Abstract
The high seroprevalence of human herpesvirus type 8 (HHV-8) in moderate or severe cirrhotics appears to be associated with male sex, hepatitis B virus (HBV) infection, alcoholism, and disease severity. The status of HHV-8 infection in mild cirrhotics remains unclear. Plasma samples collected from 93 mild cirrhotics and 93 age- and sex-matched healthy controls were analyzed for HHV-8 antibody and HHV-8 DNA. Mild cirrhotics had higher seropositivity for HHV-8 antibodies than healthy controls (P=0.0001). Univariate logistic regression analysis revealed that an age ≥55 years (odds ratio (OR) 2.88, P=0.02), hepatitis C virus (HCV) infection (OR 3.42, P=0.01), and hepatitis activity (OR 4.10, P=0.004) were associated with HHV-8 seropositivity in cirrhotics. Stepwise multivariate logistic regression analysis confirmed that age ≥55 years (adjusted OR (aOR) 1.92, P=0.04) and hepatitis activity (aOR 3.55, P=0.005) were independent factors. The rate of hepatitis activity was higher in HCV-infected than in HBV-infected patients (P<0.0001) and in women than in men (P=0.0001). Cirrhotics who were seropositive for HHV-8 or HCV or had hepatitis activity were significantly older (P=0.02, <0.0001 and <0.0001, respectively). Plasma samples from all participants were negative for HHV-8 DNA. HHV-8 antibody titers in mild cirrhotics also markedly exceeded those in controls (P<0.0001), as did those in patients ≥55 years old vs. younger patients (P=0.01), those in patients with vs. without HCV infection (P=0.0008), and those in patients with vs. without hepatitis activity (P=0.0005). Mild cirrhotics had high HHV-8 seroprevalence and HCV infection, and, in particular, old age and hepatitis activity were predictors.
Emerging Microbes & Infections (2017) 6, e45; doi:10.1038/emi.2017.32; published online 7 June 2017
Introduction
To date, HHV-8 DNA has been found consistently in all types of Kaposi’s sarcoma (KS).Citation1 This neoplasm occasionally develops in human immunodeficiency virus (HIV) non-infected patients with variable immunologic abnormalities.Citation2, Citation3 Immunologic abnormalities have been documented in cirrhotic patientsCitation4, Citation5 and are strongly associated with cirrhosis severity.Citation5, Citation6 In a previous study, we found that the seroprevalence of HHV-8 in patients with moderate or severe cirrhosis was significantly greater than that in healthy controls.Citation7, Citation8, Citation9 It appeared to be associated with cirrhosis severity, sex, and disease etiologies.Citation7 However, the prevalence of HHV-8 infection in patients with mild cirrhosis has not been described, nor is it clear whether HHV-8 prevalence is associated with hepatitis activity. This study aimed to assess the prevalence of HHV-8 infection in Child–Pugh class A cirrhotics with or without hepatocellular carcinoma (HCC) and with or without hepatitis activity and to compare it to the prevalence in healthy individuals and in the class B or C cirrhotics reported previously.
MATERIALS AND METHODS
Study subjects and sample collection
After obtaining written informed consent from all participants, plasma samples were collected from 93 healthy controls (60 men and 33 women) and 93 Child–Pugh class A cirrhotics with (23 men and nine women) or without (37 men and 24 women) HCC. The age- and sex-matched healthy controls were selected from persons who received routine health examinations during the same period as the cirrhotics were admitted and were proven to be free of cirrhosis and other serious illnesses. All participants were negative for anti-HIV antibodies.
Cirrhosis was diagnosed by biopsy or by clinical diagnostic criteria, including twice-documented ultrasonographic evidence of a coarse and nodular parenchyma, irregular surface, and dull margins with either splenomegaly, ascites, hepatic encephalopathy or varices.Citation10 Disease severity was assessed according to the Child–Pugh scoring system.Citation11 Diagnosis of HCC was supported by histologic findings or based on unequivocal imaging and laboratory data following the diagnostic criteria published by the European Association for the Study of Liver Disease in 2001.Citation12
Cirrhotics with positive tests for serum hepatitis B virus surface antigen (HBsAg) were considered to be HBV-infected; those with positive serum anti-HCV antibodies were considered to be HCV-infected. Alcohol-related cirrhotics were defined as those who had consumed at least 80 g of alcohol daily for at least the previous five years. Hepatitis activity was defined as a plasma alanine aminotransferase (ALT) level ≥1.5-fold higher than the upper limit of the normal range (ULNR).
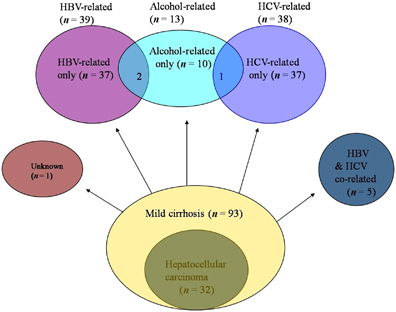
Cirrhotics were divided into the following subgroups: HBV-infected (n=39; including HBV-infected only (n=37) and HBV and alcohol co-related (n=2)); HCV-infected (n=38; including HCV-infected only (n=37) and HCV and alcohol co-related (n=1)); HBV and HCV co-infected (n=5); alcohol-related (n=13; including alcohol-related only (n=10), HBV and alcohol co-related (n=2), and HCV and alcohol co-related (n=1)); and unknown etiology (n=1; ).
The study protocol was approved by the Institutional Review Board of the Buddhist Dalin Tzu Chi Hospital, Taiwan (B09702035).
Immunofluorescence assay for detection of anti-HHV-8 antibody
A commercially available immunofluorescence assay (IFA) kit (Advanced Biotechnologies, Columbia, MD, USA) was used to detect HHV-8 immunoglobulin G (IgG) antibodies against the lytic antigens in plasma samples according to the manufacturer’s instructions. This assay used HHV-8-infected primary effusion lymphoma cell lines. Human plasma at various dilutions was brought into contact with fixed and infected cells, and was examined with a fluorescence microscope. Samples that displayed fluorescence at a dilution of 1:40 were considered positive. Participants with a positive result by IFA were considered to be HHV-8 positive. Maximum HHV-8 antibody dilutions were determined by an end-point IFA.
Chemiluminescence immunoassay for HBsAg and anti-HCV and anti-HIV antibodies
Plasma samples were assayed for HBsAg and anti-HCV, anti-HIV-1, and anti-HIV-2 antibodies with the Vitros HBsAg, anti-HCV, and anti-HIV 1+2 reagent packs, respectively, with controls and calibrators (Ortho-Clinical Diagnostics, High Wycombe, England) and the Vitros ECi immunodiagnostic system (Ortho-Clinical Diagnostics, Rochester, NY, USA) according to the manufacturer’s instructions.
Assay for ALT
Plasma samples were assayed for ALT with the ALT Liquid Reagent with controls and calibrators (Roche Diagnostics, Mannheim, Germany) and the Roche Integra 800 system (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions.
DNA extraction and amplification of HHV-8 DNA
HHV-8 DNA was extracted and polymerase chain reaction (PCR)-amplified from the plasma as previously reported.Citation8
Statistical analysis
Differences in the mean values of continuous variables between two groups of patients were analyzed by the t-test. A χ2 or Fisher’s exact test was used to assess the significance of between-group differences in categorical variables. The comparison of anti-HHV-8 titers in plasma between two groups was analyzed by the Mann–Whitney test. Univariate logistic regression was used for analysis of the possible factors predicting the HHV-8 seropositivity in Child–Pugh class A cirrhotics. All variables were entered into stepwise multivariate logistic regression analysis. Statistical significance was set at P<0.05. Statistical analyses were performed using SPSS v. 17.0 software for Windows (Chicago, IL, USA).
RESULTS
HHV-8 antibody and DNA
The prevalence of HHV-8 antibodies was much greater in mildly cirrhotic patients (46 out of 93) than in healthy controls (21 out of 93) (P=0.0001; ). HHV-8 antibody titers in mildly cirrhotic patients also markedly exceeded those in controls (P<0.0001; ). The three patients who showed the highest positive dilutions (1:640) were male. Seropositivity was not associated with clinical manifestations of HHV-8 infection, such as KS, primary effusion lymphoma or multicentric Castleman disease.
Table 1 Mean age and positivity and maximal titers of plasma HHV-8 antibodies in healthy controls and mild cirrhotics
Cirrhosis patients of both genders had a higher HHV-8 seropositivity (26 out of 60 or 43.3% in male and 20 out of 33 or 60.6% in female patients) than healthy controls (15 out of 60 or 25% in male and six out of 33 or 18.2% in female controls; P=0.03 and 0.0004, respectively; χ2 test). There were no gender differences in HHV-8 seropositivity in either controls or patients, except in the HBV-infected patients for which the rate was significantly greater in female (6 out of 10, 60%) than in male patients (six out of 29, 20.7%; P=0.02; χ2 test). Cirrhosis patients who were seropositive for HHV-8 or HCV, or had hepatitis activity, were significantly older (P=0.02, <0.0001, and <0.0001, respectively; ). In contrast, HBV-infected cirrhotics were significantly younger (P=0.001; ).
Table 2 Comparisons of mean age and maximal titers and positivity of plasma HHV-8 antibodies among various subgroups of patients with Child–Pugh class A cirrhosis
We performed HHV-8 DNA PCR analyses on all collected plasma samples. All participants were negative for HHV-8 DNA by this analysis.
Positivity for HHV-8 antibody by age
Mildly cirrhotic patients 55 years of age or older had higher seropositivity and titers of HHV-8 antibodies than younger patients (P=0.01, both χ2 test and Mann–Whitney test, respectively) ().
HHV-8 seropositivity by HCC
HHV-8 seropositivity in cirrhotics without HCC (30 out of 61, 49.2%) was similar to that in HCC patients (16 out of 32, 50%; ).
Positivity for HHV-8 antibody by disease etiology
HHV-8 seropositivity in cirrhotics with HCV infection was significantly greater than in those without (P=0.007; χ2 test; ). In contrast, patients with HBV infection had lower HHV-8 seropositivity than those without HBV infection (P=0.008; χ2 test; ). The seropositive rate in HBV and HCV co-infected patients was 100% (five out of five); the one patient of unknown etiology was also seropositive for HHV-8. The HHV-8 antibody titers in cirrhotics with HCV infection were much greater than in those without HCV infection (P=0.0008; Mann–Whitney test; ).
Hepatitis activity and HHV-8 seropositivity
In 38 HCV-infected patients, 27 had hepatitis activity (71.1%), and 19 of them were seropositive for HHV-8 (70.4%). In contrast, none of the 39 HBV-infected patients had plasma ALT levels ≥1.5-fold higher than ULNR (P<0.0001; χ2 test), and 12 of them were seropositive for HHV-8 (30.8%). Three of the five patients with HBV and HCV co-infection showed hepatitis activity, and none of the 13 alcohol-related patients had plasma ALT levels ≥1.5-fold higher than ULNR. Of 33 female patients, 19 had hepatitis activity (57.6%); in contrast, 11 of 60 male patients had hepatitis activity (18.3%, P=0.0001; χ2 test). In patients without HCC, 17 had hepatitis activity (27.9%, 17 out of 61) and 12 were seropositive for HHV-8 (70.6%, 12 out of 17). In patients with HCC, 13 had hepatitis activity (40.6%, 13 out of 32) and 10 were seropositive for HHV-8 (76.9%, 10 out of 13). Hence, HHV-8 seropositivity was greater in all cirrhotics with hepatitis activity (22 out of 30, 73.3%) compared to those without hepatitis activity (24 out of 63, 38.1%; P=0.002; χ2 test; ). The HHV-8 antibody titers in cirrhotics with hepatitis activity were also greater than in those without hepatitis activity (P=0.0005; Mann–Whitney test; ).
Associated factors for HHV-8 seropositivity
Univariate logistic regression analysis revealed that age ≥55 years old (odds ratio (OR) 2.88, P=0.02), HCV infection (OR 3.42, P=0.01), and hepatitis activity (OR 4.10, P=0.004) were predicting factors for HHV-8 seropositivity in Child–Pugh class A cirrhotics (). Stepwise multivariate logistic regression analysis further confirmed that age ≥55 years old (adjusted odds ratio (aOR) 1.92, P=0.04) and hepatitis activity (aOR 3.55, P=0.005) were independent predictors ().
Table 3 Predictors for HHV-8 seropositivity in Child–Pugh class A cirrhotics analyzed by univariate logistic regression analysis
Table 4 Predictor for HHV-8 seropositivity in patients with Child–Pugh class A cirrhosis analyzed by stepwise multivariate logistic regression analysis
Table 5 HHV-8 seropositivities in patients with variant severity of cirrhosis
DISCUSSION
HHV-8 seroprevalence in cirrhotics appeared to be associated with disease severity. In our earlier study concerning patients without hepatitis activity, the seroprevalence of HHV-8 infection in those with Child–Pugh class B cirrhosis (42%) was lower than that in those with class C cirrhosis (47%).Citation8 In the present study, among Child–Pugh class A cirrhotics without hepatitis activity, the seropositive rate (38.1%) was even lower.
Our recent study concerning HHV-8 seroprevalence in patients with end-stage renal disease found that one of the two healthy controls with the highest IFA antibody titer (1:160) was positive by enzyme-linked immunosorbent assay.Citation13 If the cut-off point of the HHV-8 antibody titer in the present study was set at 1:160, patients with mild cirrhosis still had significantly higher HHV-8 seropositive rate than controls (P=0.0003; ).
KS is found mainly in elderly persons, and an age-related slightly increased risk for classic KS has been found among individuals from Asia and Africa.Citation14, Citation15 In the present study, HHV-8-seropositive patients were significantly older than seronegative patients. Patients with HCV infection or hepatitis activity were also significantly older than those without. HHV-8 seropositivity in patients with HCV infection or hepatitis activity was also significantly greater than in those without. In addition, patients aged 55 or older had significantly higher HHV-8 seropositivity than younger patients. Stepwise multivariate logistic regression analysis confirmed that age ≥55 years was an independent risk factor for HHV-8 seropositivity. Thus, old age seems to have an important role in HHV-8 infection in patients with mild cirrhosis, particularly in patients with HCV infection or hepatitis activity.
Mildly cirrhotic patients with HCC in the present study showed similar HHV-8 seropositivity as patients without HCC did. These findings support the hypothesis that HHV-8 seropositivity in cirrhotics is associated with cirrhosis severity, not with HCC.Citation9
HHV-8 seropositivity in patients with class A cirrhosis in the present study is greater than in healthy controls and even greater than in patients with class B or C cirrhosis (). This is mainly attributable to the significantly higher positive rate among HCV-infected patients in this group (63.2%) than in the more severe groups (32%–34%) (). Abnormal cellular immunities have been found in patients with chronic HCV infection.Citation16, Citation17, Citation18, Citation19, Citation20 Furthermore, inflammatory activity is more important in HCV-infected cirrhotics than in the HBV-infected.Citation21, Citation22 In the present study, many more HCV-infected patients (27 out of 38, 71.1%) showed hepatitis activity than HBV-infected patients (0 out of 39), and HHV-8 seropositivity in patients with hepatitis activity was as high as 73.3%. Hence, the significantly higher seropositivity in patients with mild cirrhosis appears to be associated with the inflammatory properties of HCV.
This significant decrease in HHV-8 seropositivity in HCV-infected patients with more severe cirrhosis () indicates that there may be seroreversion to HHV-8 antibody in cirrhotics, particularly in HCV-infected patients. In a study of patients after bone marrow transplantation, half of the 20 recipients who initially had positive results for HHV-8 antibody seroreverted within one year.Citation23 The incidence of seroreversion in patients undergoing hemodialysis is 16.4 out of 100 person-years. Patients 50 years of age and younger have an increased probability for seroreversion than those older than 50 years.Citation24 However, seroreversion of antibody activity to HHV-8 in patients with cirrhosis has not been described.
It has been reported that all cases with hepatitis activity in asymptomatic HCV carriers occurred during the first 5 years after diagnosis. The presence of the C100-3 antibody and anti-human T cell leukemia virus-I antibody are two independent and significant predictors of hepatitis activity in asymptomatic HCV RNA carriers.Citation25 However, no study concerning hepatitis activity or other hepatitis-associated antibodies in HCV-infected mildly cirrhotic patients has been documented. The relationship between HHV-8 antibody and hepatitis activity in mildly cirrhotic patients, particularly in HCV-infected patients, needs to be investigated.
Like HCV infection, patients with chronic HBV infection have been found to have impaired cellular immunities.Citation26, Citation27 In the present study, among HBV-infected low-grade cirrhotics, HHV-8 seropositivity in women was significantly greater than that in men (P=0.02). Hence, gender seems to have a role in this subgroup.
In contrast to patients with moderate or severe cirrhosis,Citation7, Citation8 most of the HCV-infected patients with mild cirrhosis in the present study were female (22 out of 38). However, most of the female patients were HCV-infected (22 out of 33). This is compatible with the observation of an increasing HCV prevalence in women, most likely reflecting the increase in hepatitis C over hepatitis B.Citation22 Hence, the high HHV-8 seroprevalence in female patients in the present study may be due to the fact that two-thirds of them are HCV-infected. We also found that a significantly larger proportion of female compared to male patients had hepatitis activity, mainly due to a high prevalence of HCV infection. Whether a female sex hormone may contribute to hepatitis activity deserves further exploration.
HHV-8 has two modes of infection—latency and lytic replication. Few viral genes are expressed during latent infection, and the HHV-8 genome is maintained as an episome.Citation28, Citation29 During the lytic cycle, virtually all HHV-8 genes are expressed, resulting in the generation of infectious progeny virions that destroy the host cell.Citation30 In the present study, we found anti-lytic phase antibodies at titers as high as 1:640; it is possible that the virus was actively transducing and translating its messages. Many studies have reported that HHV-8 DNA is detected more frequently and at higher copy numbers in the plasma of KS patients compared with controls with asymptomatic HHV-8 infection.Citation31, Citation32, Citation33, Citation34 In addition, the method used in this study can only detect 5–10 copies per mL of HHV-8 DNA and may be not sensitive enough to detect low levels of HHV-8 DNA. This may be the reason why none of the plasma specimens were positive for HHV-8 DNA. The question of whether or not patients such as those who participated in this study have HHV-8 DNA at levels less than 5–10 copies per mL will be resolved only if the detection sensitivity can be increased.
HHV-8 is a B-lymphotropic virus. HHV-8 DNA is found principally in circulating B cells in infected subjects.Citation35, Citation36 Hence, HHV-8 DNA may be detected at higher copy numbers in peripheral blood mononuclear cells (PBMCs) than in plasma samples. The second limitation of our study is that no PBMCs from patients were analyzed for HHV-8 DNA. We cannot be sure whether PBMCs from mildly cirrhotic patients contain HHV-8 DNA.
In our study, stepwise multivariate logistic regression analysis also demonstrated that hepatitis activity in mildly cirrhotic patients was an independent predictor for HHV-8 seropositivity. Univariate logistic regression analysis showed that HCV infection was one risk factor able to enhance the prediction of HHV-8 infection. HHV-8 infection appeared to have a role in hepatitis activity in mildly cirrhotic patients. Nevertheless, a third limitation of the present study was that no patients with hepatitis activity received biopsies that proved the presence of HHV-8 DNA in their liver tissues. The role of HHV-8 infection in hepatitis and its influence on the evolution of HCV infection need to be clarified.
In 1972, Triger et al demonstrated a highly significant increase in the frequency of high antibody titers (≥1/128) to measles and/or rubella viruses in 15 patients (14 women and one man) with chronic active hepatitis.Citation37 Two years later, Laitinen and VaheriCitation38 also reported that seven patients (five women and two men) with chronic active hepatitis had high measles and/or rubella antibody titers without preceding rubella or measles infection. Highly significant increases in high-titer antibodies to HHV-8 in patients with chronic active hepatitis have not been reported to date. Without biopsy in patients with hepatitis activity in the present study, they cannot be proven to have chronic active hepatitis. Therefore, we cannot definitely state whether the increased HHV-8 antibody titers in mildly cirrhotic patients with hepatitis activity are due to atypical viral infections or to atypical immune responses. However, similar to the above two reports, in the present study, most of the mildly cirrhotic patients with hepatitis activity who were seropositive for HHV-8 antibodies were female. Female hormones may have a role in increasing viral antibody titers in these patients. This needs to be further studied.
In conclusion, both the seropositive rates and antibody titers for HHV-8 were increased in patients with mild cirrhosis and were even greater than those in patients with moderate or severe cirrhosis. This indicates that there may be seroreversion to HHV-8 antibodies in moderately or severely cirrhotic patients, particularly in HCV-infected patients. Old age and hepatitis activity are two independent predictors for HHV-8 seropositivity in mildly cirrhotic patients, and HCV infection is a risk factor that can enhance the prediction of HHV-8 infection. The definite relationships between HHV-8 infection and HCV infection, in particular, old age or hepatitis activity in mildly cirrhotic patients, as well as the impact of HHV-8 infection on the disease evolution, remain to be elucidated.
Acknowledgments
This work was supported by grants DTCRD95(2)-04 and DTCRD 97-04 from the Buddhist Dalin Tzu Chi Hospital, Chiayi County, Taiwan.
References
- Su CC, Li CF, Liao YLet al.Immunohistochemical and molecular assessment of human herpesvirus type 8 in gastrointestinal tumors. J Clin Pathol 2005;58: 856–859.
- Al-Khader AA, Shaheen FA.Posttransplant complications encountered in renal transplantation in the Middle East. Transplant Proc 2004;36: 180–183.
- Rady PL, Hodak E, Yen Aet al.Detection of human herpesvirus-8 DNA in Kaposi’s sarcomas from iatrogenically immunosuppressed patients. J Am Acad Dermatol 1998;38: 429–437.
- Rimola A, Soto R, Bory Fet al.Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology 1984;4: 53–58.
- Lombardo L, Capaldi A, Poccardi Get al.Peripheral blood CD3 and CD4 T-lymphocyte reduction correlates with severity of liver cirrhosis. Int J Clin Lab Res 1995;25: 153–156.
- Leevy CB, Elbeshbeshy HA.Immunology of alcoholic liver disease. Clin Liver Dis 2005;9: 55–66.
- Chou AL, Huang WW, Tsao SMet al.Human herpesvirus type 8 in patients with cirrhosis: Correlation with sex, alcoholism, hepatitis B virus, disease severity, and thrombocytopenia. Am J Clin Pathol 2008;130: 231–237.
- Chou AL, Huang WW, Lin MNet al.Human herpesvirus type 8 in patients with cirrhosis independent of thrombocytopenia. J Clin Pathol 2010;63: 254–258.
- Su CC, Tseng KC, Hsieh TCet al.High seroprevalence of human herpesvirus type 8 in patients with hepatocellular carcinoma. Eur J Clin Microbiol Infect Dis 2015;34: 55–62.
- Huang JF, Yu ML, Lee CMet al.Sustained virological response to interferon reduces cirrhosis in chronic hepatitis C: a 1386-patient study from Taiwan. Aliment Pharmacol Ther 2007;25: 1029–1037.
- Pugh RN, Murray-Lyon IM, Dawson JLet al.Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60: 646–649.
- Bruix J, Sherman M, Llovet JMet al.EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35: 421–430.
- Su CC, Tsai JP, Lin MNet al.High seroprevalence of human herpesvirus type 8 in patients with end-stage renal disease in Taiwan. J Clin Virol 2013;58: 89–93.
- Su CC, Lu JJ, Perng CLet al.Evolution of human herpesvirus type 8-associated gastric Kaposi’s sarcoma following corticosteroid treatment for asthma. J Formos Med Assoc 2006;105: 155–159.
- Guttman-Yassky E, Dubnov J, Kra-Oz Zet al.Classic Kaposi sarcoma. Which KSHV-seropositive individuals are at risk? Cancer 2006;106: 413–419.
- Sarobe P, Lasarte JJ, Zabaleta Aet al.Hepatitis C virus structural proteins impair dendritic cell maturation and inhibit in vivo induction of cellular immune responses. J Virol 2003;77: 10862–10871.
- Tseng CT, Klimpel GR.Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med 2002;195: 43–49.
- Crotta S, Stilla A, Wack Aet al.Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med 2002;195: 35–41.
- Sugimoto K, Ikeda F, Stadanlick Jet al.Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 2003;38: 1437–1448.
- Auffermann-Gretzinger S, Keeffe EB, Levy S.Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 2001;97: 3171–3176.
- Liang TJ, Heller T.Pathogenesis of hepatitis C associated hepatocellular carcinoma. Gastroenterology 2004;127: S62–S71.
- Benvegnu L, Alberti A.Patterns of hepatocellular carcinoma development in hepatitis B virus and hepatitis C virus related cirrhosis. Antiviral Res 2001;52: 199–207.
- Rosenzwajg M, Fery N, Bons Vet al.Human herpes virus 8 (HHV8) serology in allogeneic bone marrow transplant recipients. Bone Marrow Transplant 1999;24: 351–354.
- Zavitsanou A, Sypsa V, Petrodaskalaki Met al.Human herpesvirus 8 infection in hemodialysis patients. Am J Kidney Dis 2006;47: 167–170.
- Tsuji K, Yamasaki K, Yamanishi Met al.Risk of alanine aminotransferase flare-up among asymptomatic hepatitis C virus RNA carriers: a 10-year follow-up study. J Gastroenterol Hepatol 2001;16: 536–540.
- Chang JJ, Lewin SR.Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol 2007;85: 16–23.
- Wang K, Fan X, Fan Yet al.Study on the function of circulating plasmacytoid dendritic cells in the immunoactive phase of patients with chronic genotype B and C HBV infection. J Viral Hepat 2007;14: 276–282.
- Bechtel JT, Liang Y, Hvidding Jet al.Host range of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol 2003;77: 6474–6481.
- Vieira J, O’Hearn P, Kimball Let al.Activation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol 2001;75: 1378–1386.
- Ganem D.KSHV infection and the pathogenesis of Kaposi’s sarcoma. Annu Rev Pathol 2006;1: 273–296.
- Broccolo F, Bossolasco S, Careddu AMet al.Detection of DNA of lymphotropic herpesviruses in plasma of human immunodeficiency virus-infected patients: frequency and clinical significance. Clin Diagn Lab Immunol 2002;9: 1222–1228.
- Cannon MJ, Dollard SC, Black JBet al.Risk factors for Kaposi’s sarcoma in men seropositive for both human herpesvirus 8 and human immunodeficiency virus. AIDS 2003;17: 215–222.
- Lin L, Lee JY, Kaplan LDet al.Effects of chemotherapy in AIDS-associated non-Hodgkin’s lymphoma on Kaposi’s sarcoma herpesvirus DNA in blood. J Clin Oncol 2009;27: 2496–2502.
- Johnston C, Orem J, Okuku Fet al.Impact of HIV infection and Kaposi sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS One 2009;4: e4222.
- Ambroziak JA, Blackbourn DJ, Herndier BGet al.Herpes-like sequences in HIV infected and uninfected Kaposi’s sarcoma patients. Science 1995;268: 582–583.
- Mesri EA, Cesarman E, Arvanitakis Let al.Human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med 1996;183: 2385–2390.
- Triger DR, MacCallum FO, Kurtz JBet al.Raised antibody titres to measles and rubella viruses in chronic active hepatitis. Lancet 1972;1: 665–667.
- Laitinen O, Vaheri A.Very high measles and rubella virus antibody titres associated with hepatitis, systemic lupus erythematosus, and infectious mononucleosis. Lancet 1974;1: 194–197.