Abstract
Emerging Microbes & Infections (2017) 6, e10; doi:10.1038/emi.2016.138; published online 22 February 2017
Dear Editor,
Swine are considered an important host of influenza A virus (IAV), allowing for rapid reassortment that can produce novel viruses leading to human infections resulting in outbreaks and pandemics.Citation1 Therefore, zoonotic transmission of IAV creates a major public health threat. A small portion of the United States swine population (~1.5%) is raised for youth education in small farm settings and exhibited at agricultural fairs, which encourages increased human-animal interaction, creating an important interface for zoonotic IAV transmission (Bliss et al,Citation2 Journal of the American Veterinary Medical Association, in press). However, being a small niche, exhibition swine are often overlooked as active participants in disease transmission and pathogen dissemination.Citation3 Nevertheless, most documented cases of swine-to-human IAV transmission have been associated with exposure to swine during agricultural fairs.Citation4, Citation5 In 2012, 309 confirmed cases of H3N2 variant infection were reported, with >90% of individuals infected that year reporting swine contact at agricultural fairs.Citation6
Active surveillance has revealed that when IAV is detected in swine at an agricultural fair, by the last day of the fair >60% of swine may be infected.Citation7 Conversely, it has been estimated that 1.5% of pigs arrive at fairs with active infection.Citation2 These point-in-time estimates illustrate the rapid IAV transmission that is occurring in swine at agricultural fairs, which appears faster than predicted by a typical direct contact transmission model.Citation8 This led us to investigate potential underlying mechanisms that can enhance viral spread in agricultural fairs. Identifying routes of IAV transmission in these settings will allow animal health officials to focus efforts on developing control strategies that will likely limit viral spread and lessen the public health risk.
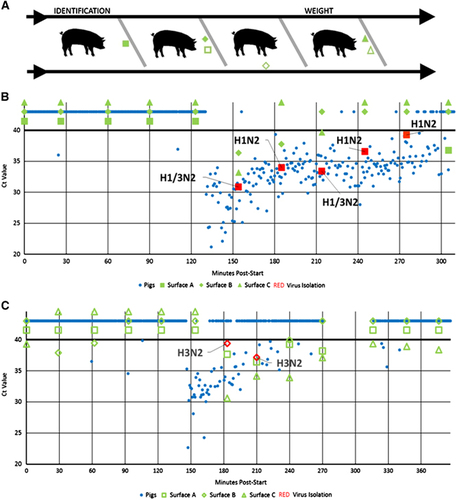
Individual pigs are typically weighed and identified upon arrival at agricultural fairs to ensure they meet show standards. This corralling commonly occurs at a central location within the barn and is accomplished by moving pigs single-file through a chute (Figure 1A). Previous testing has identified IAV contamination on environmental surfaces in live animal markets,Citation9 leading Bliss et al to hypothesize that entry-day corralling may contribute to viral transmission during fairs via viral contamination of chute surfaces.Citation2 The present study investigated IAV contamination of chute surfaces during corralling activities.
Prior to corralling, three swine-contact surfaces in the chute were identified for sampling (A, B, C) at six agricultural fairs, identified as Fairs 1–6. Each surface was wiped with cotton gauze (Convidien LLC, Mansfield, MA, USA) immediately before commencement of corralling, and approximately every 30 min thereafter. Samples were placed in 5 mL of viral transport mediaCitation10 and frozen until testing. Results of another study sought to determine IAV prevalence in the pig population by nasal wipe collection as pigs proceeded through the chute using previously described methods (The Ohio State University Animal Use Protocol 2009A0134-R2) and were used for comparison.Citation11
All samples were tested in parallel for IAV with real-time reverse transcription-polymerase chain reaction (rRT-PCR) and virus isolation in Madin-Darby Canine Kidney (MDCK) cells; recovered isolates were subtyped using previously described protocols.Citation2
In total, 236 environmental samples were collected at Fairs 1–6. IAV was detected via rRT-PCR in 26 (11.0%) environmental samples at two fairs, Fairs 1 and 2. Seven (3.0%) IAV isolates were recovered from environmental samples at Fairs 1 and 2. IAV-positive pigs were found at the same two fairs. IAV was not detected in the environment or pigs at the remaining four fairs. At Fair 1, 10 of 33 (30.3%) environmental samples tested positive via rRT-PCR for IAV and five (15.2%) isolates were recovered (Figure 1B). At Fair 2, 17 of 42 (40.5%) environmental samples tested positive via rRT-PCR for IAV and two (4.8%) isolates were recovered (Figure 1C). The nucleotide sequences for six of the seven IAV environmental isolates were obtained and are available on GenBank (Supplementary Table S1). Although no human cases were reported in association with these fairs, the genotypes of the recovered IAV isolates have been associated with variant influenza infections in humans.
Time of sample collection from the start of corralling (minutes post-start) was recorded (or estimated when data were missing) for environmental and individual pig samples. All environmental samples taken prior to the start of corralling tested negative for IAV. Comparing individual pig data shows that before environmental surfaces tested positive, several IAV-positive pigs with low cycle threshold (Ct) values at Fair 1 moved through the chute (135 min post-start; Figure 1A). Similarly, at Fair 2, virus was found on environmental surfaces immediately after the majority of IAV-positive pigs had moved through the chute (150 min post-start; Figure 1B). At both fairs, environmental subtypes matched those of the pigs with a mixture of H1N2 and H1/3N2 subtypes and an H3N2 subtype at Fairs 1 and 2, respectively.
While length of exhibition, direct contact between pigs, and large pig populations have been proposed as enhancing IAV transmission during agricultural fairs,Citation11 this study provides insight into how corralling activities can potentially drive high IAV prevalence. The recovery of viable IAVs from environmental surfaces during corralling illustrates that this activity can increase virus transmission in exhibition swine. Previous testing found pigs sampled during movement through the chute have a higher IAV prevalence compared with when arriving pigs are sampled on trailers or in respective pens. As IAV-positive pigs move through the chute, nasal secretions are left behind on pig-contact surfaces allowing each subsequent pig to contact residual virus, thereby creating an indirect transmission pathway.Citation2 Although a few swine samples were detected with high Ct values during the early part of corralling at both Fair 1 and Fair 2, once pigs with low Ct values (that is, truly positive and actively shedding virus) proceeded through the chute, virus was detected on environmental surfaces. Pigs moving through the same chute after these low Ct value pigs were more likely to test positive via rRT-PCR, albeit at high Ct values, than pigs moving through prior to the low Ct value pigs. It is presumed that most high Ct count positive pigs were not yet infected at time of sampling, but rather IAV was deposited on the pigs’ snouts during transit through the chute and this initial inoculation was subsequently detected by the nasal wipe sampling. Exposure of naïve pigs to IAV during the entry process could explain subsequent infection, accelerated transmission and increase in IAV prevalence by the close of the fairs, five to seven days later.
With clear epidemiological links between IAV in swine at agricultural fairs and outbreaks in humans attending the same fairs,Citation5, Citation12 an environment for IAV transmission is created by increased swine-human contact. Precautions must be taken to reduce IAV transmission and prevalence to protect public health. Limiting swine-human contact during agricultural fairs would likely decrease swine-to-human IAV transmission but would not impact the increase in IAV-positive swine. Show officials should use mitigation procedures, such as rinsing with water, cleaning, and wiping down environmental surfaces, where mechanical force is likely to reduce the amount of virus with which subsequent pigs may contact. Where rinsing fails or is not feasible, disinfecting chutes with animal-safe disinfectants will likely decrease viral burden on these surfaces significantly. Such cleaning and disinfecting procedures would be expected to decrease virus transmission between pigs upon fair entry, thereby decreasing IAV prevalence in swine during the fair, and ultimately reduce the public health threat.
This study demonstrates that swine-contact surfaces used during corralling are important fomites in indirect transmission of IAV. It also provides insight into the role of environmental surfaces in IAV transmission during and after swine exhibitions. With evidence of virus contamination of environmental surfaces, mitigation strategies targeting IAV control during this and similar processes is paramount.
Supplementary Table S1
Download MS Excel (9.7 KB)Acknowledgments
The funding for this project has been provided in part by the Centers of Excellence for Influenza Research and Surveillance, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services contract HHSN272201400006C; Centers for Disease Control and Prevention, Department of Health and Human Services, under Cooperative Agreement U38OT000143; and The Ohio State University Undergraduate Research Office. We would like to thank Animal Influenza Ecology and Epidemiology Research Program personnel Amber Kihm, Grant Price, Nick Bortolani, Christie Hammons, Nola Bliss, Alison Martin, and Josh Lorbach for dedicating their time to sampling efforts. We also thank Sue Trock (Centers for Disease Control and Prevention) for her assistance in this study. This study would not be possible without the continued support of Tony Forshey (Ohio Department of Agriculture) and Bret Marsh (Indiana State Board of Animal Health).
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
References
- Brown IH.The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol 2000;74: 29–46.
- Bliss N, Nelson SW, Nolting JM, Bowman AS.Prevalence of influenza A virus in exhibition swine during arrival at agricultural fairs. Zoonoses Public Health 2016;63: 477–485.
- Nelson MI, Wentworth DE, Das SRet al.Evolutionary dynamics of influenza A viruses in US exhibition swine. J Infect Dis 2016;213: 173–182.
- Wong KK, Greenbaum A, Moll MEet al.Outbreak of influenza A (H3N2) variant virus infection among attendees of an agricultural fair, Pennsylvania, USA, 2011. Emerg Infect Dis 2012;18: 1937–1944.
- Bowman AS, Nelson SW, Page SLet al.Swine-to-human transmission of influenza A(H3N2) virus at agricultural fairs, Ohio, USA, 2012. Emerg Infect Dis 2014;20: 1472–1480.
- Jhung MA, Epperson S, Biggerstaff Met al.Outbreak of variant influenza A(H3N2) virus in the United States. Clin Infect Dis 2013;57: 1703–1712.
- Bowman AS, Nolting JM, Nelson SW, Slemons RD.Subclinical influenza virus A infections in pigs exhibited at agricultural fairs, Ohio, USA, 2009-2011. Emerg Infect Dis 2012;18: 1945–1950.
- Torremorell M, Allerson M, Corzo C, Diaz A, Gramer M.Transmission of influenza A virus in pigs. Transbound Emerg Dis 2012;59(Suppl 1): 68–84.
- Choi MJ, Torremorell M, Bender JBet al.Live animal markets in Minnesota: A potential source for emergence of novel influenza A viruses and interspecies transmission. Clin Infect Dis 2015;61: 1355–1362.
- Edwards JL, Nelson SW, Workman JDet al.Utility of snout wipe samples for influenza A virus surveillance in exhibition swine populations. Influenza Other Respir Viruses 2014;8: 574–579.
- Bowman AS, Workman JD, Nolting JM, Nelson SW, Slemons RD.Exploration of risk factors contributing to the presence of influenza A virus in swine at agricultural fairs. Emerg Microbes Infect 2014;3: e5.
- Bowman AS, Sreevatsan S, Killian MLet al.Molecular evidence for interspecies transmission of H3N2pM/H3N2v influenza A viruses at an Ohio agricultural fair, July 2012. Emerg Microbes Infect 2012;1: e33.