Abstract
Clostridium difficile is the major causative agent of nosocomial antibiotic-associated diarrhea. In a 2009 outbreak of C. difficile-associated diarrhea that was recorded in a major Costa Rican hospital, the hypervirulent NAP1 strain (45%) predominated together with a local genotype variant (NAPCR1, 31%). Both strains were fluoroquinolone-resistant and the NAPCR1 genotype, in addition, was resistant to clindamycin and rifampicin. We now report on the genotypes and antibiotic susceptibilities of 68 C. difficile isolates from a major Costa Rican hospital over a 2-year period without outbreaks. In contrast to our previous findings, no NAP1 strains were detected, and for the first time in a Costa Rican hospital, a significant fraction of the isolates were NAP9 strains (n=14, 21%). The local NAPCR1 genotype remained prevalent (n=18, 26%) and coexisted with 14 strains (21%) of classic hospital NAP types (NAP2, NAP4, and NAP6), eight new genotypes (12%), four environmental strains classified as NAP10 or NAP11 (6%), three strains without NAP designation (4%) and seven non-toxigenic strains (10%). All 68 strains were resistant to ciprofloxacin, 88% were resistant to clindamycin and 50% were resistant to moxifloxacin and rifampicin. Metronidazole and vancomycin susceptibilities were universal. The NAPCR1 and NAP9 strains, which have been associated with more severe clinical infections, were more resistant to antibiotics than the other strains. Altogether, our results confirm that the epidemiology of C. difficile infection is dynamic and that A−B+ strains from the NAP9 type are on the rise not only in the developed world. Moreover, our results reveal that the local NAPCR1 strains still circulate in the country without causing outbreaks but with equally high antibiotic-resistance rates and levels.
Introduction
Clostridium difficile has become the leading cause of nosocomial diarrhea in adults.Citation1 Clinical manifestations of C. difficile infections (CDI) vary from asymptomatic to fulminant colitis, including pseudomembranous colitis (PMC) or antibiotic-associated diarrhea. There may be complications, such as toxic megacolon, colonic perforation and a few extraintestinal manifestations.Citation2
Most disease-causing isolates of C. difficile produce one or two toxins, i.e., TcdA and TcdB. These toxins enter intestinal epithelial cells and glycosylate various families of cytoplasmic GTPases,Citation3 which leads to actin depolymerization with the loss of internal cell architecture, apoptosis, villus destruction and a mucosal inflammatory response.Citation4, Citation5 The genes encoding toxins A and B (tcdA and tcdB) are part of a so-called pathogenicity locus (PaLoc), which also includes tcdR (a sigma factor that promotes the transcription of both of the toxin genes), tcdE (a potential holin) and tcdC (a potential negative regulator of tcdA and tcdB).Citation6 Although deletions in tcdC have been claimed to favor TcdA and TcdB hypersecretion,Citation7 this conjecture remains controversial because, as Cartman et al.Citation8 demonstrated, these deletions have little effect in significantly increasing toxin production. Furthermore, variations in the combined repetitive oligopeptide domain of TcdB have been associated with increases in the virulence of epidemic strains.Citation9 A minority of toxigenic C. difficile strains also produce a third toxin, known as binary toxin Clostridium difficile toxin (CDT), that is encoded by cdtA and cdtB at the CdtLoc locus and is separated from the PaLoc.Citation10 CDT is composed of an ADP-ribosyltransferase that blocks actin polymerization and a binding component that is involved in toxin delivery.Citation11 Although it is believed that CDT affects the cytoskeleton and enhances the adhesion and colonization of C. difficile,Citation10 its role in CDI remains controversial.Citation12
Various methods have been used to type C. difficile strains. While pulsed-field gel electrophoresis (PFGE) is predominantly used in North America, ribotyping by PCR is most often used in Europe. PFGE NAP1 strains correspond to ribotype 027 and harbor toxin A, toxin B, CDT, a 18-bp mutation in tcdC and a point mutation in this gene at position 117. In contrast, most TcdA-negative and TcdB-positive isolates belong to NAP9 and correspond to PCR ribotype 017.Citation13
The effects, severity, complications, recurrence and even death rate of CDI have increased since 2003 in accordance with the increased isolation rates of hypervirulent strains, such as NAP1 and NAP9.Citation14, Citation15 The NAP1 strains have been associated with higher sporulation rates and greater resistance to antimicrobials, especially fluoroquinolones,Citation14, Citation16 whereas the NAP9 strains possess a TcdB that is capable of exerting a variant cytopathic effect.Citation17
During a C. difficile outbreak in a major Costa Rican hospital in 2009, the hypervirulent NAP1 strain (45%) and the NAPCR1 strains (31%) were the predominant genotypes. Both types of strains were resistant to ciprofloxacin, moxifloxacin and levofloxacin, and the NAPCR1 strains were also resistant to clindamycin and rifampicin. NAP9 and the other seven classical nosocomial strains were present but in minor proportions.Citation18, Citation19 Since this outbreak, the distribution of C. difficile genotypes in other Costa Rican hospitals has not been reported. To determine whether the NAP1 and NAPCR1 genotypes were dominant in a non-pediatric hospital over a two-year period without C. difficile outbreaks, 68 isolates from diarrheic patients were genotyped using PFGE and the antimicrobial susceptibilities of the isolates were tested. This information contributes to an understanding of CDI epidemiology worldwide and has the potential to guide local prevention efforts and treatment strategies.
Materials and methods
Isolates and bacteriological procedures
This study included 68 C. difficile isolates that were obtained from the diarrheal stools of non-pediatric patients who were admitted to a major hospital in Costa Rica with 633 beds between October 2010 and August 2012. All patients had been identified as having hospital-acquired CDI according to the criteria from the Infectious Diseases Society of America.Citation20 Toxins A and B were detected in the stool samples by the hospital's clinical laboratory, and the samples with positive results were inoculated onto cefoxitin–cycloserine fructose agar plates (CCFA, Oxoid, Hampshire, UK). Yellow colonies on CCFA were cryopreserved at −80 °C in brain–heart infusion broth with 20% glycerol and sent to the Laboratory of Research in Anaerobic Bacteriology at the University of Costa Rica for further analysis and identification. There, the strains were subcultured in selective C. difficile moxalactam norfloxacin medium (Oxoid) and later on Brucella agar plates (BD Diagnostics, Franklin Lakes, NJ, USA) supplemented with 5% lysed horse blood (Oxoid) and 1 μg/mL vitamin K (Sigma-Aldrich, St. Louis, MO, USA) and (blood agar vitamin K) under an atmosphere composed of 90% N2, 5% H2 and 5% CO2 in an anaerobic chamber (Bactron II; ShellLab, Cornelius, OR, USA) at 37 °C for 48 h. The identities of the isolates were phenotypically confirmed using selective media, the rapID 32A system (bioMériuex, Marcy-l'Étoile, France) and chartreuse fluorescence on blood agar vitamin K under long-wave ultraviolet light and genotypically confirmed through PCR-based detection of the C. difficile marker tpi and molecular typing by PFGE.Citation21
Molecular typing
Genomic DNA from each strain was obtained from overnight cultures in brain–heart infusion broth (Oxoid) using the InstaGene reagent (Bio-Rad, Hercules, CA, USA). Fragments of tcdA, tcdB, cdtB and tcdC were amplified by PCR using known primers and conditions.Citation21 A NAP1/027 strain (tcdA+, tcdB+, 18 bp-deletion in tcdC, cdtB+), a NAP7 strain (tcdA+, tcdB+, tcdC deletion>18 pb, cdtB+), an A−B+ strain (tcdA−, tcdB+, wild-type tcdC, cdtB−) and the non-toxigenic C. difficile strain ATCC 700057 (tcdA−, tcdB−, tcdC−, cdtB−) were used as controls.
For the PFGE typing, we obtained chromosomal SmaI macrorestriction patterns with a published methodCitation19 and a CHEF-DRIII variable angle system. Gel pictures were analyzed with BioNumerics v4.6 (Applied Maths, Austin, TX, USA) and compared with the databases of the National Microbiology Laboratory of Public Health Agency of Canada.
Antibiotic susceptibility testing
The minimum inhibitory concentrations (MIC) for clindamycin, ciprofloxacin, moxifloxacin, rifampicin, metronidazole and vancomycin were determined using E-test strips (AB bioMérieux, Askim, Sweden) and Brucella agar plates containing 5% blood, 1 μg/mL vitamin K and 5 μg/mL hemin according to established guidelines.Citation22 For susceptibility categorization, we used the resistance breakpoints recommended by the CLSI;Citation23 i.e., 8 μg/mL for clindamycin, ciprofloxacin and moxifloxacin and 32 μg/mL for metronidazole. For rifampicin and vancomycin, we adopted the breakpoints recommended in the document M100-S21 for Staphylococcus aureus because no values have been defined for anaerobic bacteria; these values were 4 μg/mL for rifampicin and 16 μg/mL for vancomycin.
Results
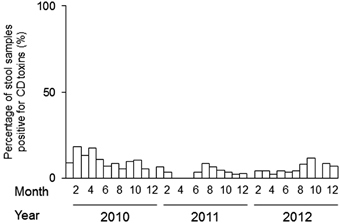
No outbreaks were reported from October 2010 to August 2012 in the hospital under study (). Our genotyping procedure revealed that 28 isolates were positive for tcdA and tcdB, negative for cdtB and carried wild-type tcdC; these results were expected for classic hospital strains. Eighteen isolates exhibited the characteristic NAPCR1 pattern (i.e., tcdA+, tcdB+, cdtB− and tcdC with a deletion), 14 exhibited the A−B+ strain pattern (i.e., tcdA−, tcdB+, cdtB− and wild-type tcdC) and 1 isolate exhibited all 3 toxins and a deletion in tcdC. Seven isolates were non-toxigenic ().
Table 1 Antibiotic resistances of various Clostridium difficile genotypes recovered between October 2010 and August 2012 at a hospital without a history of outbreaks during the period under study
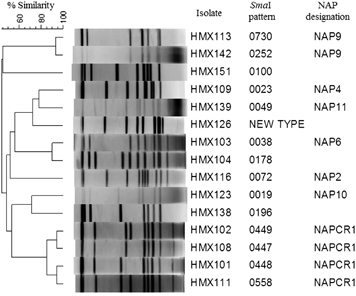
Although we observed a variety of genotypes ( and ), the local NAPCR1 genotype predominated (n=18, 26%). The NAP9 genotype was the second-most prevalent genotype (n=14, 21%), followed by the 14 isolates (21%) from the other traditional hospital pulsotypes of NAP2 (n=2), NAP4 (n=9), and NAP6 (n=3). Environment- or community-associated pulsotypes, such as NAP10 and NAP11, were observed (n=4, 6%) as were new pulsotypes (n=8, 12%) and known pulsotypes with no NAP designations (100, 196 and 178; n=3, 4%). Interestingly, no NAP1 strains were detected.
Antimicrobial susceptibility tests revealed that 88% of the isolates were resistant to clindamycin with very high MICs (>256 μg/mL, ). Half of the isolates were resistant to moxifloxacin and rifampicin (MIC >32 μg/mL; ). The MICs for metronidazole and vancomycin were rather low, and although all isolates were susceptible to both antibiotics, the MIC90 values were twice the MIC50 values ().
Table 2 MICs and resistances of 68 isolates of Clostridium difficile recovered between October 2010 and August 2012 from a Costa Rican hospital without a history of outbreaks during the period under study
All isolates from the two most common genotypes (i.e., NAPCR1 and NAP9) were resistant to clindamycin, moxifloxacin and rifampicin (). Among all of the clindamycin-resistant strains, only those from the NAPCR1 and NAP9 genotypes exhibited MICs >256 μg/mL. The remaining hospital, community and non-toxigenic isolates and the strains from the new genotypes exhibited low antibiotic-resistance levels ().
Discussion
We detected a predominance of C. difficile NAPCR1 and NAP9 strains in the diarrheal stool samples of patients admitted to a hospital in which no C. difficile outbreaks had occurred during the period under study. This knowledge is relevant from the clinical perspective because both genotypes have been associated with more severe cases of CDI.Citation19, Citation24, Citation25
NAPCR1 strains have circulated in various Costa Rican hospitals since 2003 (López-Ureña D et al., 2003, unpublished data) and have had major roles in the 2009 C. difficile outbreak in the San Juan de Dios Hospital.Citation18 Here, we found NAPCR1 strains quite frequently in a group of clinical isolates from another hospital and confirmed the widespread distribution of these strains and their dominance even in the absence of outbreaks. Moreover, the identification of NAPCR1 PFGE types reveals the ongoing evolution of this lineage and this species over a short time.
The worldwide prevalence of clinically significant NAP9 strains seems to be increasing,Citation26 particularly in Asian countries.Citation27 Our results reinforce this view because this genotype was the second-most prevalent group. Many studies have found these strains in humansCitation24, Citation28 and in animals.Citation29 NAP9 strains have been found once in Costa RicaCitation18 and in Latin America,Citation27 where they seem to be gradually replacing other circulating genotypes. As observed in many countries,Citation30, Citation31 our A−B+ strains were homogeneous, did not carry cdtAB, and harbored intact tcdC alleles. In contrast, in Australia, the tcdA−, tcdB+ strains are cdtB+.Citation13 Furthermore, because our NAP9 strains were clindamycin-resistant, they may share a clonal origin with the strains that caused epidemics in Canada, the Netherlands, Ireland and Poland.Citation13 We now know that these strains belong to the RT017 group (data not shown), but further studies are being performed to confirm that their sequence type is indeed ST37 or ST86.Citation32
Although NAP1 strains were previously isolated during a C. difficile outbreak at another Costa Rican hospital, we only found a single tcdA+, tcdB+ and cdtB+ strain with a tcdC deletion in this study. This strain did not give rise to the 001 macrorestriction pattern associated with NAP1 in our previous reportsCitation18, Citation19 but rather exhibited a PFGE pattern without a NAP designation (i.e., a 0196 macrorestriction pattern). Other NAP strains coexisted including common inhabitants of hospital environments, such as NAP2, NAP4 and NAP6 strains,Citation33 and NAP10 and NAP11 strains with potential zoonotic or community origins.Citation34
Despite the marked increase in the recovery of clindamycin-resistant anaerobic strains in Costa Rica in the last decade,Citation35, Citation36 this antibiotic is still the first-choice antibiotic for infections by anaerobic bacteria in Costa Rica and other geographic areas.Citation37 The alarming clindamycin resistance level of C. difficile observed in this study (88%) is slightly lower than the level recorded during a 2009 outbreak at another major hospital (97%)Citation18 but is still much higher than the values reported from other latitudes.Citation38, Citation39 Almost half of the strains, all of which belonged to genotypes NAPCR1 and NAP9, had MICs >256 μg/mL, whereas the remaining strains had MICs=8 μg/mL. These findings indicate that only certain lineages acquire highly efficient mechanisms of resistance to clindamycin.
Fluoroquinolone resistance is increasing in epidemic strains of C. difficile primarily due to the emergence of chromosomal mutations in DNA gyrase genes.Citation38, Citation39, Citation40 As described in 2010, all strains from this study were resistant to ciprofloxacin with MICs ⩾32 μg/mL.Citation18 Moreover, half of the isolates were resistant to moxifloxacin. All of the NAPCR1 and NAP9 strains were resistant to both quinolones. Older fluoroquinolones, such as ciprofloxacin, exhibited moderate or poor activity against C. difficile, and the third- and fourth-generation fluoroquinolones, such as moxifloxacin, were effective against these bacteria. However, recent studies indicate that the rates of resistance to moxifloxacin in C. difficile have increased dramatically in different countries.Citation41, Citation42, Citation43
Resistance to rifampicin is not unusual in C. difficile (6%–39%), especially in multidrug-resistant strains.Citation44, Citation45 The reported MIC values of ⩽0.002 μg/mL for susceptible strains and >32 μg/mL for resistant strains largely match our findings.Citation44, Citation46 Up to 50% of our strains were rifampicin-resistant, particularly those from the NAPCR1 and NAP9 genotypes, which were also resistant to clindamycin, ciprofloxacin and moxifloxacin. Interestingly, all of the isolates that were resistant to moxifloxacin were also rifampicin-resistant, and this association should be explored further.
The antibiotic-resistance levels of the less abundant genotypes were rather low, but they supported the previously reported increased resistance to fluoroquinolone of non-epidemic C. difficile strainsCitation40 and the ciprofloxacin resistance that is present in virtually all strains of C. difficile.
All of the isolates were susceptible to metronidazole and vancomycin. In agreement with the known metronidazole MIC of C. difficile,Citation20 our MICs were invariably <2.0 μg/mL. However, a gradual change in the pattern of sensitivity to this antibiotic might be occurring within our strains because the MIC90 for metronidazole was twice the MIC50. A similar situation might be occurring in terms of their vancomycin susceptibility because 12 strains (18%), six of which were classified as NAPCR1, exhibited reduced vancomycin susceptibility with MICs ⩾2 μg/mL. These data strongly support the presence of continuous monitoring programs both in clinics and the community.
In this study, a local genotype of C. difficile was the most prevalent strain in a set of 68 isolates that were recovered in a hospital over a 2-year period without outbreaks. This genotype has previously been found to be dominant in another hospital during an outbreak. The second-most prevalent genotype was NAP9, which is in line with its increased prevalence in the USA,Citation31 Europe,Citation24, Citation31 AsiaCitation25 and Australia.Citation13 The NAPCR1 and NAP9 genotypes exhibited high levels of antibiotic resistance, which reflects the use of antibiotics to which the strains have developed resistance and the association between CDIs and increased antibiotic use. The high levels of resistance to several antibiotics among the predominant genotypes could favor their persistence in the hospital environments and dominance over other genotypes. Constant characterization of circulating C. difficile isolates in terms of their population structures and antibiotic resistances not only improves our understanding of the epidemiology of CDI but also guides sanitary authorities and physicians in efforts to reduce the burden associated with this emerging pathogen.
Acknowledgments
We thank Dr Michael R Mulvey, Dr George Golding and Tim Du (the National Microbiology Laboratory, Winnipeg, MB, Canada) for access to the NAP types database and technical assistance with the PFGE and ribotyping analyses. Pablo Vargas and Robin Cárdenas (the Laboratory of Research in Anaerobic Bacteriology, University of Costa Rica, San José, Costa Rica) are acknowledged for their technical assistance. This work was supported by the National Rector’s Council, Costa Rica (CONARE) and the Vice-Rectory for Research of the University of Costa Rica through grants VI-803-B1-654 and VI-803-B3-003.
- SlimingsC,RileyT.Antibiotics and hospital-acquired Clostridium difficile infection: Update of systematic review and meta-analysis.J Antimicrob Chemother2014; 69:881–891.
- ShivashankarR,KhannaS,KammerPPet al.Clinical factors associated with development of severe-complicated Clostridium difficile infection.Clin Gastroenterol Hepatol2013; 11:1466–1471.
- AktoriesK.Bacterial protein toxins that modify host regulatory GTPases.Nat Rev Microbiol2011; 9:487–498.
- KimH,KokkotouE,NaXet al.Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase.Gastroenterology2005; 129:1875–1888.
- ChumblerNM,FarrowMA,LapierreLAet al.Clostridium difficile toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism.PLoS Pathog2012; 8:e1003072.
- DingleKE,ElliottB,RobinsonEet al.Evolutionary history of the Clostridium difficile pathogenicity locus.Genome Biol Evol2014; 6:36–52.
- DupuyB,GovindR,AntunesAet al.Clostridium difficile toxin synthesis is negatively regulated by TcdC.J Med Microbiol2008; 57:685–689.
- CartmanST,KellyML,HeegDet al.Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdc genotype and toxin production.Appl Environ Microbiol2012; 78:4683–4690.
- LanisJM,HeinlenLD,JamesJAet al.Clostridium difficile 027/BI/NAP1 encodes a hypertoxic and antigenically variable form of TcdB.PLoS Pathog2013; 9:e1003523.
- GerdingDN,JohnsonS,RupnikMet al.Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance.Gut Microbes2014; 5:15–27.
- SchwanC,StecherB,TzivelekidisTet al.Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria.PLoS Pathog2009; 5:e1000626.
- KuehneSA,ColleryMM,KellyMLet al.Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain.J Infect Dis2014; 209:83–86.
- ElliottB,SquireMM,TheanSet al.New types of toxin A-negative, toxin B-positive strains among clinical isolates of Clostridium difficile in Australia.J Med Microbiol2011; 60:1108–1111.
- MerriganM,VenugopalA,MallozziMet al.Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production.J Bacteriol2010; 192:4904–4911.
- KimJ,PaiH,SeoM-Ret al.Clinical and microbiologic characteristics of tcdA-negative variant Clostridium difficile infections.BMC Infect Dis2012; 12:109.
- RazaviB,ApisarnthanarakA,MundyLM.Clostridium difficile: emergence of hypervirulence and fluoroquinolone resistance.Infection2007; 35:300–307.
- Chaves-OlarteE,FreerE,ParraAet al.R-Ras glucosylation and transient RhoA activation determine the cytopathic effect produced by toxin B variants from toxin A-negative strains of Clostridium difficile.J Biol Chem2003; 278:7956–7963.
- Quesada-GómezC,RodríguezC,Gamboa-CoronadoMDMet al.Emergence of Clostridium difficile NAP1 in Latin America.J Clin Microbiol2010; 48:669–670.
- Quesada-GómezC,López-UreñaD,Acuña-AmadorLet al.Emergence of an outbreak-associated Clostridium difficile variant with increased virulence.J Clin Microbiol2015; 53:1216–1226.
- CohenSH,GerdingDN,JohnsonSet al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA).Infect Control Hosp Epidemiol2010; 31:431–455.
- SpigagliaP,MastrantonioP.Comparative analysis of Clostridium difficile clinical isolates belonging to different genetic lineages and time periods.J Med Microbiol2004; 53:1129–1136.
- Letournel-GlomaudC,HoussayeS,MilhaihaLet al.E-test antibiotics susceptibility of strict anaerobic bacteria.Anaerobe2003; 9:281–284.
- Clinical and Laboratory Standards InstituteMethods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria,Approved Standard, CLSI document M11-A8,CLSI: Wayne, PA, USA.2012.Available athttp://shop.clsi.org/site/Sample_pdf/M11A8_sample.pdf.
- DrudyD,HarnedyN,FanningSet al.Emergence and control of fluoroquinolone-resistant, toxin A–negative, toxin B–positive Clostridium difficile.Infect Control2007; 28:932–940.
- DuP,CaoB,WangJet al.Sequence variation in tcdA and tcdB of Clostridium difficile: ST37 with truncated tcdA is a potential epidemic strain in China.J Clin Microbiol2014; 52:3264–3270.
- CollinsD,HawkeyP,RileyT.Epidemiology of Clostridium difficile infection in Asia.Antimicrob Resist Infect Control2013; 2:21.
- GoorhuisA,LegariaMC,van den BergRJet al.Application of multiple-locus variable-number tandem-repeat analysis to determine clonal spread of toxin A-negative Clostridium difficile in a general hospital in Buenos Aires, Argentina.Clin Microbiol Infect2009; 15:1080–1086.
- AlfaMJ,KabaniA,LyerlyDet al.Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea.J Clin Microbiol2000; 38:2706–2714.
- ThakurS,SandfossM,Kennedy-StoskopfSet al.Detection of Clostridium difficile and Salmonella in feral swine population in North Carolina.J Wildl Dis2011; 47:774–776.
- JohnsonS,SambolSP,BrazierJSet al.International typing study of toxin A-negative, toxin B-positive Clostridium difficile variants.J Clin Microbiol2003; 41:1543–1547.
- Van den BergRJ,ClaasECJ,OyibDHet al.Characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates from outbreaks in different countries by amplified fragment length polymorphism and PCR ribotyping.J Clin Microbiol2004; 42:1035–1041.
- GriffithsD,FawleyW,KachrimanidouMet al.Multilocus sequence typing of Clostridium difficile.J Clin Microbiol2010; 48:770–778.
- MartinH,WilleyB,LowDEet al.Characterization of Clostridium difficile strains isolated from patients in Ontario, Canada, from 2004 to 2006.J Clin Microbiol2008; 46:2999–3004.
- LessaFC.Community-associated Clostridium difficile infection: how real is it?Anaerobe2013; 24:121–123.
- MolinaJ,BarrantesG,Quesada-GómezCet al.Phenotypic and genotypic characterization of multidrug-resistant BacteroidesParabacteroides spp., and Pseudoflavonifractor from a Costa Rican hospital.Microb Drug Resist2014; 20:478–484.
- Quesada-GómezC,Rodríguez-CavalliniE,RodríguezC.Scarce detection of mobile erm genes associated with tetQ in Bacteroides and Parabacteroides from Costa Rica.Anaerobe2013; 21:18–21.
- RashidM-U,WeintraubA,NordCE.Development of antimicrobial resistance in the normal anaerobic microbiota during one year after administration of clindamycin or ciprofloxacin.Anaerobe2015; 31:72–77.
- HuangH,WeintraubA,FangHet al.Antimicrobial resistance in Clostridium difficile.Int J Antimicrob Agents2009; 34:516–522.
- TicklerIA,GoeringRV,WhitmoreJDet al.Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013.Antimicrob Agents Chemother2014; 58:4214–4218.
- SpigagliaP,BarbantiF,LouieTet al.Molecular analysis of the gyrA and gyrB quinolone resistance-determining regions of fluoroquinolone-resistant Clostridium difficile mutants selected in vitro.Antimicrob Agents Chemother2009; 53:2463–2468.
- BarbutF,MastrantonioP,DelméeMet al.Prospective study of Clostridium difficile infections in Europe with phenotypic and genotypic characterisation of the isolates.Clin Microbiol Infect2007; 13:1048–1057.
- BourgaultA-M,LamotheF,LooVGet al.In vitro susceptibility of Clostridium difficile clinical isolates from a multi-institutional outbreak in Southern Québec, Canada.Antimicrob Agents Chemother2006; 50:3473–3475.
- HuangH,WuS,WangMet al.Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes.Int J Antimicrob Agents2009; 33:339–342.
- CurrySR,MarshJW,ShuttKAet al.High frequency of rifampin resistance identified in an epidemic Clostridium difficile clone from a large teaching hospital.Clin Infect Dis2009; 48:425–429.
- SpigagliaP,BarbantiF,MastrantonioP.Multidrug resistance in European Clostridium difficile clinical isolates.J Antimicrob Chemother2011; 66:2227–2234.
- O’ConnorJR,GalangMA,SambolSPet al.Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile.Antimicrob Agents Chemother2008; 52:2813–2817.