Dear Editor,
Tuberculosis (TB) remains a leading cause of morbidity and mortality globally despite the availability of the TB therapy.Citation1 The current TB therapy is lengthy and suboptimal, requiring a treatment time of at least six months for drug-susceptible TB and 9–12 months (shorter Bangladesh regimen) or 18–24 months (regular regimen) for multi-drug-resistant tuberculosis (MDR-TB).Citation1 The lengthy therapy makes patient compliance difficult, which frequently leads to emergence of drug-resistant strains. The requirement for the prolonged treatment is thought to be due to dormant persister bacteria which are not effectively killed by the current TB drugs, except rifampin and pyrazinamide (PZA) which have higher activity against persisters.Citation2, Citation3 Therefore, new therapies should address the problem of insufficient efficacy against Mycobacterium tuberculosis persisters, which could cause relapse of clinical disease.Citation4 PZA is a critical frontline TB drug that kills persister bacteria,Citation5, Citation6, Citation7 and shortens the TB treatment from 9–12 months to six months.Citation6, Citation7 Although, several new TB drugs are showing promise in clinical studies, none can replace PZA, as they all have to be used together with PZA.Citation7 Because of the essentiality of PZA and the high cost of developing new drugs, in this study, we explored the idea of identifying drugs that enhance the anti-persister activity of PZA as an economic alternative approach to developing new drugs for improved treatment by screening a clinical compound library against old M. tuberculosis cultures enriched with persisters.
M. tuberculosis strain H37Ra was cultured in 7H9 medium (pH 6.8) with 10% albumin-dextrose-catalase (ADC) and 0.05% Tween 80 for three months. Then, the culture was washed and resuspended in acidic 7H9 medium (pH 5.5) without ADC. Bacterial suspension (~107 colony forming unit (CFU)/mL) was exposed to 100 μg/mL PZA and transferred to 96-well microplates for drug screens with a clinical drug library. The drug library consisting of 1524 compounds Citation8 was added to the 3-month-old cells at a final concentration of 50 μM in the drug screen. The plates were incubated in a 37 °C incubator without shaking. At three, five or seven days post drug exposure, a 96-pin replicator was used to transfer the bacterial suspension onto 7H11 agar plates to monitor the bacterial survival as described.Citation9 The combination of N,N'- dicyclohexylmethanediimine (DCCD) (100 μg/mL) and PZA was used as a positive control based on our previous study,Citation10 and 5% dimethyl sulfoxide in each plate was included as a negative control.
The screen identified 130 molecules from the clinical compound library that showed anti-persister activity in the PZA combination screen, where 83 of them are FDA-approved drugs (Supplementary Table S1). On the basis of the results of the primary screen, we selected these active drug candidates for rescreens using the same method above, and the results were found to be reproducible. The 130 compounds included 21 antibiotics, 24 antibacterials, 11 antiseptics, five antineoplastics, nine antifungals, ten anthelmintics, eight antiinflammatories, three vitamins and 39 other drugs including antimalarial, antihistamine, antiulcerative and anti-hypertensive agents (Supplementary Table S1). The current anti-TB drugs isoniazid, streptomycin and para-aminosalicylate had limited activity. However, rifampin, clofazimine, and the second-line drugs amikacin, ofloxacin, levofloxacin, moxifloxacin, gatifloxacin, as well as other fluoroquinolones tosufloxacin, enrofloxacin and fleroxacin had good activity. Tetracycline drugs (tetracycline, doxycycline, minocycline, meclocycline, chlortetracycline) had significant activity. Nitroxoline (5-nitro-8-hydroxyquinoline), an oral antibiotic which is used to treat urinary tract infections in Europe and has good activity against biofilm infections, was found to be quite active. Other hydroxyquinoline drugs such as clioquinol and cloxyquin, sulfa drug dapsone, and antimalarial drug atovaquone, and antifungal nifuroxime also had activity. Moreover, azole drugs (miconazole, clotrimizole, oxiconazole, sulconazole) had good activity against the 3-month-old M. tuberculosis culture, which is consistent with our previous findings.Citation11 Anti-inflammatory agents such as nonsteroidal anti-inflammatory drugs (NSAIDs) acemetacin, indomethacin, meclofenamic acid, tolfenamic acid, flufenamic acid, meloxicam had good activity, which is in keeping with our previous observation that salicylate (aspirin) and ibuprofen could enhance PZA activity.Citation12 Acid inhibitor omeprazole and lansoprazole used to treat peptic ulcer also had activity in the screen. Interestingly, membrane-active essential oils such as olive oil, cod liver oil, storax and Thymol (2-isopropyl-5-methylphenol), a natural monoterpene phenol derivative of cymene, which is isomeric with carvacrol known to have antibacterial activity via membrane disruption, were also found to have good activity (Supplementary Table S1). Monesin, thiostrepton, silver, antihelmintic drugs (closantel, oxantel, tetramisole, pyrvinium pamoate), as well as antiseptic agents thonzonium bromide, pyrithione zinc, benzalkonium chloride, cetylpyridinium, methylbenzalkonium chloride, were also found in this screen to have good activity against the 3-month-old M. tuberculosis culture. However, while these agents may be useful for mechanistic studies, they are either too toxic to be used or topical agents not easily bioavailable.
In an independent screen with lower 10 μM drug concentration of the clinical compound library combined with 100 μg/mL PZA treated for three days, 37 hits were identified to enhance PZA activity (see bold type drugs in Supplementary Table S1), including clinically used drugs clofazimine, rifampin, clotrimazole, doxycycline, tosufloxacin, fleroxacin, nitroxoline, nifuroxime, diacerein, tolfenamic acid, pipemidic acid, benzbromarone, rose bengal and cod liver oil and so on. The overlapping drug candidates identified from two different screens at 10 and 50 μM respectively further confirm the reliability of the resutls.
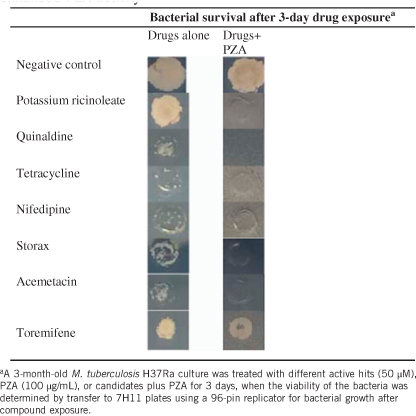
Since most of the 130 active hits at 50 μM had varying activity against the 3-month-old M. tuberculosis alone, it would be very time-consuming to evaluate each individual hit for their enhancement of PZA activity. Therefore, we focused our attention on drug candidates which had limited activity alone, but in combination with PZA produced no surviving bacteria with the 3-month-old M. tuberculosis culture (Table ). This allowed us to identify three drug candidates (tetracycline, nifedipin and acemetacin). Non-drug molecules (potassium ricinoleate, quinaldine and storax) were also identified. However, the DCCD positive control did not show the expected result of enhancement of PZA activity due to high concentration (100 μg/mL), which killed the bacteria completely when used alone. Ricinoleic acid (12-hydroxy-9-cis-octadecenoic acid) is a fatty acid that has membrane-disruptive activity, and is quite safe for use in food even at 2.4 g/day. The finding that ricinoleic acid could enhance PZA activity is consistent with previous observation that weak acids such as fatty acid could enhance PZA.Citation13 Acemetacin is a NSAID that enhanced PZA activity. Nifedipine, a standard anti-hypertensive drug, was found to enhance PZA activity (Table ). Quinaldine (2-methylquinoline), which is an antimalarial drug and has been used to prepare dyes, was found to have activity. Storax, composed of 33%–50% storesin, an alcoholic resin, in free and as cinnamic esters, is derived from plant Liquidambar orientalis and its derivatives (resinoid, essential oils) are used as flavors and fragrances. It is of interest to note that storax enhanced PZA activity against the 3-month-old M. tuberculosis culture (Table ). Toremifene, a selective estrogen receptor modulator as a negative control drug, did not enhance PZA activity (Table ).
Different mechanisms by which the identified compounds enhance PZA activity may be involved. In addition, the compounds that enhance PZA activity could in turn shed light on how PZA works. Bacterial membrane is known to be an important persister drug target.Citation10, Citation14 In this context, it is worth noting that NSAID weak acids such as acemetacin, indomethacin, meclofenamic acid, tolfenamic acid, as well as fatty acid ricinoleate and cinnamic acid (Storax) may perturb the membrane and lower the membrane potential which could synergize with the active component of PZA, pyrazinoic acid (POA), to disrupt the membrane energy more effectively. Other drug candidates such as azole drug clotrimazole could enhance PZA activity through a different membrane perturbation mechanism. In addition, the observation that PZA enhancement by DNA damaging agents such as fluoroquinolones (tosufloxacin and fleroxacin), reactive nitrogen (nitroxoline, nifuroxime), and anticancer drugs carboplatin and decitabine, could indicate that PZA may affect DNA as part of its complex mechanisms of action in addition to inhibition of trans-translation (RpsA) and energy production (PanD).Citation7 The enhancement of PZA activity by clofazimine, which is now used as part of the 9-month short course MDR-TB treatment, may be due to its activity on respiratory chain and production of reactive oxygen species,Citation15 which can in turn damage DNA.
Despite the identification of many potentially interesting hits that could enhance PZA activity, there are some limitations of this study. First, only a subset of the hits from this clinical compound library are FDA-approved drugs, while others are either non-FDA-approved or too toxic for human use (Supplementary Table S1). Second, the drug concentration (50 μM) used for the PZA enhancement screen may be too high which could lead to identification of some hits that may not enhance PZA activity but due to their own direct activity on mycobacteria. However, our independent screen at the lower drug concentration (10 μM) confirmed many of the hits identified at the higher concentration (50 μM) (Table ). Further studies are needed to confirm the findings of this study by using lower drug concentrations specific to their blood concentrations achievable clinically and perform detailed colony count assays after drug exposure in combination with PZA at different times for each drug candidate. This will be a tedious and time-consuming process that can be done in future studies. Third, our study is in vitro and animal studies are required to confirm the findings of this study.
In summary, M. tuberculosis persisters pose considerable challenges to the treatment of TB.Citation2, Citation4 Because of its unique ability to kill M. tuberculosis persisters, PZA is an indispensable drug that plays a critical role in shortening the treatment and reducing relapse.Citation4, Citation6, Citation7 Because clinically used drugs have relatively clear safety and pharmacokinetic profiles in humans, identifying clinical drugs that enhance PZA activity represents a rapid and efficient approach to developing more effective treatment. Our finding that various hits, especially FDA-approved drugs, could enhance PZA activity is of particular interest and may have implications for improved treatment of drug-susceptible and drug-resistant TB. Further studies are needed to determine if these drug candidates can shorten TB treatment in vivo in animal models and if so in patients.
Supplementary Table S1
Download PDF (213 KB)Acknowledgments
Hongxia Niu was sponsored by the China Scholarship Council. Ying Zhang was partially supported by NIH grants AI108535 and AI099512.
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
References
- World Health Organization. Global tuberculosis report 2016.Geneva: WHO. Available at http://www.who.int/tb/publications/global_report/en/ (accessed 13 October 2016).
- Zhang Y.The magic bullets and tuberculosis drug targets. Annu Rev Pharmacol Toxicol 2005;45: 529–564.
- Smith CV, Sharma V, Sacchettini JC.TB drug discovery: addressing issues of persistence and resistance. Tuberculosis 2004;84: 45–55.
- Zhang Y, Yew WW, Barer MR.Targeting persisters for tuberculosis control. Antimicrob Agents Chemother 2012;56: 2223–2230.
- Zhang Y, Permar S, Sun Z.Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J Med Microbiol 2002;51: 42–49.
- Zhang Y, Mitchison D.The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 2003;7: 6–21.
- Zhang Y, Shi W, Zhang Wet al.Mechanisms of pyrazinamide action and resistance. Microbiol Spectr 2013;2: 1–12.
- Chong CR, Chen X, Shi Let al.A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol 2006;2: 415–416.
- Byrne ST, Gu P, Zhou Jet al.Pyrrolidine dithiocarbamate and diethyldithiocarbamate are active against growing and nongrowing persister Mycobacterium tuberculosis. Antimicrob Agents Chemother 2007;51: 4495–4497.
- Zhang Y, Wade MM, Scorpio Aet al.Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother 2003;52: 790–795.
- Byrne ST, Denkin SM, Gu Pet al.Activity of ketoconazole against Mycobacterium tuberculosis in vitro and in the mouse model. J Med Microbiol 2007;56 (Pt 8): 1047–1051.
- Byrne ST, Denkin SM, Zhang Y.Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J Antimicrob Chemother 2007;59: 313–316.
- Wade MM, Zhang Y.Effects of weak acids, UV and proton motive force inhibitors on pyrazinamide activity against Mycobacterium tuberculosis in vitro. J Antimicrob Chemother 2006;58: 936–941.
- Hurdle JG, O'Neill AJ, Chopra Iet al.Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Micro 2011;9: 62–75.
- Yano T, Kassovska-Bratinova S, Teh JSet al.Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem 2011;286: 10276–10287.