et al.Expression of the Epstein-Barr virus BamHI A fragment in nasopharyngeal carcinoma: evidence for a viral protein expressed in vivo. J Virol1991;65: 6252–6259.
- Decaussin G, Sbih-Lammali F, de Turenne-Tessier M et al.Expression of BARF1 gene encoded by Epstein-Barr virus in nasopharyngeal carcinoma biopsies. Cancer Res2000;60: 5584–5588.
- Seto E, Yang L, Middeldorp J et al.Epstein-Barr virus (EBV)-encoded BARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J Med Virol2005;76: 82–88.
- Old LJ, Boyse EA, Oettgen HF et al.Precipitating antibody in human serum to an antigen present in cultured burkitt's lymphoma cells. Proc Natl Acad Sci USA1966;56: 1699–1704.
- Henle W, Henle G, Ho HC et al.Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck neoplasms, and control groups. J Natl Cancer Inst1970;44: 225–231.
- Henle G, Henle W.Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer1976;17: 1–7.
- Raab-Traub N, Flynn K.The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell1986;47: 883–889.
- Lin JC, Wang WY, Chen KY et al.Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med2004;350: 2461–2470.
- Lo YM, Chan LY, Chan AT et al.Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res1999;59: 5452–5455.
- Chan AT, Lo YM, Zee B et al.Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst2002;94: 1614–1619.
- Leung SF, Chan AT, Zee B et al.Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer2003;98: 288–291.
- Wang WY, Twu CW, Chen HH et al.Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res2010;16: 1016–1024.
- An X, Wang FH, Ding PR et al.Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer2011;117: 3750–3757.
- Wang WY, Twu CW, Lin WY et al.Plasma Epstein-Barr virus DNA screening followed by (1)(8)F-fluoro-2-deoxy-D-glucose positron emission tomography in detecting posttreatment failures of nasopharyngeal carcinoma. Cancer2011;117: 4452–4459.
- Desgranges C, de-The G, Wolf H et al.Further studies on the detection of the Epstein-Barr virus DNA in nasopharyngeal carcinoma biopsies from different parts of the world. IARC Sci Publ1975;11 (Pt 2): 191–193.
- Cosmopoulos K, Pegtel M, Hawkins J et al.Comprehensive profiling of Epstein-Barr virus microRNAs in nasopharyngeal carcinoma. J Virol2009;83: 2357–2367.
- Wong AM, Kong KL, Tsang JW et al.Profiling of Epstein-Barr virus-encoded microRNAs in nasopharyngeal carcinoma reveals potential biomarkers and oncomirs. Cancer2012;118: 698–710.
- Zhang G, Zong J, Lin S et al.Circulating Epstein-Barr virus microRNAs miR-BART7 and miR-BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int J Cancer2015;136: E301–E312.
- Sivachandran N, Thawe NN, Frappier L.Epstein-Barr virus nuclear antigen 1 replication and segregation functions in nasopharyngeal carcinoma cell lines. J Virol2011;85: 10425–10430.
- Mansouri S, Pan Q, Blencowe BJ et al.Epstein-Barr virus EBNA1 protein regulates viral latency through effects on let-7 microRNA and dicer. J Virol2014;88: 11166–11177.
- Sheu LF, Chen A, Meng CL et al.Enhanced malignant progression of nasopharyngeal carcinoma cells mediated by the expression of Epstein-Barr nuclear antigen 1 in vivo. J Pathol1996;180: 243–248.
- O'Neil JD, Owen TJ, Wood VH et al.Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription factor pathway in nasopharyngeal carcinoma cells and enhances angiogenesis in vitro. J Gen Virol2008;89: 2833–2842.
- Sivachandran N, Sarkari F, Frappier L.Epstein-Barr nuclear antigen 1 contributes to nasopharyngeal carcinoma through disruption of PML nuclear bodies. PLoS Pathog2008;4: e1000170.
- Valentine R, Dawson CW, Hu C et al.Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappaB pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol Cancer2010;9: 1.
- Cao JY, Mansouri S, Frappier L.Changes in the nasopharyngeal carcinoma nuclear proteome induced by the EBNA1 protein of Epstein-Barr virus reveal potential roles for EBNA1 in metastasis and oxidative stress responses. J Virol2012;86: 382–394.
- Wang L, Tian WD, Xu X et al.Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer2014;120: 363–372.
- Li B, Huang G, Zhang X et al.Increased phosphorylation of histone H3 at serine 10 is involved in Epstein-Barr virus latent membrane protein-1-induced carcinogenesis of nasopharyngeal carcinoma. BMC Cancer2013;13: 124.
- Lo AK, Lo KW, Ko CW et al.Inhibition of the LKB1-AMPK pathway by the Epstein-Barr virus-encoded LMP1 promotes proliferation and transformation of human nasopharyngeal epithelial cells. J Pathol2013;230: 336–346.
- Yu PH, Chou SF, Chen CL et al.Upregulation of endocan by Epstein-Barr virus latent membrane protein 1 and its clinical significance in nasopharyngeal carcinoma. PLoS One2013;8: e82254.
- Kondo S, Seo SY, Yoshizaki T et al.EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res2006;66: 9870–9877.
- Aga M, Bentz GL, Raffa S et al.Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene2014;33: 4613–4622.
- Pathmanathan R, Prasad U, Sadler R et al.Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med1995;333: 693–698.
- Dawson CW, Tramountanis G, Eliopoulos AG et al.Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem2003;278: 3694–3704.
- Liu HP, Chen CC, Wu CC et al.Epstein-Barr virus-encoded LMP1 interacts with FGD4 to activate Cdc42 and thereby promote migration of nasopharyngeal carcinoma cells. PLoS Pathog2012;8: e1002690.
- Chen CC, Liu HP, Chao M et al.NF-kappaB-mediated transcriptional upregulation of TNFAIP2 by the Epstein-Barr virus oncoprotein, LMP1, promotes cell motility in nasopharyngeal carcinoma. Oncogene2014;33: 3648–3659.
- Tsai CN, Tsai CL, Tse KP et al.The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the downregulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc Natl Acad Sci USA2002;99: 10084–10089.
- Yoshizaki T, Sato H, Furukawa M et al.The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci USA1998;95: 3621–3626.
- Horikawa T, Yoshizaki T, Sheen TS et al.Association of latent membrane protein 1 and matrix metalloproteinase 9 with metastasis in nasopharyngeal carcinoma. Cancer2000;89: 715–723.
- Lee DC, Chua DT, Wei WI et al.Induction of matrix metalloproteinases by Epstein-Barr virus latent membrane protein 1 isolated from nasopharyngeal carcinoma. Biomed Pharmacother2007;61: 520–526.
- Deng W, Pang PS, Tsang CM et al.Epstein-Barr virus-encoded latent membrane protein 1 impairs G2 checkpoint in human nasopharyngeal epithelial cells through defective Chk1 activation. PLoS One2012;7: e39095.
- Guo L, Tang M, Yang L et al.Epstein-Barr virus oncoprotein LMP1 mediates survivin upregulation by p53 contributing to G1/S cell cycle progression in nasopharyngeal carcinoma. Int J Mol Med2012;29: 574–580.
- Xu Y, Shi Y, Yuan Q et al.Epstein-Barr Virus encoded LMP1 regulates cyclin D1 promoter activity by nuclear EGFR and STAT3 in CNE1 cells. J Exp Clin Cancer Res2013;32: 90.
- Kondo S, Wakisaka N, Muramatsu M et al.Epstein-Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines. J Virol2011;85: 11255–11264.
- Port RJ, Pinheiro-Maia S, Hu C et al.Epstein-Barr virus induction of the Hedgehog signalling pathway imposes a stem cell phenotype on human epithelial cells. J Pathol2013;231: 367–377.
- Busson P, McCoy R, Sadler R et al.Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol1992;66: 3257–3262.
- Heussinger N, Buttner M, Ott G et al.Expression of the Epstein-Barr virus (EBV)-encoded latent membrane protein 2A (LMP2A) in EBV-associated nasopharyngeal carcinoma. J Pathol2004;203: 696–699.
- Rovedo M, Longnecker R.Epstein-barr virus latent membrane protein 2B (LMP2B) modulates LMP2A activity. J Virol2007;81: 84–94.
- Kong QL, Hu LJ, Cao JY et al.Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog2010;6: e1000940.
- Lan YY, Hsiao JR, Chang KC et al.Epstein-Barr virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9. J Virol2012;86: 6656–6667.
- Allen MD, Young LS, Dawson CW.The Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility. J Virol2005;79: 1789–1802.
- Iwakiri D, Sheen TS, Chen JY et al.Epstein-Barr virus-encoded small RNA induces insulin-like growth factor 1 and supports growth of nasopharyngeal carcinoma-derived cell lines. Oncogene2005;24: 1767–1773.
- Wong HL, Wang X, Chang RC et al.Stable expression of EBERs in immortalized nasopharyngeal epithelial cells confers resistance to apoptotic stress. Mol Carcinog2005;44: 92–101.
- Seto E, Ooka T, Middeldorp J et al.Reconstitution of nasopharyngeal carcinoma-type EBV infection induces tumorigenicity. Cancer Res2008;68: 1030–1036.
- Ye Y, Zhou Y, Zhang L et al.EBV-miR-BART1 is involved in regulating metabolism-associated genes in nasopharyngeal carcinoma. Biochem Biophys Res Commun2013;436: 19–24.
- Lei T, Yuen KS, Xu R et al.Targeting of DICE1 tumor suppressor by Epstein-Barr virus-encoded miR-BART3* microRNA in nasopharyngeal carcinoma. Int J Cancer2013;133: 79–87.
- Lo AK, To KF, Lo KW et al.Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc Natl Acad Sci USA2007;104: 16164–16169.
- Choy EY, Siu KL, Kok KH et al.An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med2008;205: 2551–2560.
- Chan JY, Gao W, Ho WK et al.Overexpression of Epstein-Barr virus-encoded microRNA-BART7 in undifferentiated nasopharyngeal carcinoma. Anticancer Res2012;32: 3201–3210.
- Cai LM, Lyu XM, Luo WR et al.EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene 27 October 2014.doi 10.1038/onc.2014.341.
- Hsu CY, Yi YH, Chang KP et al.The Epstein-Barr virus-encoded microRNA MiR-BART9 promotes tumor metastasis by targeting E-cadherin in nasopharyngeal carcinoma. PLoS Pathog2014;10: e1003974.
- Xie YJ, Long ZF, He XS.Involvement of EBV-encoded BART-miRNAs and dysregulated cellular miRNAs in nasopharyngeal carcinoma genesis. Asian Pac J Cancer Prev2013;14: 5637–5644.
- Chen LC, Wang LJ, Tsang NM et al.Tumour inflammasome-derived IL-1beta recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med2012;4: 1276–1293.
- Ferradini L, Miescher S, Stoeck M et al.Cytotoxic potential despite impaired activation pathways in T lymphocytes infiltrating nasopharyngeal carcinoma. Int J Cancer1991;47: 362–370.
- Hu H, Tang KF, Chua YN et al.Expression of interleukin-18 by nasopharyngeal carcinoma cells: a factor that possibly initiates the massive leukocyte infiltration. Hum Pathol2004;35: 722–728.
- Giannini A, Bianchi S, Messerini L et al.Prognostic significance of accessory cells and lymphocytes in nasopharyngeal carcinoma. Pathol Res Pract1991;187: 496–502.
- Zong YS, Zhang CQ, Zhang F et al.Infiltrating lymphocytes and accessory cells in nasopharyngeal carcinoma. Jpn J Cancer Res1993;84: 900–905.
- Tang KF, Tan SY, Chan SH et al.A distinct expression of CC chemokines by macrophages in nasopharyngeal carcinoma: implication for the intense tumor infiltration by T lymphocytes and macrophages. Hum Pathol2001;32: 42–49.
- Beaulieu AD, Paquin R, Gosselin J.Epstein-Barr virus modulates de novo protein synthesis in human neutrophils. Blood1995;86: 2789–2798.
- McColl SR, Roberge CJ, Larochelle B et al.EBV induces the production and release of IL-8 and macrophage inflammatory protein-1 alpha in human neutrophils. J Immunol1997;159: 6164–6168.
- Fiola S, Gosselin D, Takada K et al.TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J Immunol2010;185: 3620–3631.
- Iwakiri D, Zhou L, Samanta M et al.Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J Exp Med2009;206: 2091–2099.
- Granucci F, Zanoni I, Pavelka N et al.A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med2004;200: 287–295.
- Isobe Y, Sugimoto K, Yang L et al.Epstein-Barr virus infection of human natural killer cell lines and peripheral blood natural killer cells. Cancer Res2004;64: 2167–2174.
- Gaudreault E, Fiola S, Olivier M et al.Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J Virol2007;81: 8016–8024.
- Ariza ME, Glaser R, Kaumaya PT et al.The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J Immunol2009;182: 851–859.
- Glaser R, Litsky ML, Padgett DA et al.EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology2006;346: 205–218.
- Gosselin J, Flamand L, D′Addario M et al.Infection of peripheral blood mononuclear cells by herpes simplex and Epstein-Barr viruses. Differential induction of interleukin 6 and tumor necrosis factor-alpha. J Clin Invest1992;89: 1849–1856.
- Martel-Renoir D, Grunewald V, Touitou R et al.Qualitative analysis of the expression of Epstein-Barr virus lytic genes in nasopharyngeal carcinoma biopsies. J Gen Virol1995;76 (Pt 6): 1401–1408.
- Feng P, Ren EC, Liu D et al.Expression of Epstein-Barr virus lytic gene BRLF1 in nasopharyngeal carcinoma: potential use in diagnosis. J Gen Virol2000;81: 2417–2423.
- Ning S.Innate immune modulation in EBV infection. Herpesviridae2011;2: 1.
- Larochelle B, Flamand L, Gourde P et al.Epstein-Barr virus infects and induces apoptosis in human neutrophils. Blood1998;92: 291–299.
- Savard M, Belanger C, Tardif M et al.Infection of primary human monocytes by Epstein-Barr virus. J Virol2000;74: 2612–2619.
- Tardif M, Savard M, Flamand L et al.Impaired protein kinase C activation/translocation in Epstein-Barr virus-infected monocytes. J Biol Chem2002;277: 24148–24154.
- Li L, Liu D, Hutt-Fletcher L et al.Epstein-Barr virus inhibits the development of dendritic cells by promoting apoptosis of their monocyte precursors in the presence of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood2002;99: 3725–3734.
- Severa M, Giacomini E, Gafa V et al.EBV stimulates TLR- and autophagy-dependent pathways and impairs maturation in plasmacytoid dendritic cells: implications for viral immune escape. Eur J Immunol2013;43: 147–158.
- Alcami A, Koszinowski UH.Viral mechanisms of immune evasion. Trends Microbiol2000;8: 410–418.
- Randall RE, Goodbourn S.Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol2008;89: 1–47.
- Roberge CJ, Larochelle B, Rola-Pleszczynski M et al.Epstein-Barr virus induces GM-CSF synthesis by monocytes: effect on EBV-induced IL-1 and IL-1 receptor antagonist production in neutrophils. Virology1997;238: 344–352.
- Roberge CJ, Poubelle PE, Beaulieu AD et al.The IL-1 and IL-1 receptor antagonist (IL-1Ra) response of human neutrophils to EBV stimulation. Preponderance of IL-Ra detection. J Immunol1996;156: 4884–4891.
- Hannum CH, Wilcox CJ, Arend WP et al.Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature1990;343: 336–340.
- Savard M, Belanger C, Tremblay MJ et al.EBV suppresses prostaglandin E2 biosynthesis in human monocytes. J Immunol2000;164: 6467–6473.
- Gosselin J, Menezes J, D’Addario M et al.Inhibition of tumor necrosis factor-alpha transcription by Epstein-Barr virus. Eur J Immunol1991;21: 203–208.
- D’Addario M, Ahmad A, Morgan A et al.Binding of the Epstein-Barr virus major envelope glycoprotein gp350 results in the upregulation of the TNF-alpha gene expression in monocytic cells via NF-kappaB involving PKC, PI3-K and tyrosine kinases. J Mol Biol2000;298: 765–778.
- Fachiroh J, Stevens SJ, Haryana SM et al.Combination of Epstein-Barr virus scaffold (BdRF1/VCA-p40) and small capsid protein (BFRF3/VCA-p18) into a single molecule for improved serodiagnosis of acute and malignant EBV-driven disease. J Virol Methods2010;169: 79–86.
- Thorley-Lawson DA, Poodry CA.Identification and isolation of the main component (gp350-gp220) of Epstein-Barr virus responsible for generating neutralizing antibodies in vivo. J Virol1982;43: 730–736.
- Gu AD, Xie YB, Mo HY et al.Antibodies against Epstein-Barr virus gp78 antigen: a novel marker for serological diagnosis of nasopharyngeal carcinoma detected by xMAP technology. J Gen Virol2008;89: 1152–1158.
- Joab I, Nicolas JC, Schwaab G et al.Detection of anti-Epstein-Barr-virus transactivator (ZEBRA) antibodies in sera from patients with nasopharyngeal carcinoma. Int J Cancer1991;48: 647–649.
- Chien YC, Chen JY, Liu MY et al.Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med2001;345: 1877–1882.
- Paramita DK, Fatmawati C, Juwana H et al.Humoral immune responses to Epstein-Barr virus encoded tumor associated proteins and their putative extracellular domains in nasopharyngeal carcinoma patients and regional controls. J Med Virol2011;83: 665–678.
- de Turenne-Tessier M, Jolicoeur P, Middeldorp JM et al.Expression and analysis of the Epstein-Barr virus BARF1-encoded protein from a tetracycline-regulatable adenovirus system. Virus Res2005;109: 9–18.
- Paramita DK, Fachiroh J, Artama WT et al.Native early antigen of Epstein-Barr virus, a promising antigen for diagnosis of nasopharyngeal carcinoma. J Med Virol2007;79: 1710–1721.
- Imai S, Nishikawa J, Takada K.Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J Virol1998;72: 4371–4378.
- Shannon-Lowe CD, Neuhierl B, Baldwin G et al.Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc Natl Acad Sci USA2006;103: 7065–7070.
- Altman JD, Moss PA, Goulder PJ et al.Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996. 274: 94-96. J Immunol2011;187: 7–9.
- Scotet E, Peyrat MA, Saulquin X et al.Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur J Immunol1999;29: 973–985.
- Landais E, Saulquin X, Houssaint E.The human T cell immune response to Epstein-Barr virus. Int J Dev Biol2005;49: 285–292.
- Lee SP, Chan AT, Cheung ST et al.CTL control of EBV in nasopharyngeal carcinoma (NPC): EBV-specific CTL responses in the blood and tumors of NPC patients and the antigen-processing function of the tumor cells. J Immunol2000;165: 573–582.
- Lin X, Gudgeon NH, Hui EP et al.CD4 and CD8 T cell responses to tumour-associated Epstein-Barr virus antigens in nasopharyngeal carcinoma patients. Cancer Immunol Immunother2008;57: 963–975.
- Moss DJ, Chan SH, Burrows SR et al.Epstein-Barr virus specific T-cell response in nasopharyngeal carcinoma patients. Int J Cancer1983;32: 301–305.
- Steven NM, Annels NE, Kumar A et al.Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med1997;185: 1605–1617.
- Angelini DF, Serafini B, Piras E et al.Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog2013;9: e1003220.
- Fogg MH, Wirth LJ, Posner M et al.Decreased EBNA-1-specific CD8+ T cells in patients with Epstein-Barr virus-associated nasopharyngeal carcinoma. Proc Natl Acad Sci USA2009;106: 3318–3323.
- Li J, Zeng XH, Mo HY et al.Functional inactivation of EBV-specific T-lymphocytes in nasopharyngeal carcinoma: implications for tumor immunotherapy. PLoS One2007;2: e1122.
- Martorelli D, Houali K, Caggiari L et al.Spontaneous T cell responses to Epstein-Barr virus-encoded BARF1 protein and derived peptides in patients with nasopharyngeal carcinoma: bases for improved immunotherapy. Int J Cancer2008;123: 1100–1107.
- Li J, Chen QY, Mo H et al.Immunophenotyping at the time of diagnosis distinguishes two groups of nasopharyngeal carcinoma patients: implications for adoptive immunotherapy. Int J Biol Sci2011;7: 607–617.
- Brown DM.Cytolytic CD4 cells: Direct mediators in infectious disease and malignancy. Cell Immunol2010;262: 89–95.
- Maini MK, Gudgeon N, Wedderburn LR et al.Clonal expansions in acute EBV infection are detectable in the CD8 and not the CD4 subset and persist with a variable CD45 phenotype. J Immunol2000;165: 5729–5737.
- Munz C, Bickham KL, Subklewe M et al.Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med2000;191: 1649–1660.
- Leen A, Meij P, Redchenko I et al.Differential immunogenicity of Epstein-Barr virus latent-cycle proteins for human CD4(+) T-helper 1 responses. J Virol2001;75: 8649–59.
- Minarovits J.Epigenotypes of latent herpesvirus genomes. Curr Top Microbiol Immunol2006;310: 61–80.
- Khanna R, Busson P, Burrows SR et al.Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res1998;58: 310–314.
- Levitskaya J, Coram M, Levitsky V et al.Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature1995;375: 685–688.
- Levitskaya J, Sharipo A, Leonchiks A et al.Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA1997;94: 12616–12621.
- Zaldumbide A, Ossevoort M, Wiertz EJ et al.In cis inhibition of antigen processing by the latency-associated nuclear antigen I of Kaposi sarcoma herpes virus. Mol Immunol2007;44: 1352–13560.
- Blake N, Lee S, Redchenko I et al.Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity1997;7: 791–802.
- Long HM, Taylor GS, Rickinson AB.Immune defence against EBV and EBV-associated disease. Curr Opin Immunol2011;23: 258–264.
- Blake N, Haigh T, Shaka’a G et al.The importance of exogenous antigen in priming the human CD8+ T cell response: lessons from the EBV nuclear antigen EBNA1. J Immunol2000;165: 7078–7087.
- Voo KS, Fu T, Wang HY et al.Evidence for the presentation of major histocompatibility complex class I-restricted Epstein-Barr virus nuclear antigen 1 peptides to CD8+ T lymphocytes. J Exp Med2004;199: 459–470.
- Tellam J, Connolly G, Green KJ et al.Endogenous presentation of CD8+ T cell epitopes from Epstein-Barr virus-encoded nuclear antigen 1. J Exp Med2004;199: 1421–1431.
- Rowe M, Khanna R, Jacob CA et al.Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein-Barr virus latent membrane protein-1: coordinate up-regulation of peptide transporters and HLA-class I antigen expression. Eur J Immunol1995;25: 1374–1384.
- Murray PG, Constandinou CM, Crocker J et al.Analysis of major histocompatibility complex class I, TAP expression, and LMP2 epitope sequence in Epstein-Barr virus-positive Hodgkin's disease. Blood1998;92: 2477–2483.
- Tudor CS, Dawson CW, Eckhardt J et al.c-Myc and EBV-LMP1: two opposing regulators of the HLA class I antigen presentation machinery in epithelial cells. Br J Cancer2012;106: 1980–1988.
- Smith C, Wakisaka N, Crough T et al.Discerning regulation of cis- and trans-presentation of CD8+ T-cell epitopes by EBV-encoded oncogene LMP-1 through self-aggregation. Blood2009;113: 6148–6152.
- Zuo J, Thomas W, van Leeuwen D et al.The DNase of gammaherpesviruses impairs recognition by virus-specific CD8+ T cells through an additional host shutoff function. J Virol2008;82: 2385–2393.
- Wu CC, Liu MT, Chang YT et al.Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res2010;38: 1932–1949.
- Li D, Qian L, Chen C et al.Down-regulation of MHC class II expression through inhibition of CIITA transcription by lytic transactivator Zta during Epstein-Barr virus reactivation. J Immunol2009;182: 1799–1809.
- Horst D, van Leeuwen D, Croft NP et al.Specific targeting of the EBV lytic phase protein BNLF2a to the transporter associated with antigen processing results in impairment of HLA class I-restricted antigen presentation. J Immunol2009;182: 2313–2324.
- Croft NP, Shannon-Lowe C, Bell AI et al.Stage-specific inhibition of MHC class I presentation by the Epstein-Barr virus BNLF2a protein during virus lytic cycle. PLoS Pathog2009;5: e1000490.
- Yao M, Ohshima K, Suzumiya J et al.Interleukin-10 expression and cytotoxic-T-cell response in Epstein-Barr-virus-associated nasopharyngeal carcinoma. Int J Cancer1997;72: 398–402.
- Vockerodt M, Haier B, Buttgereit P et al.The Epstein-Barr virus latent membrane protein 1 induces interleukin-10 in Burkitt's lymphoma cells but not in Hodgkin's cells involving the p38/SAPK2 pathway. Virology2001;280: 183–198.
- Kitagawa N, Goto M, Kurozumi K et al.Epstein-Barr virus-encoded poly(A)(-) RNA supports Burkitt's lymphoma growth through interleukin-10 induction. EMBO J2000;19: 6742–50.
- You RI, Chang YC, Chen PM et al.Apoptosis of dendritic cells induced by decoy receptor 3 (DcR3). Blood2008;111: 1480–1488.
- Chang YC, Chen TC, Lee CT et al.Epigenetic control of MHC class II expression in tumor-associated macrophages by decoy receptor 3. Blood2008;111: 5054–5063.
- Ho CH, Chen CL, Li WY et al.Decoy receptor 3, upregulated by Epstein-Barr virus latent membrane protein 1, enhances nasopharyngeal carcinoma cell migration and invasion. Carcinogenesis2009;30: 1443–1451.
- Shimakage M, Sakamoto H.Macrophage involvement in Epstein-Barr virus-related tumors. Exp Ther Med2010;1: 285–291.
- Fang W, Zhang J, Hong S et al.EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget2014;5: 12189–12202.
- Gondek DC, Lu LF, Quezada SA et al.Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol2005;174: 1783–1786.
- Chen ML, Pittet MJ, Gorelik L et al.Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA2005;102: 419–424.
- Cao X, Cai SF, Fehniger TA et al.Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity2007;27: 635–646.
- Lau KM, Cheng SH, Lo KW et al.Increase in circulating Foxp3+CD4+CD25(high) regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer2007;96: 617–622.
- Marshall NA, Vickers MA, Barker RN.Regulatory T cells secreting IL-10 dominate the immune response to EBV latent membrane protein 1. J Immunol2003;170: 6183–6189.
- Dukers DF, Meij P, Vervoort MB et al.Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol2000;165: 663–670.
- Fogg M, Murphy JR, Lorch J et al.Therapeutic targeting of regulatory T cells enhances tumor-specific CD8+ T cell responses in Epstein-Barr virus associated nasopharyngeal carcinoma. Virology2013;441: 107–113.
- Fries KL, Miller WE, Raab-Traub N.Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol1996;70: 8653–8659.
- Liu MY, Shih YY, Li LY et al.Expression of the Epstein-Barr virus BHRF1 gene, a homologue of Bcl-2, in nasopharyngeal carcinoma tissue. J Med Virol2000;61: 241–250.
- Lo AK, Lo KW, Tsao SW et al.Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia2006;8: 173–180.
- Tang YL, Lu JH, Cao L et al.Genetic variations of EBV-LMP1 from nasopharyngeal carcinoma biopsies: potential loss of T cell epitopes. Braz J Med Biol Res2008;41: 110–116.
- Horst D, Burrows SR, Gatherer D et al.Epstein-Barr virus isolates retain their capacity to evade T cell immunity through BNLF2a despite extensive sequence variation. J Virol2012;86: 572–577.
- Tsai MH, Raykova A, Klinke O et al.Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep2013;5: 458–470.
- Lin CL, Lo WF, Lee TH et al.Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res2002;62: 6952–6958.
- Comoli P, Pedrazzoli P, Maccario R et al.Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol2005;23: 8942–8949.
- Straathof KC, Bollard CM, Popat U et al.Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood2005;105: 1898–1904.
- Louis CU, Straathof K, Bollard CM et al.Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother2010;33: 983–990.
- Lutzky VP, Corban M, Heslop L et al.Novel approach to the formulation of an Epstein-Barr virus antigen-based nasopharyngeal carcinoma vaccine. J Virol2010;84: 407–417.
- Taylor GS, Jia H, Harrington K et al.A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res2014;20: 5009–5022.
- Hui EP, Taylor GS, Jia H et al.Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res2013;73: 1676–1688.
- Smith C, Tsang J, Beagley L et al.Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res2012;72: 1116–1125.
- Wu NC, De La Cruz J, Al-Mawsawi LQ et al.HIV-1 quasispecies delineation by tag linkage deep sequencing. PLoS One2014;9: e97505.