Abstract
Rift Valley fever (RVF) outbreaks have occurred across eastern Africa from 1912 to 2010 approximately every 4–15 years, most of which have not been accompanied by significant epidemics in human populations. However, human epidemics during RVF outbreaks in eastern Africa have involved 478 deaths in 1998, 1107 reported cases with 350 deaths from 2006 to 2007 and 1174 cases with 241 deaths in 2008. We review the history of RVF outbreaks in eastern Africa to identify the epidemiological factors that could have influenced its increasing severity in humans. Diverse ecological factors influence outbreak frequency, whereas virus evolution has a greater impact on its virulence in hosts. Several factors could have influenced the lack of information on RVF in humans during earlier outbreaks, but the explosive nature of human RVF epidemics in recent years mirrors the evolutionary trend of the virus. Comparisons between isolates from different outbreaks have revealed an accumulation of genetic mutations and genomic reassortments that have diversified RVF virus genomes over several decades. The threat to humans posed by the diversified RVF virus strains increases the potential public health and socioeconomic impacts of future outbreaks. Understanding the shifting RVF epidemiology as determined by its evolution is key to developing new strategies for outbreak mitigation and prevention of future human RVF casualties.
Emerging Microbes and Infections (2016) 5, e58; doi:10.1038/emi.2016.57; published online 22 June 2016
Introduction
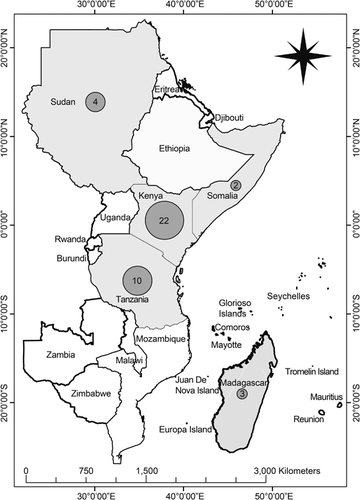
Rift Valley fever (RVF), caused by the Rift Valley fever virus (RVFV; genus: Phlebovirus, family: Bunyaviridae), is an arboviral disease primarily of domesticated animals that also causes mild to life-threatening disease in humans. The virus is a negative-sense, single-stranded RNA virus, and its name is derived from the Great Rift Valley of Kenya, where the disease was first recognized and characterized in 1912Citation1 and first described in 1931 after a highly fatal epizootic in Kenya in 1930.Citation2 Subsequently, RVF epizootics/epidemics have occurred (Figure 1) in 4–15-year cycles in association with flooding above the normal rainfall in many flood-prone habitats.Citation3
RVFV is inherently complex, posing a significant challenge to outbreak prediction, mitigation and management.Citation4 Characteristically, once RVFV is introduced into permissive ecologies, the virus becomes endemic/enzootic, making the region vulnerable to periodic outbreaks with the potential to spread further into non-endemic environments that have favorable conditions.Citation5, Citation6 For example, in Tanzania, the number of villages affected by RVF outbreaks increased from two in 1930 to 175 in 2006 and 2007, and the number of cases increased 11-fold from 1026 in 1930 to 11 339 in 1978 and another 12-fold to 136 750 in 2007 in 21/28 districts.Citation5 Similarly, in Kenya, RVF outbreaks were limited to 1/69 administrative districts from 1912 to 1950, but in 2006 and 2007, 38/69 districts were affected.Citation6 Furthermore, in both Kenya and Tanzania, the districts that formed the primary cluster of the 1977–1978 and 1997–1998 outbreaks became part of the relatively larger cluster of outbreaks in 2006/2007.Citation5, Citation6 As the virus causes severe outbreaks with large-scale livestock losses and, more recently, significant impacts on human health, it poses an emerging zoonotic threat to vulnerable African communities. Although the virus is endemic to sub-Saharan Africa (SSA), it has the potential for global spread, as it has already crossed significant natural geographic barriers such as the Indian Ocean, the Sahara Desert and the Red Sea to reach naive ecologies.Citation5
As a zoonotic disease, RVF disproportionately affects vulnerable communities with poor resilience to economic and environmental challenges through its wider societal effects. The RVFV has been associated with congenital abnormalities (stillbirths, mummified fetuses, defects of the central nervous system, musculoskeletal problems, hydranencephaly, hydroencephaly, porencephaly, arthrogryposis and cerebellar hypoplasia) in fetal or neonatal ruminants.Citation7 During the outbreaks in Africa, vertical transmission of RVFV has been reported in mosquito vectors, ruminants and, most recently, humans.Citation8 This clearly reflects the burden of this emerging zoonotic infectious disease to humans, especially among women and pregnant women.Citation9
Despite its enormous public health and socioeconomic impacts, RVF is often neglected by major global donors and disease control programs.Citation4 However, in recent years, the classification of RVFV as a potential bio-/agro-terrorism agent has triggered global interest, particularly in the area of vaccine development and diagnostics.Citation4 We review the history of RVF outbreaks in East Africa and the epidemiological risk factors that could have influenced RVF epidemiology in past outbreaks. In the context of the persistent and intensifying epidemics in humans, we specifically examine the genetic evolution of RVFV as well as the socioeconomic impacts of these outbreaks in East Africa.
History
In the first six decades after the viral disease was described (1931–1996), human RVF cases during livestock RVF outbreaks in East Africa were rare and generally mild.Citation10 Although the first human death due to RVF was reported in 1934, this was in a laboratory worker who was investigating RVF cultures,Citation11 presumably because of exposure to very high titers. Widespread epizootics in the region were not accompanied by similar epidemics of the disease in human populations for >50 years.Citation10
Of the 23 RVF outbreaks in Kenya between 1912 and 2007 (), 14 affected 3–38 of the 69 districts nationally, whereas nine were localized to 1–2 of the 69 districts.Citation6 Twenty of both the national and localized RVF outbreaks in Kenya (1912–1989) scarcely involved human populations.Citation25 For instance, RVF epizootics from 1950 to 1951 in Kenya resulted in the deaths of an estimated 100 000 sheep, but without significant impact on human health.Citation5 Thereafter, RVF epizootics re-occurred in 1961 and 1964, and from 1989 to 1991 in farms that were <20 km from the site of the 1931 outbreak.Citation5 These outbreaks primarily involved domestic sheep and cattle, especially those imported to Kenya.Citation10 During the 1989 epizootics, 12 of the 30 herdsmen with detectable RVFV IgG (four with RVFV-specific IgM) had handled the affected animals,Citation10 but neither experienced clinical disease typical of RVF nor could recall any illness during the outbreak period. A previous report revealed low levels of RVFV antibody in human populations,Citation26 despite several reported cases of epizootics in East Africa in the early 1900s.Citation2, Citation27 In addition, the RVF epizootic in 1989 in Kenya occurred at a farm and involved 80%–90% of the cattle, but only two out of 26 herdsmen had detectable RVF antigen and had no clinical illness.Citation10
Table 1 RVF outbreaks in eastern Africa, 1912–2010
Similarly, Tanzania has experienced nine RVF epizootics between 1930 and 1997 without significant morbidity and mortality in humans.Citation3, Citation5 However, in Sudan, RVF epizootics that resulted in 100% morbidity and 40% mortality in livestock did coincide with human cases in 1973 and 1976.Citation13 However, these human cases were limited to two laboratory staff who had contact with the infected animals and developed a mild disease that resolved spontaneously within two weeks with neither complication nor death.Citation13 In Sudan, RVF was also implicated in a 1988–1989 outbreak of acute febrile illness that was primarily attributed to Sandfly fever Naples virus, a Phlebovirus that is closely related to RVF. However, this assessment was based on the fact that 26% of the 185 patients had RVFV IgG antibodies,Citation28 which are indicative of past RVF infections and cannot be used to identify causative agents of current infections.
Since the first major RVF outbreak in humans in 1977 in Egypt,Citation29 there have been several RVF outbreaks with significant impact on human health in eastern Africa. The 1997–1998 RVF outbreaks, which occurred in Kenya, Somalia and Tanzania simultaneously, resulted in an estimated 89 000 human infections and 478 deaths in Kenya and Somalia, but no human involvement was reported in Tanzania.Citation3, Citation5, Citation16, Citation17 These estimates were based on clinical features such as acute onset of fever and headache associated with hemorrhage (hematochezia, hematemesis and bleeding from other mucosal sites), but specimen from fatal cases were not available. However, 47% (17/36) of the blood samples from other ill patients who were tested at the National Institute of Virology, South Africa, and the Center for Disease Control and Prevention, USA, had acute RVFV infections detected by IgM antibodies, virus isolation, reverse-transcriptase PCR for viral nucleic acid or immunohistochemistry.Citation16 From 2006 to 2007, RVF outbreaks re-occurred with significant mortality in humans () in Kenya,Citation18, Citation19, Citation20, Citation30 SomaliaCitation20 and Tanzania,Citation5, Citation29 and eventually extended to Sudan in 2007 and 2008,Citation21, Citation22 and Madagascar in 2008.Citation23 RVF outbreaks re-occurred in Sudan in 2010, affecting both animals (abortion in ewes and does) and humans with a history of contact with aborted fetal material,Citation9, Citation24 but little information is available about these outbreaks. The RVF epidemiology during the 2007–2008 outbreaks in eastern Africa changed from being originally associated with livestock to considerably infecting humans and resulting in high fatality rates.Citation9 As the human RVF cases identified in more recent outbreaks have been more severe in terms of their associated morbidity and mortality, we postulate that insufficient surveillance and case finding, lack of diagnostic capabilities, poor health infrastructure, under-recognition, underreporting and underestimation by medical personnel and inadequate public awareness may not necessarily be the major determinants of the insignificant impact of RVF on human populations reported in East Africa in the early 1900s.
Human RVF outbreaks in other African countries in the 1900s were not significantly more extensive than what occurred in East Africa. In South Africa, symptomatic human RVF infection started in 1950 and 1951 with one case (no death) and in 1974 and 1975 with 110 cases (1 death),Citation31 but Mauritania and Senegal reported 220 deaths in 1987Citation32 and 300–400 cases (six deaths) in 1997 and 1998.Citation33 In Madagascar, the first and less severe RVF epizootic occurred in 1979 and re-occurred from 1990 to 1991Citation15 with a significant loss of animals, but no human involvement. However, another RVF epizootic in Madagascar in 2008 involved 476 human cases and 19 deaths (case fatality rate (CFR)=4%).Citation23 Interestingly, human involvement in the affected countries was initially limited to those who had contact with infected animals,Citation34 but was later extended with increasing severity to those who could not recall any contact with any animals in the subsequent epidemics.Citation35
In 1977 and 1978, RVF outbreaks occurred in Egypt, resulting in 20 000–200 000 estimated human cases with 600 deaths.Citation9, Citation29 In Saudi Arabia and Yemen, RVF outbreaks in 2000 involved 2171 human infections and 245 deaths.Citation36 It has been suggested that the RVF outbreaks in Saudi Arabia and Egypt were imported from Kenya and Sudan, respectively, through infected animals. The report that the same RVF virus strain was implicated in the 1997–1998 RVF outbreaks in Kenya as in the 2000 outbreaks in Saudi Arabia and YemenCitation36 seems to correlate with the 89 000 estimated human infections (478 deaths) in Kenya.Citation5, Citation16, Citation17 However, it is unclear how the same virus that caused only mild illness in humans with no complications in Sudan was implicated with a high incidence rate of human infection and death (75 000 estimated with 698 confirmed infections and 222 deaths) after it was imported to Egypt.Citation9 Notably, the 1989 RVF epizootics in Kenya were confirmed by virus isolation, plaque reduction neutralization tests and indirect fluorescent antibody tests, whereas earlier confirmatory tests were less stringent.Citation12 Although each country in East Africa now has a coordinated inter- and intra-sectoral outbreak response and disease mitigation strategies in place, the enormous negative impacts of each RVF outbreak demands new and systematic approaches to preventing or reducing the resultant outcomes if and when outbreaks do recur. An improved prediction algorithm with a lead period of >six months as well as integrated and sustainable approaches between different governmental sectors and organizations within and between countries are necessary to holistically address future RVF outbreaks.
Epidemiological risk factors associated with past RVF outbreaks in East Africa
Ecological factors
Unlike the majority of arboviruses that adapt to a narrow range of vectors, the RVFV infects a wide range of vectors including mosquitoes (with Aedes and Culex as the major vectors), flies and ticks.Citation37 Interestingly, different species of vectors have different roles in sustaining the transmission of RVFV in an environment.Citation38 Usually, flooded dambos (low-lying areas of soil) in East Africa induce the hatching of transovarially infected eggs of Aedes mosquitoes that are dormant in the soil, which serve as primary vectors (eggs can remain viable for several decades).Citation39 Hatched infectious mosquitoes transmit the virus to nearby livestock and wildlife vertebrate hosts, which serve as amplifiers of the virus, infecting more mosquitoes, and thereafter secondary vectors of the virus (Culex, Anopheles and Mansonia mosquitoes) amplify the transmission of the virus to non-infected domestic animals and humans.Citation40 Flooding in areas with a high density of livestock and/or wildlife creates a conducive environment for RVF transmission,Citation18 and under such conditions, the virus is maintained within the ecosystem. Other environmental factors such as canopy cover, dissolved oxygen, pH, turbidity, organic matter, salinity and temperature also influence the abundance of various mosquito vector species and arbovirus transmission.Citation41
RVF epidemiology in East Africa is closely associated with the ecological factors prevalent in the Great Rift Valley (a long depression in the earth that runs down the eastern side of Africa), which traverses Ethiopia and Kenya to northern Tanzania with two branches that form the eastern and western drainage ecosystems. The western branch runs through Tanzania and Uganda, and the eastern branch runs through Kenya and Tanzania.Citation42 Interestingly, the ten explosive RVF outbreaks in Tanzania between 1930 and 2007 occurred in the eastern wing of the Great Rift Valley of northern Tanzania.Citation5 The clay and loamy soil texture of the Rift Valley support long periods of water retention (flooding) and render it suitable for breeding primary mosquito vectors and the survival of their RVFV-infected eggs.Citation5 A significant association has been observed between RVF outbreaks from 1930 to 2007 and the clay and loamy soil textures in the eastern Rift Valley ecosystem of Tanzania, where clustering of RVF outbreaks has persistently and predominantly been detected.Citation3, Citation22 Unlike sandy soil, clay soil texture supports the retention of water for long periods of time, and thereby contributes to the flooding and wetness of the habitat, making it suitable for the breeding and survival of mosquito vectors.Citation5, Citation9
Climatic factors
Climate determines the geographic and temporal distribution and the life cycles of arthropod vectors as well as the dispersion and evolution of associated arboviruses. It also defines the efficiency with which arboviruses are transmitted from arthropods to vertebrate hosts.Citation43 Climatic variables indirectly affect vector abundance and distribution, and their ability to vector arboviral diseases.Citation44 One vector species may be displaced by another with a different vectorial capacity in response to environmental changes, such as deforestation, expansion in irrigation or increase in brackish water breeding sites due to rises in sea level.Citation45
Historical outbreaks in East Africa have been linked to periods of abnormally high rainfall with a few localized exceptions, such as the 1989 Kenyan outbreak that was related to local heavy rainfall at the focus of the outbreak.Citation10 Such conditions depend on El Niño/Southern Oscillation (ENSO)-related climate anomalies that are based on a combination of satellite measurements of elevated sea-surface temperatures and satellite-derived normalized difference vegetation index data.Citation46 These data were used to retrospectively predict RVF outbreaks in Kenya between 1950 and 1998,Citation47 and prospectively predicted the 2006–2007 RVF outbreaks in the Horn of Africa with 2–6-month lead times.Citation47 The association of RVF outbreaks with ENSO anomalies, which are increasing with climate change,Citation48 also has implications for the duration of future inter-epidemic periods (IEPs).
During past RVF outbreaks in East Africa, excessive rainfall that averaged 1720 mm (as experienced during El Niño years) in Kenya, Somalia and Tanzania led to flooding and increased vegetation cover that favored a high vector density and species diversity.Citation14 The flooding caused several lakes, rivers and dambos in the epicenters of RVF outbreaks to overflow.Citation49 Notably, a large proportion of RVFV, which remain dormant within the eggs of mosquitoes in the soil for several years, are hatched during flooding, resulting in intensified transmission of the virus to herds and human hosts within the environment during these outbreaks. However, the absence of RVF outbreaks during some IEPs in Kenya (ranging from one to seven years), despite heavy rainfall and flooding, indicates that other unknown drivers of RVF may exist in vulnerable ecologies. Nonetheless, IEPs may signal the time required for herd immunity to fall to levels permissive for the spread of the virus.Citation5, Citation9 Therefore, there is a need to definitively define all of the drivers of RVF outbreaks in vulnerable ecologies if future RVF outbreaks are to be abated.
The impact of rainfall on the presence, absence, size and persistence of breeding sites depends upon the local evaporation rates, soil type, slope of terrain and the proximity of large bodies of water (e.g., rivers, lakes and ponds), whereas wind has a significant effect on vector distribution.Citation50 Overall, high relative humidity favors most metabolic processes in vectors towards their prolonged survival, whereas low humidity tends to decrease their daily survival rate due to dehydration and dessication.Citation50 In some cases, low humidity and high temperatures accelerate the metabolic rate of a vector, increasing biting rates and frequency of blood feeding (in an attempt to compensate for high levels of water loss), which lead to enhanced egg production and increases in vector populations.Citation50 However, extremely high temperatures may be detrimental to vector populations.Citation50 Consequently, the geographical range or distribution of vectors tends to be limited by a minimum and maximum temperature/humidity. Although there is no clearly defined pattern of average annual maximum or minimum temperatures associated with RVF outbreaks, they have shown a tendency to cease as the maximum and minimum monthly temperatures decline.Citation5 Moreover, higher extrinsic incubation temperatures of RVF mosquito vector eggs (Aedes taeniorhynchus and Culex pipiens) have been shown to result in earlier RVFV dissemination and transmission,Citation51 which should increase their replication, transmission and evolutionary rates. We therefore speculate that global climate change may select for adaptive changes in the RVFV that may also influence its host range, virulence, pathogenicity and/or transmission efficacy.
Human behavioral factors
Land use changes such as deforestation, irrigation for farming, application of fertilizer in farms and the building of residential houses are strongly linked to the emergence and re-emergence of arboviral disease.Citation52 The majority of the RVF epizootics from 2006 to 2007 was livestock in pastoral and agro-pastoral farming systems.Citation16, Citation53 In addition, one of the RVF outbreaks in Sudan was linked to the construction of the dam of Merowe on the Nile River basin, as it provided new breeding sites for RVF vectors.Citation22 The inability of mosquitoes to fly more than a few hundred meters during their lifetime may limit their role in long-range disease dissemination.Citation54 However, uncontrolled livestock, human movement and/or the importation of animals can contribute to the spatiotemporal spread of RVF outbreaks from endemic to naive areas.Citation5 Generally, human RVF cases are uncommon in the absence of animal disease occurrence.Citation5 Nevertheless, through serosurveys, inter-epidemic human RVFV transmission has been reported in East Africa in the absence of reported or observed outbreaks.Citation55 However, because these studies were restricted to human populations, it is difficult to ascertain that an animal infection did not precede human exposure in the studied environment.
RVFV has been detected in both livestockCitation56, Citation57 and waterbuck during IEPs.Citation6 As wildlife, which has been considered to be a possible reservoir of RVF, live in close proximity to livestock, they may contribute to the amplification of the virus during epizootics.Citation6 Human RVF is significantly associated with direct contact with tissue, tissue fluids and mucous membranes of infected animals or animal products as well as with infectious fomites, small droplets and mosquito bites. Generally, RVFV infects humans by either inoculation (parenteral route) through a wound from contaminated surgical instruments or contact with broken skin or inhalation of aerosols produced during the slaughter or birthing of infected animals.Citation21 Previous studies have revealed that direct contact or handling of infected animals and being a herdsperson or veterinarian were significantly associated with acute RVFV infection in humans.Citation16, Citation30, Citation34 Touching an aborted animal fetus was found to be associated with severe RVF disease (hemorrhage, encephalitis or ocular disease), possibly due to an exposure to high quantities of RVFV through aerosolization of the virus when handling a carcass.Citation16, Citation58 This speculation is supported by a report that showed that laboratory workers acquired RVF through aerosol transmission.Citation55 In another report, consuming or handling products from sick animals was significantly associated with severe disease and death.Citation30 However, no study has shown that RVFV is transmitted through the oral–fecal route. Therefore, ingestion of uncooked infected animals or unpasteurized milk may not necessarily drive transmission, as previously reported.Citation30, Citation34 Nonetheless, as consuming or handling products from sick animals has been found to be significantly associated with acute RVF infection, severe illness and death,Citation17 inhalation of infectious aerosols in the process of preparing, handling and consuming these uncooked infected animals or their products may be the major mode of transmission of RVFV to humans. Indeed, aerosol exposure to RVFV causes earlier and more severe neuropathology in the mice.Citation59 The transmission of RVFV to humans through mosquito bites is limited, but it may be very difficult to quantify because the bites may have gone unnoticed.Citation14
Other human behavioral factors that favor RVF spread during outbreaks include poor inter-ministerial collaboration and inadequate surveillance of livestock and human populations due to limited resources.Citation36, Citation58 The severity of RVF epidemics has been exacerbated by delays in recognizing the risk factors and in making timely decisions to prevent and control the disease.Citation60 In addition, insufficient data on the herd immunity level in both animal and human populations, and the lack of entomological surveillance to identify risk-prone areas in some vulnerable ecologiesCitation61 may contribute to frequent RVF outbreaks.
The delayed implementation of outbreak responses and other disease mitigation strategies tends to exacerbate the impacts of a disease on lives, livelihoods and local, national and regional economies. Due to limited resources, the inability of veterinary authorities and vaccine manufacturers to maintain stocks of vaccine, which have short shelf-lives and could expire before they are used or sold, has contributed to the spread of the disease and the associated socioeconomic impact.Citation3 Although the existing livestock vaccineCitation62 has a shelf-life of ~4 years, the IEPs between major outbreaks can be up to 10 years or even 20 years.Citation3 However, the procurement of vaccines when epidemics/epizootics have already started could be time-consuming, allowing the disease to spread in the meantime. The production of effective livestock vaccines that have long shelf-lives to conform to the episodic nature of RVF has become a necessity. The prediction algorithm that successfully predicted the 2006–2007 RVF outbreaks in AfricaCitation47 resulted in a lead period that was not long enough for a sustainable and effective implementation of disease mitigation strategies.Citation47
Genetic evolution of RVFV
The widespread epizootics of RVF among ruminants in the early twentieth century were not accompanied by similar epidemics in human populations.Citation14 The evolutionary rate of RVFV has diversified an ancestral virus that existed 120–130 years ago into multiple extant RVFV strains.Citation63 Therefore, the past 100+ years since RVFV was first identified have been sufficient for major evolutionary changes to occur through mutation and genetic reassortment. However, low genetic diversity of the virus was reported during the 1977, 1983 and 2006–2007 epidemics in Egypt, MauritaniaCitation63 and East Africa,Citation40 respectively. According to Bird et al.,Citation63 RVFV genetic diversity primarily involves the accumulation of mutations at an average of 2.9 × 10−4 substitutions per site per year, with some evidence of RNA segment reassortment.
The apparent stability of the RVFV genome has been attributed to high vertical transmission rates and the negative selection pressure exerted by diverse alternating vertebrate hosts and arthropod vectors. However, the low nucleotide diversity observed may be due to the limited diversification time from a recent common ancestor rather than the stability of the genome,Citation64 especially considering the low replication rates during IEPs when large proportions of the virus are dormant in mosquito eggs. Indeed, the genetic diversity of RVFV isolates has computationally coalesced to a single putative ancestral sequence from ~1880–1890,Citation65 only 40–50 years before RVF was first identified in 1931.Citation2 However, purifying negative selection may lead to an underestimation of the age of viral lineages.Citation66 Because RVFV can persist in dormant eggs for long periods of time, outbreaks in endemic areas are associated with an intensified transmission of multiple lineages, albeit with low divergenceCitation40, Citation64 and larger epidemic disease incidence.Citation66 In contrast, RVF outbreaks in naive ecologies (Egypt in 1977, Mauritania in 1983, and Saudi Arabia and Yemen in 2000) have been associated with newly introduced single lineages of RVFV with minimal genetic diversityCitation63 through animal movement and mosquito vectors. A mathematical model to assess the spread of RVF revealed that a lack of herd immunity allowed the virus to expand geographically, leading to longer transmission intensification periods and delayed infection rates before it became an epidemic, thus involving many dispersed vulnerable ecologies.Citation65
A possible instability of the RVFV genome has been demonstrated by a report showing that the glycoprotein (Gn) (associated with virus attachment and entry into cells) sequences of the isolates involved in the 2006–2007 outbreak had as high as 2.2% amino-acid substitutions compared with isolates from the outbreaks between 1944 and 2002.Citation40 Six of these amino-acid substitutions represented distinct mutations from all known historical viruses, and one of these, a glycine-to-arginine substitution at position 216 of the Gn protein, was found only in isolates from the virulent human infections.Citation40 The emergence of potentially more virulent and pathogenic virus genotypes can occur within short time frames, resulting from the of only a few key amino acids.Citation40 This speculation is supported by reports of other arboviruses in which amino-acid substitutions significantly have been shown to alter their behavior and virulence in recent decades. In the USA, neuroinvasive and non-virulent strains of West Nile virus differ by five amino acids in their envelope proteins.Citation67 Similarly, a single nucleotide change in the envelope protein (E1-A226V) of the Chikungunya virusCitation68 has been associated, in part, with increased fitness in a new vector species (Aedes albopictus) in a region that lacked the typical Aedes aegypti vector, as well as with viral infectivity and dissemination and transmission rates.Citation69 Thus, any slight change in the genetic constitution of an arbovirus should not be ignored. Indeed, a single amino-acid change in a virus genome can act through multiple phenotypic effects to create an epidemic.Citation70 Therefore, we speculate that the relatively small changes in RVFV genomes and the accumulation of these changes over several decades could have influenced its virulence (as estimated by the incidence and CFRs), host preferences, vector competence and transmission rates. Such changes could facilitate its establishment in new ecologies following its introduction through animal movement and adaptation to available vector populations.
In addition, the evolution of RVFV strains may be enhanced by its segmented genome’s propensity to undergo segment reassortment in nature. Partial sequences of all three RNA segments have shown evidence of reassortment in SSA RVFV isolates.Citation71 The convergence of some lineages within genome segments implies that reassortment has played an evolutionary role in the history of RVFV.Citation40 Co-infection with different RVFV strains may facilitate the formation of reassortant RVFV chimera that may proliferate in the environment as they are taken up and transmitted by vectors.Citation72 A reassortant RVFV was reported to differ from all other isolates during the 1977 RVF outbreak in Egypt, but it showed similarities with strains that caused human deaths.Citation64 Interestingly, such reassortment has also been hypothesized to be responsible for the increased virulence of RVF in humans in an original report.Citation29 If attenuated RVF vaccines with unattenuated segments are used for vaccination during outbreaks, the chances of co-infection between wild and vaccine strains may be increased. Consequently, the diversification of RVFV genotypes through reassortment into chimeric viruses with unpredictable levels of virulence could be accelerated. It is therefore important to use vaccines in which all three segments are attenuatedCitation73 and/or are replication deficient.Citation74 In addition, limiting the vaccination of livestock to during IEPs could decrease the chances of co-infection between wild and vaccine strains of RVFV.
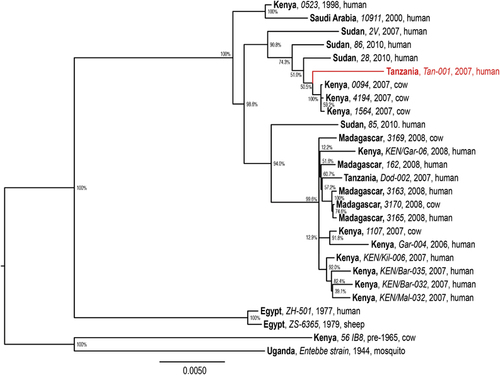
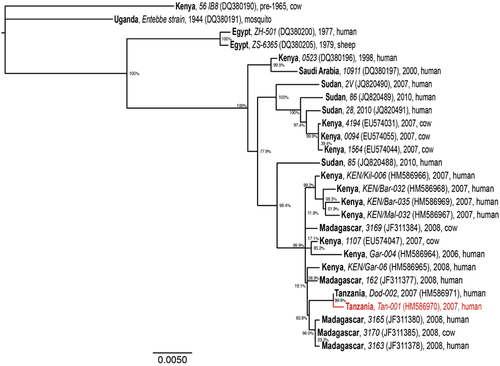
The molecular phylogeny of RVFV genomes (Figure 2) demonstrates the diversification of distinct RVFV lineages within East Africa, especially within the past decade, during which RVF outbreaks have had a greater impact on human health. We found that the genomic phylogeny (Figure 2) corresponds closely with those of the S and L segments (not shown); however, the M segment phylogeny (Figure 3) reveals clear reassortment of the Tanzanian Tan-001 isolate from 2007, for which only the M segment clusters with the other Tanzanian isolate (Dod-002) among isolates from Madagascar. The isolate’s L and S segments, as shown in the full genome phylogeny (Figure 2), cluster among the isolates from Kenya and Sudan. In contrast, all three segments of the Dod-002 isolate cluster among isolates from Madagascar. Reassortant RVFVs have also been reported among Kenyan isolatesCitation63 and with increased pathogenicity in humans in South Africa.Citation64 The Madagascar isolate 3169 might also be a reassortant, as its full genome is most closely related to other Madagascar RVF viruses (Figure 2), whereas its M segment clusters among Kenyan isolates (Figure 3). This latter case is much more subtle and inconclusive, but it shows that reassortment may be occurring more frequently among more closely related RVF genomes than can be identified phylogenetically. The diversification of RVFV genomes through mutation and assortment mechanisms could have generated lineages that are virulent in humans,Citation40 some of which may potentially induce abortion and neonatal mortality in humans.Citation76 Although ecological factors and human behaviors may impact the frequency and location of its occurrence, genetic factors significantly impact the virulence of the virus as well as the disease severity in its hosts.
Socioeconomic impacts of RVF outbreaks in East Africa
The increase in human mortality and morbidity associated with RVF outbreaks in the past two decades has exacerbated both their social and economic impacts. Although human RVF mortality was low (CFR <1%) during the twentieth century, mortality rates rose to as high as 23%–47% during the East African 2006 and 2007 outbreaks, possibly due to the genetic evolution of the virus. Even for RVF survivors, the neurological and visual complications are likely to be lifelong, with a considerable economic impact due to a loss in disability-adjusted life years (DALYs) estimated to range between 353–11 958 and 188–6530 for 2005.Citation77 These DALY estimates for RVF were deduced from the possibly lifelong neurological and visual complications/blindness that caused substantial economic impact in RVF survivors.Citation55 Hospitalization due to arboviral diseases results in both monetary loss and loss of time, with a significant impact of deaths on families, communities and countries in the region. Similarly, the psychological distress caused by RVF on the affected families is unquantifiable, and the loss of a productive member of the family in terms of labor, income and parental care results in serious consequences. Those who were seriously affected by the outbreak claimed that RVF represented a worse threat to health than the commonly dreaded human immunodeficiency virus/acquired immune deficiency syndrome.Citation14
RVF is known to induce abortions and perinatal mortality (>95%) in herds used for meat, dairy production and income generation, leading to reduced food availability and income. These effects are more severe on poorer dwellers who lack alternative sources of livelihood.Citation23 In Tanzania, the loss of livestock during the 2006–2007 RVF outbreak was estimated to be US $ 4 243 250 for cattle and US $ 2 202 467 for goats and sheep.Citation30 The imposition of trade bans for three years on the exportation of live animals from the Horn of Africa exacerbated the impact of RVF-induced losses. In Tanzania, the export of cattle dropped by 54% during the 2006–2007 RVF outbreak, and the domestic livestock market flow dropped by 37%. In Kenya, the loss was estimated at over US $32 million.Citation78 The overall economic loss during the 2006–2007 RVF outbreaks in East Africa was estimated to exceed $60 million.Citation79 Such enormous losses are catastrophic to low-income and impoverished countries, as the governments of the affected countries are compelled to mobilize funds from different sources to contain the outbreak. In addition, the loss of earnings from the livestock trade results in a lack of finance for basic amenities such as education, health, food and shelter.Citation80 Furthermore, movement bans on animals have caused animal traders to incur additional costs by keeping the animals they purchased before the bans were enforced.Citation14 The associated loss of income for herd owners has damaged the pride, prestige, integrity and self-importance of livestock owners among their peers. Livestock traders were forced to rely on their past savings to such an extent that they lacked the financial capital to resume trading activities, even when the outbreak was contained.Citation14 Overall, the political, psychological and economic implications of RVF may have contributed to an intentional underreporting of the symptoms, thus confounding the estimates of the size and impact of outbreaks.
Figure 2 Maximum likelihood phylogenetic tree of complete RVFV genome sequences (combined L (GenBank accessions: DQ375400–1, DQ375406, DQ375410, DQ375427, DQ375429, EU574004, EU574017, EU574020, EU574029, HM586953–60, JF311371, JF311375–6 and JQ820488–91), M (GenBank accessions: DQ380190–1, DQ380196–7, DQ380200, DQ380205, JQ820488–91, EU574031, EU574044, EU574047, EU574055, HM586964–71, JF311368–9, JF311371, JF311375–6 and JQ820483–6) and S segments (GenBank accessions: DQ380145, DQ380149, DQ380156, DQ380169–70, DQ380176, EU574057, EU574086, EU574072, EU574075, HM586975–82, JF311386–7, JF311389, JF311393–4, JQ820472, JQ820474, JQ820476 and JQ820477)) analyzed using PhyML v. 3.0.Citation75 The phylogenies employed the General Time-Reversible nucleotide substitution (rate categories=4) model, in which the base frequencies and the relative substitution rates between them were estimated by maximizing the likelihood of the phylogeny. For estimating the tree topology, both nearest-neighbor interchange and sub-tree pruning and regrafting improvements were used. Country (bold), isolate identification (italics), year and host are indicated for all 27 sequences analyzed. Bootstrap values at the major nodes are expressed as percentage agreement among 1000 replicates. The branch length scale represents substitutions per site. A red sequence indicates a reassortant isolate.
Conclusions
Since its discovery in 1912 and the 1930s, RVF has undergone continued geographic expansion through East Africa with increasing human disease. Persistent and episodic outbreaks of RVF caused by different lineages of the virus that localize in particular areas have occurred in several eastern African countries. The endemic areas of RVF may periodically experience outbreaks and serve as the index foci for future outbreaks in non-endemic environments. The epidemiology of past RVF outbreaks demonstrates a complex interaction between environmental, viral and host factors. The RVFV that primarily caused the epizootics in East Africa in the twenty-first century has evolved from an ancestral virus that existed 100–200 years ago into multiple extant strains that have caused increased disease severity in humans. The evolution of RVFV through mutation and reassortment and the accumulation of these changes over several decades may have changed the disease epidemiology, increasing its geographic distribution and severity in human populations. Therefore, understanding RVF epidemiology and its clinical symptoms in humans, as determined by its evolution, is an essential step in developing new strategies for outbreak mitigation and prevention of future human RVF casualties. The enormous human health and socioeconomic impacts, including basic food security, that are imposed by RVF make it imperative to adopt more stringent and effective measures to mitigate its impacts.
Figure 3 Maximum likelihood phylogenetic tree of complete RVFV M segment sequences. Country (bold), isolate identification (italics), GenBank accession (in brackets), year and host are indicated for all 27 sequences analyzed. Bootstrap values at the major nodes are expressed as percentage agreement among 1000 replicates. The branch length scale represents substitutions per site. A red sequence indicates a reassortant isolate.
This work was supported by stipends paid to Marycelin Baba from the Institute of International Education Scholar Rescue Fund (IIE-SRF) and the German Academic Exchange Service (DAAD; ref: CBID/DAAD-PDF-MB/LET0514). We wish to extend our appreciation to the University of Maiduguri, Nigeria, for supporting this work at International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya. The technical support of Benard Kulohoma (ICIPE) and David Makori (ICIPE) are highly appreciated. We are grateful to Maamun Jeneby (Institute of Primate Research, Nairobi, Kenya) and Geofrey Jagero (ICIPE) for their moral and technical support.
- StordyRJ.Annual Report Department of Agriculture, British East Africa: 1912–1913.London: HMSO.1913,35.
- DaubneyR,HudsonJ,GarnhamP.Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep cattle and man from East Africa.J Pathol Bacteriol1931; 34:545–579.
- FyumagwaR,EzekielM,NyakiAet al.Response to Rift Valley fever in Tanzania: challenges and opportunities.Tanzan J Health Res2011; 13(Suppl 1):1–9.
- DarO,McIntyreS,HogarthSet al.Rift Valley fever and a new paradigmof research and development for zoonotic disease control.Emerg Infect Dis2013; 19:189–193.
- SindatoC,KarimuriboED,PfeifferDUet al.Spatial and temporal pattern of Rift Valley fever outbreaks in Tanzania; 1930 to 2007.PLoS ONE2014; 9:e88897.
- MurithiRM,MunyuaP,IthondekaPMet al.Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts.Epidemiol Infect2011; 139:372–380.
- JavanbakhtJ,MardjanmehrS,TavasolyAet al.Neuropathological microscopic features of abortions induced by Bunyavirus or Flavivirus infections.Diagn Pathol2014; 9:223.
- AdamI,KarsanyMS.Case report: Rift Valley fever with vertical transmission in a pregnant Sudanese woman.J Med Virol2008; 80:929.
- HimeidanYE,KwekaEJ,MahgoubMMet al.Recent outbreaks of Rift Valley fever in East Africa and the Middle East.Front Public Health2014; 2:169.
- LoganTM,DaviesFG,LinthicumKJet al.Rift Valley fever antibody in human sera collected after an outbreak in domestic animals in Kenya.Trans R Soc Trop Med Hyg1992; 86:202–203.
- SchwentkerFF,RiversTM.Rift Valley fever in man.J Exp Med1934; 59:305–313.
- DaviesFG,LoganTM,BinepalYet al.Rift Valley fever virus activity in East Africa in 1989.Vet Rec1992; 130:247–249.
- EisaM,Kheir el-SidED,ShomeinAMet al.An outbreak of Rift Valley fever in the Sudan—1976.Trans R Soc Trop Med Hyg1980; 74:417–419.
- SindatoC,KarimuriboE,MboeraL.The epidemiology and socio-economic impact of Rift Valley fever epidemics in Tanzania: a review.Tanzan J Health Res2011; 13(Suppl 1):1–16.
- MorvanJ,SaluzzoJF,FontenilleDet al.Rift Valley fever on the east coast of Madagascar.Res Virol1991; 142:475–482.
- WoodsCW,KarpatiAM,GreinTet al.World Health Organization Hemorrhagic Fever Task Force. An outbreak of Rift Valley fever in Northeastern Kenya, 1997-98.Emerg Infect Dis2002; 8(2):138–144.
- Centers for Disease Control and Prevention (CDC).Rift Valley fever—East Africa 1997–1998.MMWR Morb Mortal Wkly Rep1998; 47:261–264.
- NgukuP,SharifS,MutongaDet al.An investigation of a major outbreak of Rift Valley fever in Kenya, 2006-2007: clues and enigmas concerning Rift Valley fever outbreaks and their prevention.Am J Trop Med Hyg2010; 83(Suppl 2):5–13.
- Centers for Disease Control and Prevention (CDC).Rift Valley fever outbreak—Kenya, November 2006–January 2007.MMWR Morb Mortal Wkly Rep2007; 56:73–76.
- World Health OrganizationRift Valley fever in Kenya, Somalia and the United Republic of Tanzania. In:Global Alert and Response.Geneva: WHO.2007Available athttp://www.who.int/csr/don/2007_05_09/en/accessed 29 May 2015.
- AdamAA,KarsanyMS,AdamI.Manifestations of severe Rift Valley fever in Sudan.Int J Infect Dis2010; 14:e179–e180.
- HassanOA,AhlmC,SangRet al.The 2007 Rift Valley fever outbreak in Sudan.PLoS Negl Trop Dis2011; 5:e1229.
- AndriamandimbySF,Randrianarivo-SolofoniainaAE,JeanmaireEMet al.Rift Valley fever during rainy seasons, Madagascar, 2008 and 2009.Emerg Infect Dis2010; 16:963–970.
- AradaibIE,EricksonBR,ElagebRMet al.Rift Valley fever, Sudan, 2007 and 2010.Emerg Infect Dis2013; 19:246–253.
- DaviesFG.Observations on the epidemiology of Rift Valley fever in Kenya.J Hyg (Lond)1975; 75:219–230.
- JohnsonBK,OchengD,GichogoAet al.Antibodies against haemorrhagic fever viruses in Kenya populations.Trans R Soc Trop Med Hyg1983; 77:731–733.
- MetselaarD,HendersonBE,KiryaGBet al.Isolation of arboviruses in Kenya, 1966-1971.Trans R Soc Trop Med Hyg1974; 68:114–123.
- WattsDM,el-TiganiA,BotrosBAet al.Arthropod-borne viral infections associated with a fever outbreak in the northern province of Sudan.J Trop Med Hyg1994; 97:228–230.
- MeeganJM.The Rift Valley fever epizootic in Egypt 1977-78. Description of the epizzotic and virological studies.Trans R Soc Trop Med Hyg1979; 73:618–623.
- AnyanguAS,Hannah GouldL,SharifSKet al.Risk factors for severe Rift Valley fever infection in Kenya, 2007.Am J Trop Med Hyg2010; 83(2 Suppl):14–21.
- Van VeldenDJ,MeyerJD,OlivierJet al.Rift Valley fever affecting humans in South Africa: a clinicopathological study.S Afr Med J1977; 51:867–871.
- DigoutteJP,PetersCJ.General-aspects of the 1987 Rift Valley fever epidemic in Mauritania.Res Virol1989; 140:27–30.
- CaminadeC,NdioneJA,DialloMet al.Rift Valley fever outbreaks in Mauritania and related environmental conditions.Int J Environ Res Public Health2014; 11:903–918.
- ArcherB,ThomasJ,WeyerJet al.Epidemiologic investigations into Outbreaks of Rift Valley fever in humans, South Africa, 2008–2011.Emerg Infect Dis2013; 19:1918–1925.
- McIntoshBM,RussellD,dos SantosIet al.Rift Valley fever in humans in South Africa.S Afr Med J1980; 58:803–806.
- ShoemakerT,BoulianneC,VincentMJet al.Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000-01.Emerg Infect Dis2002; 8:1415–1420.
- PepinM,BouloyM,BirdBHet al.Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention.Vet Res2010; 41:61.
- TchouassiDP,BastosAD,SoleCLet al.Population genetics of two key mosquito vectors of Rift Valley Fever virus reveals new insights into the changing disease outbreak patterns in Kenya.PLoS Negl Trop Dis2014; 8(12):e3364.
- YatesWW.The effect of drying on the viability of Aedes mosquito egg.Mosq News1945; 5:98–99.
- NderituL,LeeJS,OmoloJet al.Sequential Rift Valley fever outbreaks in eastern Africa caused by multiple lineages of the virus.J Infect Dis2011; 203:655–665.
- OfullaA,KaranjaD,OmondiRet al.Relative abundance of mosquitoes and snails associated with water hyacinth and hipp grass in the Nyanza gulf of Lake Victoria.Lakes Reserv Res Manag2010; 15:255–271.
- MorleyC.Tectonic evolution of the East African Rift System and the modifying influence of magmatism: a review.Acta Vulcanol1999; 11:1–19.
- GouldEA,HiggsS.Impact of climate change and other factors on emerging arbovirus diseases.Trans R Soc Trop Med Hyg2009; 103:109–121.
- TabachnickWJ,DayJF.The impact of climate change on vector-borne arboviral episystems. In: Singh SK editor.Viral Infections and Global Change.NJ Hoboken: John Wiley & Sons, Inc; 2014, pp22–34.
- McMichaelAJ,Campbell-LendrumDH,CorvalánCFet al.Climate Change and human health: Risks and responses.Geneva: World Health Organization.2003Available athttp://www.who.int/globalchange/publications/climchange.pdfaccessed 8 March 2016.
- LinthicumKJ,AnyambaA,TuckerCJet al.Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya.Science1999; 285:397–400.
- AnyambaA,ChretienJ-P,SmallJet al.Prediction of a Rift Valley fever outbreak.Proc Natl Acad Sci USA2009; 106:955–959.
- TimmermannAJ,OberhuberA,BacherMet al.Increased El Niño frequency in a climate model forced by future greenhouse warming.Nature1999; 398:694–697.
- SangR,KiokoE,LutomiahJet al.Rift Valley fever virus epidemic in Kenya, 2006/2007: the entomologic investigations.Am J Trop Med Hyg2010; 83(2 Suppl):28–37.
- MellorPS,LeakeCJ.Climatic and geographic influences on arboviral infections and vectors.Rev Sci Tech Int Off Epizoot2000; 19:41–54.
- TurellMJ,RossiCA,BaileyCL.Effect of extrinsic incubation temperature on the ability of Aedes taeniorhynchus and Culex pipiens to transmit Rift Valley fever virus.Am J Trop Med Hyg1985; 34(6):1211–1218.
- HassanainAM,NoureldienW,KarsanyMSet al.Rift Valley Fever among febrile patients at New Halfa hospital, eastern Sudan.Virol J2010; 7:97.
- ChengulaAA,MdegelaRH,KasangaCJ.Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania.SpringerPlus2013; 2:549.
- YamarBA,DialloD,KebeCMFet al.Aspects of bioecology of two Rift Valley fever virus vectors in Senegal (West Africa): Aedes vexans and Culex poicilipes (Diptera: Culicidae).J Med Entomol2005; 42:739–750.
- LaBeaudAD,MuchiriEM,NdzovuMet al.Interepidemic Rift Valley fever virus seropositivity, northeastern Kenya.Emerg Infect Dis2008; 14:1240–1246.
- SumayeRD,GeubbelsE,MbeyelaEet al.Inter-epidemic transmission of Rift Valley fever in livestock in the Kilombero River Valley, Tanzania: a cross-sectional survey.PLoS Negl Trop Dis2013; 7:e2356.
- LichotiJK,KiharaA,OrikoAAet al.Detection of Rift Valley fever virus interepidemic activity in some hotspot areas of Kenya by sentinel animal surveillance, 2009-2012.Vet Med Int2014; 2014:379010.
- BalkhyHH,MemishZA.Rift Valley fever: an uninvited zoonosis in the Arabian peninsula.Int J Antimicrob Agents2003; 21:153–157.
- ReedC1,LinK,WilhelmsenCet al.Aerosol exposure to Rift Valley fever virus causes earlier and more severe neuropathology in the murine model, which has important implications for therapeutic development.PLoS Negl Trop Dis2013; 7:e2156.
- MunyuaP,MurithiRM,WainwrightSet al.Rift Valley fever outbreak in livestock in Kenya, 2006-2007.Am J Trop Med Hyg2010; 83(2 Suppl):58–64.
- BreimanRF,MinjauwB,SharifSKet al.Rift Valley fever: scientific pathways toward public health prevention and response.Am J Trop Med Hyg2010; 83(2 Suppl):1–4.
- SmithburnKC.Rift Valley fever: the neurotropic adaptation of the virus and the experimental use of this modified virus as a vaccine.Br J Exp Pathol1949; 30:1–16.
- BirdBH,KhristovaML,RollinPEet al.Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry.J Virol2007; 81:2805–2816.
- GrobbelaarAA,WeyerJ,LemanPAet al.Molecular epidemiology of Rift Valley fever virus.Emerg Infect Dis2011; 17:2270–2276.
- XueL,CohnstaedtLW,ScottHMet al.A hierarchical network approach for modeling Rift Valley fever epidemics with applications in North America.PLoS ONE2013; 8:e62049.
- WertheimJO,KosakovskyPSL.Purifying selection can obscure the ancient age of viral lineages.Mol Biol Evol2011; 28:3355–3365.
- BeasleyDWC,DavisCT,WhitemanMet al.Molecular determinants of virulence of West Nile virus in North America.Arch Virol2004; 18:35–41.
- TsetsarkinKA,VanlandinghamDL,McGeeCEet al.A single mutation in chikungunya virus affects vector specificity and epidemic potential.PLoS Pathog2007; 3:e201.
- TsetsarkinKA,ChenR,LealGet al.Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes.Proc Natl Acad Sci USA2011; 108:7872–7877.
- WolfeND,DunavanCP,DiamondJ.Origins of major human infectious diseases.Nature2007; 447:279–283.
- SallAA,ZanottoPM,SeneOKet al.Genetic reassortment of Rift Valley fever virus in nature.J Virol1999; 73:8196–8200.
- TurellMJ,SaluzzoJF,TammarielloRFet al.Generation and transmission of Rift Valley fever viral reassortants by the mosquito Culex pipiens.J Gen Virol1990; 71:2307–2312.
- IkegamiT,HillTE,SmithJKet al.Rift Valley fever virus MP-12 vaccine is fully attenuated by a combination of partial attenuations in the S, M and, L segments.J Virol2015; 89:7262–7276.
- WarimweGM,LorenzoG,Lopez-GilEet al.Immunogenicity and efficacy of a chimpanzee adenovirus-vectored Rift Valley fever vaccine in mice.Virology J2013; 10:349.
- GuindonS,DufayardJF,LefortVet al.New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0.Syst Biol2010; 59:307–321.
- NiklassonB,LiljestrandJ,BergströmSet al.Rift Valley fever: a sero-epidemiological survey among pregnant women in Mozambique.Epidemiol Infect1987; 99:517–522.
- LabeaudAD,BashirF,KingCH.Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections.Popul Health Metr2011; 9:1.
- KarlMR,FrancisW.An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya.Am J Trop Med Hyg2010; 83(2 suppl):52–57.
- LittlePD.Hidden value on the hoof: cross-border livestock trade in eastern Africa. Common Market for Eastern and Southern Africa Comprehensive African Agriculture Development Program, policy brief number 2, February 2009.Midrand: CAADP, 2009.Available athttp://www.caadp.net/pdf/COMESA%20CAADP%20Policy%20Brief%202%20Cross%20Border%20Livestock%20Trade%20(2)(accessed 7 October 2015).
- HotezPJ,KamathA.Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden.PLoS Negl Trop Dis2009; 3:e412.