Dear Editor,
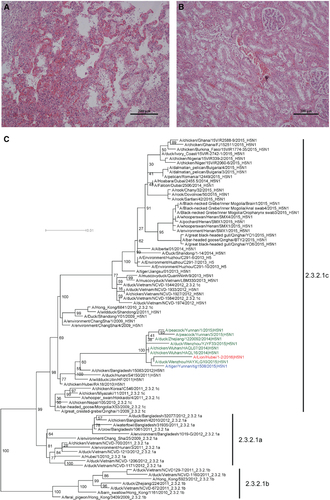
On 12 April 2016, a 3-year-old male lion died in Ezhou zoo, Hubei province, China. Clinical symptoms include droopiness and anorexia several days before death, and no improvement was observed after symptomatic treatment. The body temperature of the lion, measured the night before its death, was 38.8 °C–38.9 °C. Necropsy findings included severe congestion and edema in the lungs, slight intestinal bloating and red residual urine in the bladder. Histopathological changes consisted of renal tubular necrosis and interstitial hemorrhage in the kidneys. In addition, there were pulmonary congestion, inflammatory infiltration and interstitial proliferation in the lungs, consistent with respiratory failure ( and ).
Figure 1 Histopathological changes (A Lung, B kidney, magnification × 200) in the dead lion and phylogenetic analysis of the H5 genes (C).
No bacteria were detected in the tissues. The spleen, kidney, liver, lung, blood and urine were collected for detection of several viral pathogens using PCR or reverse transcription-PCR in which the specimens were shown to be negative for feline immunodeficiency virus, canine parvovirus, canine and feline distemper virus, and avian paramyxovirus. However, the samples tested positive for H5N1 avian influenza virus (AIV). Therefore, the supernatant of the homogenized tissues were inoculated into 10-day-old specific pathogen-free embryonated chicken eggs for virus isolation. Live virus was isolated from the lung, kidney and blood samples, and designated as A/Lion/Hubei/1-2F/2016, A/Lion/Hubei/1-2S/2016 and A/Lion/Hubei/1-2X/2016, respectively. These samples were sent for next-generation sequencing (NGS). There were no differences in the nucleotide sequences from the whole genomes of these viruses. A/Lion/Hubei/1-2F/2016 (designated 1-2F in this manuscript) was then selected for subsequent analyses, and the nucleotide sequences for all eight segments of this strain have been deposited at GenBank, under accession numbers KX571056-KX571063.
Phylogenetic analysis of the HA (), NA (Supplementary Figure S1B), NP (Supplementary Figure S1F) and NS (Supplementary Figure S1H) genes of the 1-2F isolate showed that they belonged to viruses from clade 2.3.2.1c H5N1.Citation1 The M and PB2 segments were found to be closely related to several H5N1 strains in China, which originated from an H9N2 lineage circulating in China and Southeast Asia (Supplementary Figures S1G and S1C). The PA and PB1 genes fell within a small H5N1 clade, which comes from a low pathogenic AIV gene pool existing mostly in waterfowl and wild birds (Supplementary Figures S1E and S1D). Therefore, the 1-2F isolate is a reassortant virus. Viruses belonging to this genotype were isolated from different provinces (Zhejiang, Yunnan and Hubei) in China, from different hosts (chickens, ducks, peacocks and tigers) and from different years (2014–2016), suggesting that the distribution of this H5N1 genotype is wider than previously thought.
In particular, all eight genes in 1-2F were similar to and clustered with those of a clade 2.3.2.1 H5N1 virus isolated from a tiger (A/tiger/Yunnan/tig1508/2015(H5N1)) that had died during August 2015.Citation2 Since clade 2.3.2.1 viruses are prevalent among poultry in China and have been repeatedly detected in wild birds,Citation3, Citation4, Citation5 more infections with clade 2.3.2.1 viruses in mammals can be expected.
To investigate the source of the infection, environmental samples taken from the lion and the birds in the zoo were collected for NGS analysis (Supplementary Technical Appendix). The sequencing depth was 2G per sample. H5 and N1 were not found in the environmental samples collected from birds; however, 6689 H5 and 5936 N1 reads were detected in the drinking water of the lion. These results suggested that the lion may not have acquired the H5N1 infection from the birds in the zoo. Alternatively, the lion was fed with frozen chicken carcasses, and therefore the most plausible source of the infection might be chickens, which may have been contaminated by H5N1 AIV.
Genetic analysis further showed that the HA gene of 1-2F contained multiple basic amino acids (RERRRKR) at the cleavage site. The amino-acid residues in the receptor-binding sites of HA exhibited avian characteristics (226-228 QSG). Furthermore, E627 and D701 residues in the PB2 protein suggested that the virus had not yet adapted to the mammalian host.Citation6, Citation7 A 20 amino-acid deletion of NA was detected at positions 49–68, which may increase virus adaptation to domestic fowl and virulence in mammals.Citation8 The H274 residue of NA and N31 in M2 suggested that the virus was sensitive to oseltamivir while resistant to amantadine. There were no differences in the key sites between the H5N1 AIV isolated from lions and chickens (A/chicken/Wuhan/HAQL07/2014(H5N1)), and only a difference of 1–2 amino acids when compared with viruses derived from tigers and wild birds, respectively (Supplementary Table S1).
In summary, we documented the first infection of a lion with a highly pathogenic H5N1 AIV, expanding the host range of AIV. This virus was a reassortant from circulating H5N1/H9N2 viruses and remained prevalent among poultry. Since this virus was highly virulent to the lion and that AIV has been reported to cause deaths in tigers and leopards,Citation9 we believe that captive animals are under certain risk of AIV infections, and increased surveillance is needed to track and chart the ongoing evolution of circulating AIV with respect to their potential to infect susceptible mammalian hosts.
Supplementary Methods
Download PDF (722.7 KB)Supplementary Methods
Download MS Word (37.5 KB)Supplementary Methods
Download MS Word (43 KB)This study was supported by grants from the National Research Program (2010CB530300), the National Natural Science Foundation of China (31070141 and 31100132), the Special Project of Ministry of Science and Technology (2013FY113500), and the National Mega Project on Major Infectious Disease Prevention (2012ZX10004-207), and the intramural special grant for influenza virus research from the Chinese Academy of Sciences (KJZD-EW-L09 and KJZD-EW-L15), and Shenzhen Municipal Government of China (ZDSYS201504301534057, JCYJ20160427151920801 and JCYJ20151029151932602). George F Gao is a leading principal investigator of the NSFC Innovative Research Group (81321063). Weifeng Shi was supported by the ‘Taishan Scholar’ project of Shandong Province. Yuhai Bi is supported by the Youth Innovation Promotion Association of Chinese Academy of Sciences (CAS). Gary Wong is the recipient of a Banting Postdoctoral Fellowship from the Canadian Institutes of Health Research (CIHR) and the President’s International Fellowship Initiative from the CAS. We also thank Wanpo Zhang (Huazhong Agricultural University, Wuhan, China) for preparation and observation of hematoxylin and eosin (HE) staining. The funding sources had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary information accompanies the paper on Emerging Microbes and Infections's website (http://www.nature.com/emi).
- WHO-OIE-FAO H5 Evolution Working Group.Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses.Influenza Other Respir Viruses2014; 8:384–388.
- HuT,ZhaoH,ZhangYet al.Fatal influenza A (H5N1) virus infection in zoo-housed tigers in Yunnan Province, China.Sci Rep2016; 6:25845.
- FanZ,CiY,LiuLet al.Phylogenetic and pathogenic analyses of three H5N1 avian influenza viruses (clade 2.3.2.1) isolated from wild birds in Northeast China.Infect Genet Evol2015; 29:138–145.
- BiY,ChenJ,ZhangZet al.Highly pathogenic avian influenza H5N1 Clade 2.3.2.1c virus in migratory birds, 2014-2015.Virol Sin2016; 31:300–3055.
- BiY,ZhangZ,LiuWet al.Highly pathogenic avian influenza A(H5N1) virus struck migratory birds in China in 2015.Sci Rep2015; 5:12986.
- LiZ,ChenH,JiaoPet al.Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model.J Virol2005; 79:12058–12064.
- TaftAS,OzawaM,FitchAet al.Identification of mammalian-adapting mutations in the polymerase complex of an avian H5N1 influenza virus.Nat Commun2015; 6:7491.
- MatsuokaY,SwayneDE,ThomasCet al.Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice.J Virol2009; 83:4704–4708.
- KeawcharoenJ,OraveerakulK,KuikenTet al.Avian influenza H5N1 in tigers and leopards.Emerg Infect Dis2004; 10:2189–2191.
