Dear Editor,
Listeria monocytogenes is an important food-borne pathogen causing severe invasive/non-invasive illness such as septicemia and meningitis in immunocompromised individuals, and self-limiting gastroenteritis and fever in immunocompetent adults.Citation1 In pregnant women, serious maternal–fetal infections can occur, resulting in preterm delivery, miscarriage, or stillbirth.Citation2 In some developed countries such as France, Italy, and the USA, listeriosis occurs at two to ten cases per million people per year.Citation3 In China, except for a few sporadic case reports,Citation4 no epidemiological information about clinical L. monocytogenes isolates has been reported due to a lack of surveillance on listeriosis.Citation5
In this study, 28 L. monocytogenes isolates were collected from patients in China from four cities/provinces (Beijing, Shanghai, Shanxi, and Jiangsu Province) and isolated from central nervous system infections (three cases), bacteremia (13 cases) and maternal–fetal infections (seven cases) from 2007 to 2012 (). These L. monocytogenes isolates were serotyped and characterized using several molecular typing methods.
Figure 1 Pulsed-field gel electrophoresis (PFGE)-based dendrogram was constructed by unweighted pair-group method with arithmetic means (UPGMA) to represent the genetic diversity of various L. monocytogenes strains from patients in China. Twenty-eight L. monocytogenes strains were analyzed by PFGE using Asc I and Apa I. The corresponding data, including the name of the strain (Strain_ID), the name of the case (Case_ID), city, source, isolation date (the exact isolation dates of four cases were not available), serotype, and sequence type (ST) were shown alongside the dendrogram to the right.
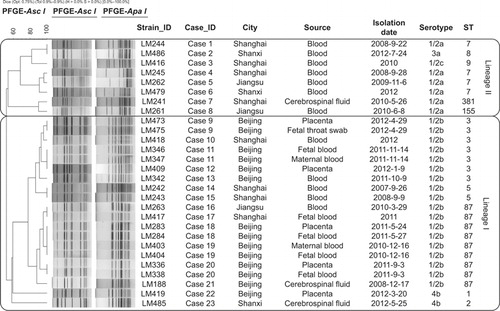
Serotyping divided the 28 clinical isolates into five serotypes, including serotype 1/2a, 1/2b, 1/2c, 3a, and 4b. The most prevalent serotype was serotype 1/2b with a frequency of 64.3%. Six (21.4%) and two (7.1%) isolates belonged to serotype 1/2a and serotype 4b respectively. Serotype 1/2c and 3a were represented by only one isolate each. All isolates were analyzed by pulsed-field gel electrophoresis (PFGE) using the Asc I and Apa I enzymes according to the PulseNet protocol,Citation6 and multi-locus sequence typing (MLST) based on seven housekeeping genes (abcZ, blgA, cat, dapE, dat, ldh, and lhkA).Citation7 By PFGE 17 Asc I pulsotypes, 16 Apa I pulsotypes, and 18 two-enzyme combined pulsotypes were identified. By MLST 10 sequence types (STs) were identified, five of which belong to lineage I and lineage II respectively (). There are three main STs: ST87, ST3, and ST7 with nine isolates (six cases), seven isolates (five cases), and four isolates (four cases) respectively. Two isolates involving two cases belonged to ST5 while the remaining STs were represented by single isolate.
The relationships of the isolates based on PFGE banding patterns are shown in . The isolates essentially grouped together by serotype and ST. There are a number of cases with two isolates from different anatomic sites with one from maternal tissue and the other from fetal tissue. All such cases showed the identical genotype by all methods. We also found identical isolates between cases. The four isolates from case 18 and case 19 are identical, but the cases were not clinically linked as the isolates were obtained from different hospitals in Beijing and different times with no evident epidemiological association. However, isolates from case 11 and case 12, which occurred in the same hospital two months apart were also identical. We further tested these isolates by multiple-locus variable-number tandem-repeat analysis using the scheme of Sperry et al.Citation8 and found that isolates from case 11 and case 12 differed in the Lm-3 locus (copy numbers were nine and six, respectively), suggesting the cases were not epidemiologically linked. In addition, isolates from cases 1 and 2 had the same pulsotype, but different serotypes and STs and are clearly unrelated.
All STs found in human isolates were also found in food-borne isolates in China (Supplementary Figure S1). However, it seems that there is a marked difference in prevalence of STs between human infections and food contaminations although the sample for human isolates in this study was small. Our previous study showed that ST3 and ST7 isolates were seldom found in contaminated food with 3.8% and 3.3% respectively.Citation5 They were clearly overrepresented among isolates from human clinical cases with 24.1% and 13.8% for ST3 and ST7 respectively. The most predominant STs in contaminated foods were ST9 (29.1%) and ST8 (10.7%),Citation5 whereas in human infections only one isolate was found for each ST. Importantly, ST87, the third most predominant ST isolated from contaminated food in China (9.2%),Citation5 is the most frequently isolated ST in human infections in this study. In addition, different contamination rates occurred in different types of food products. Wang et al.Citation9 analyzed 33 L. monocytogenes isolates from ready-to-eat (RTE) meat product in Nanjing and found that serogroups 1/2a, 3a and 1/2b, 3b were highly prevalent. Compared with the distribution of serotypes caused human infection in this study, 89.2% (25 of 28 isolates) belonged to 1/2a or 1/2b. By MLST, ST87, ST3, ST5, ST8, and ST9 found in this study were also identified in Wang’s study. Particularly, ST5 was the most predominate ST in ready-to-eat meat product in Wang’s study, although the second predominant ST (ST121) was not found in this study. Extensive research on the prevalence and diversity of L. monocytogenes isolates from human infections in China will provide more accurate framework for prediction of the public health risk associated with specific L. monocytogenes subtype.
The genotype frequency data from this study can be compared with international data. By serotype, 1/2a and 4b were prevalent in some European countries, representing 46.3% and 42.6% of human isolates from sporadic cases in Italy,Citation10 23.5% and 52.8% of listeriosis cases in Hungary respectively.Citation11 Recently, Satoko et al.Citation12 described genetic characteristics of 21 Japanese clinical L. monocytogenes isolates, 13 of which belonged to 4b. The distribution of serotypes was different in this study. Only two of 28 L. monocyotgenes isolates were serotype 4b, while 18 isolates were serotype 1/2b. However, judging whether the distribution having bias or not depends on more epidemiological studies on L. monocytogenes population isolated from human infection in China. Serotype 3a had once caused a serious outbreak transmitted by an unusual vehicle (pasteurized butter) involving 25 cases with a very high fatality rate (six patients, 24%) in Finland.Citation13 By sequence typing, a ST1 strain caused an outbreak in Sweden in 1995, a ST2 strain caused an outbreak in Italy in 1997, and ST5 strains caused outbreaks in Canada in 1996 and in USA in 2011.Citation14,Citation15,Citation16 It is interesting to note that ST87, the predominant ST in clinical listeriosis in China, was seldom linked to human infection cases in other countries with only one ST87 isolate from water reported by Ragon et al.Citation7
Our analysis of 28 clinical L. monocytogenes isolates from China showed that there seems to be a limited diversity of clinical isolates in comparison to food isolates. ST87 and ST3, both of which belong to serotype 1/2b, predominately cause the human infections. ST87 poses the highest health risk as it is the third most frequent ST isolated from food in China. Our study demonstrates the need for better surveillance of food contamination and human listeriosis in China.
Supplementary Figure
Download TIFF Image (25.2 MB)This work was supported by grants Mega Project of Research on The Prevention and Control of HIV/AIDS, Viral Hepatitis Infectious Diseases 2011ZX10004-001, 2013ZX10004-101 to Changyun Ye from the Ministry of Science and Technology, People's Republic of China.
Supplementary information of this article can be found on the Emerging Microbes & Infections' website (http://www.nature.com/emi).
- Drevets DA, Bronze MS.Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol 2008;53: 151–165.
- Lamont RF, Sobel J, Mazaki-Tovi S et al.Listeriosis in human pregnancy: a systematic review. J Perinat Med 2011;39: 227–236.
- Goulet V, Hedberg C, Le Monnier A et al.Increasing incidence of listeriosis in France and other European countries. Emerg Infect Dis 2008;14: 734–740.
- Jiao Y, Zhang W, Ma J et al.Early onset of neonatal listeriosis. Pediatr Int 2011;53: 1034–1037.
- Wang Y, Zhao A, Zhu R et al.Genetic diversity and molecular typing of Listeria monocytogenes in China. BMC Microbiol 2012;12: 119.
- Graves LM, Swaminathan B.PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int J Food Microbiol 2001;65: 55–62.
- Ragon M, Wirth T, Hollandt F et al.A new perspective on Listeria monocytogenes evolution. PLoS Pathog 2008;4: e1000146.
- Sperry KE, Kathariou S, Edwards JS et al.Multiple-locus variable-number tandem-repeat analysis as a tool for subtyping Listeria monocytogenes strains. J Clin Microbiol 2008;46: 1435–1450.
- Wang G, Qian W, Zhang X et al.Prevalence, genetic diversity and antimicrobial resistance of Listeria monocytogenes isolated from ready-to-eat meat products in Nanjing, China. Food Control 2015;50: 202–208.
- Mammina C, Aleo A, Romani C et al.Characterization of Listeria monocytogenes isolates from human listeriosis cases in Italy. J Clin Microbiol 2009;47: 2925–2930.
- Kiss R, Tirczka T, Szita G et al.Listeria monocytogenes food monitoring data and incidence of human listeriosis in Hungary, 2004. Int J Food Microbiol 2006;112: 71–74.
- Miya S, Takahashi H, Nakagawa M et al.Genetic characteristics of Japanese clinical Listeria monocytogenes isolates. PLoS One 2015;10: e0122902.
- Lyytikainen O, Autio T, Maijala R et al.An outbreak of Listeria monocytogenes serotype 3a infections from butter in Finland. J Infect Dis 2000;181: 1838–1841.
- Lomonaco S, Verghese B, Gerner-Smidt P et al.Novel epidemic clones of Listeria monocytogenes, United States, 2011. Emerg Infect Dis 2013;19: 147–150.
- Ericsson H, Eklow A, Danielsson-Tham ML et al.An outbreak of listeriosis suspected to have been caused by rainbow trout. J Clin Microbiol 1997;35: 2904–2907.
- Aureli P, Fiorucci GC, Caroli D et al.An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N Engl J Med 2000;342: 1236–1241.
