Abstract
Rickettsioses are emerging zoonotic diseases that are often neglected in many countries in Southeast Asia. Rickettsial agents are transmitted to humans through exposure to infected arthropods. Limited data are available on the exposure of indigenous community and animal farm workers to the aetiological agents and arthropod vectors of rickettsioses in Peninsular Malaysia. Serological analysis of Rickettsia conorii and Rickettsia felis was performed for 102 individuals from the indigenous community at six rural villages and 87 workers from eight animal farms in Peninsular Malaysia in a cross-sectional study. The indigenous community had significantly higher seropositivity rates for R. conorii (P<0.001) and R. felis (P<0.001), as compared to blood donors from urban (n=61). Similarly, higher seropositivity rates for R. conorii (P=0.046) and R. felis (P<0.001) were noted for animal farm workers, as compared to urban blood donors. On the basis of the sequence analysis of gltA, ompA and ompB, various spotted fever group rickettsiae closely related to R. raoultii, R. heilongjiangensis, R. felis-like organisms, R. tamurae, Rickettsia sp. TCM1, R. felis, Rickettsia sp. LON13 and R. hulinensis were identified from tick/flea samples in animal farms, indigenous villages and urban areas. This study describes rickettsial seropositivity of the Malaysian indigenous community and animal farm workers, and provides molecular evidence regarding the presence of rickettsial agents in ticks/fleas infesting domestic animals in Peninsular Malaysia.
Emerging Microbes & Infections (2017) 6, e18; doi:10.1038/emi.2017.4; published online 12 April 2017
Introduction
Rickettsioses are emerging infectious diseases that are often neglected in the tropical region. The causative agents, spotted fever group (SFG) and typhus group (TG) rickettsiae, are obligate intracellular bacteria that are transmitted to humans through arthropod vectors, mainly ticks, fleas, mites and so on.Citation1 Rickettsia conorii, R. sibirica, R. japonica, R. honei, R. heilongjiangensis, R. tamurae and R. raoultii are tick-borne SFG rickettsiae that have been reported in Asia,Citation2 whereas flea-borne rickettsioses are usually caused by R. typhi and R. felis.Citation3
Infections caused by SFG rickettsiae and R. felis may present as acute febrile illnesses burdening many populations in Southeast Asia. In a Malaysian serological survey conducted 15 years ago, SFG rickettsioses (previously known as tick typhus) have been reported as the most frequent infection among febrile hospitalized patients in rural areas of Peninsular Malaysia. The antibody prevalence of SFG rickettsiae (R. honei, TT118 strain) varied widely from 1.7% in urban blood donors to 42.5% in rural febrile patients.Citation4, Citation5 In addition, a high rickettsial seropositivity rate (~50.0%) was also reported among rubber estate workers,Citation6 suggesting the endemicity of the disease in this region. It is postulated that people may acquire rickettsioses through exposure to infected ticks and fleas in the living and working environment.
People who live at the fringe of the forest or rural areas, including the indigenous community and animal farm workers, are regarded as populations who are at high risk of acquiring rickettsioses.Citation7 The indigenous community in Malaysia (also referred as Orang Asli or ‘original people’) constitutes a minority group (0.6%) of the total population in Malaysia.Citation8 They stay in huts or settlements that are surrounded by primary or secondary forest, and engage in activities involving agriculture, hunting and collection of forest products. Their nomadic lifestyle and close contact with peri-domestic animals have increased the risk of contracting scrub typhus,Citation9 and potential tick- and flea-borne diseases. However, animal farm workers are at risk of multiple tick-borne diseases due to frequent exposure to ticks either from the animals or vegetation.Citation10, Citation11 Few studies have investigated the extent of exposure of these populations to rickettsioses in Southeast Asia. In addition, little information on the potential vectors and maintenance hosts of rickettsiae is available in this region.
This study was conducted to determine the serologic status of Malaysian indigenous community and animal farm workers and provides molecular evidence regarding the presence of rickettsial agents in ticks/fleas infesting domestic animals in Peninsular Malaysia. Serum samples collected from urban blood donors were also included for comparison purpose. R. conorii and R. felis are the SFG rickettsiae that have been reported in most Asian countries including China,Citation12 Korea,Citation13 Laos,Citation14 TaiwanCitation15 and Thailand.Citation16 Hence, the two rickettsial species were used as antigens in this serological assessment study. For vector surveillance, animal ectoparasites, mainly ticks and fleas, were collected from peri-domestic animals (cats, dogs, chickens, cattle and goats) from each study site for the detection of rickettsial DNA by using specific PCR assays followed by sequence analysis. It is hoped that the information derived from this study will be beneficial for surveillance, prevention and control of tick- and flea-borne rickettsioses in this region.
Materials and Methods
Ethics statements
Ethical approval was obtained from the University Malaya Medical Centre (Ethics committee reference number: 944.20) for serological assessment of human serum samples. Prior to the commencement of the sample collection, permission was obtained from the Department of Orang Asli Development (JAKOA) and Department of Veterinary Services, Ministry of Agriculture and Agro-based Industry (DVS), Malaysia (reference number: JPV/PSTT/100-8/1). An oral briefing on the objective and methodology of the study was given to participants. Consent was obtained either in written form or verbally followed by thumb prints (for those who were illiterate). Parents or guardians of children under the age of 18 provided informed consent on their behalf. All data from the studied populations were strictly anonymized.
Study population
Serum samples of 102 individuals residing at six rural villages who participated in a cross-sectional study (October 2012 to February 2013) to determine risk factors associated with dengue fever,Citation17 and the seroprevalence of tick-borne viral diseases,Citation18 were used. Details of the consent, sample collection, sampling scheme and population have been previously described.Citation17, Citation18 The rural villages were mostly located at forest fringe areas and in close proximity to rubber or oil palm estates.
Serum samples were collected from 87 farm workers (February 2013 to September 2013) who were based on eight farms, designated as Farm 1–8 located in six states in Peninsular Malaysia; they included a cattle and a goat farm in Negeri Sembilan (n=24), two cattle farms in Pahang (n=18), one sheep farm in Kedah (n=7), and one cattle farm each in Kelantan (n=14), Terengganu (n=7) and Johore (n=17).Citation19 For comparison, serum samples from 61 healthy blood donors residing in an urban area (Kuala Lumpur or Selangor) were kindly provided by the blood bank of the University Malaya Medical Centre for serological analysis.
Serological analysis
The sera were analysed for lgG antibodies against R. conorii (strain Malish 7) and R. felis (strain LSU) using indirect immunofluorescence assay (IFA) kits (Fuller Laboratories, Fullerton, CA, USA) in accordance with the manufacturer’s instructions. In brief, serum samples were first diluted (1:64) in phosphate-buffered saline (PBS) and 10 μL of each diluted serum sample was added to an antigen well on the IFA slide. After incubation at 37 °C for 30 min in a humidified chamber, the wells were washed with PBS and distilled water prior to incubation with IgG conjugate for 30 min. The slides were examined under × 400 magnification. Samples were regarded as positive when bright apple-green fluorescence of rickettsial antigens was observed (additional files; Supplementary Figures S1 and S2). The positive and negative sera provided in the IFA kits were used as controls. A past infection was indicated whenever there was an IgG titre of ≥1:64 without a fourfold or greater increase of titres.
Statistical analysis
For comparison of seropositivity rates among different study groups, statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) software program, version 22 (SPSS Inc., Chicago, IL, USA). Initial data entry was cross-checked (by KLK and MGK) in order to ensure that the data were entered correctly. χ2 and Kruskal–Wallis rank test were used to determine statistical significance between age, gender and study groups (indigenous population, animal farm workers and blood donors). The level of statistical significance was determined at P≤0.05 and 95% confidence interval (CI). Pairwise comparisons within the study and age groups were performed using Games–Howell post hoc tests of the SPSS software. A P-value of ≤0.05 was considered statistically significant.
Collection of ticks and fleas
Ticks were collected using tweezers from the ear, eyes, flank, abdomen, tail and perineal regions of animals. Fleas were collected using combing method. Ticks were identified to the genus level according to Walker et al.Citation20 and Geevarghese and Mishra.Citation21 Molecular identification of tick species was performed using primers targeting tick 16S rRNA gene regions.Citation22 All the fleas were identified as Ctenocephalides felis or C. orientis on the basis of morphometric characteristics.Citation23 All the samples were preserved at −80 °C prior to DNA extraction.
DNA extraction of tick/flea samples
The samples were processed according to Duh et al.Citation24 with slight modification. In brief, ticks and fleas were first thawed and then immersed in 5% sodium hypochlorite and 70% ethanol before washing with sterile distilled water. The samples were then triturated using surgical blades and DNA was extracted using QIAamp DNA mini kits (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instruction.
PCR amplification
Three rickettsial-specific genes, that is, citrate synthase gene (gltA),Citation25 190-kDa outer membrane protein gene (ompA)Citation26 and 135-kDa outer membrane protein gene (ompB),Citation27 were targeted for amplification from tick and flea samples. These PCR assays have been widely used for the detection of SFG rickettsiae in arthropod vectors.Citation28, Citation29, Citation30 Due to the large sample size of cattle ticks and fleas, the gltA PCR assay was used for screening of rickettsial DNA. Any positive samples were then subjected to further amplification using primers targeting ompA and ompB. All three rickettsial PCR assays were used for detection of rickettsiae from ticks collected from rural villages and urban areas.
All PCR assays were performed in a final volume of 20 μL containing 2 μL of DNA template, × 1 ExPrime Taq DNA polymerase (GENET BIO, Daejeon, South Korea) and 0.2 μM of each primer, in a Veriti thermal cycler (Applied Biosystems, Foster City, CA, USA). DNA extracted from R. conorii antigen slides (Fuller Laboratories, Fullerton, CA, USA) was used as positive control for all the PCR assays. Sterile distilled water was used as the negative control in each PCR reaction. PCR products were purified using GeneAll Expin Combo GP kit (GeneAll Biotechnology, Seoul, Korea) prior to sequencing on an ABI PRISM 377 Genetic Analyzer (Applied Biosystems), using both forward and reverse primers of each PCR assay. The sequences obtained were subjected to BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to search for homologous sequences in the GenBank database.
Phylogenetic analysis of tick- and flea-borne rickettsiae
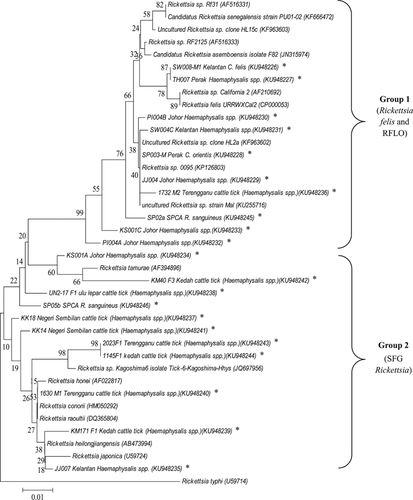
To determine the phylogenetic placement of the rickettsiae identified in this study, a dendrogram was constructed based on gltA sequences (375 nucleotides) using the neighbour-joining method of MEGA software.Citation31 Reference sequences for R. raoultii, Rickettsia sp. Rf31, Rickettsia sp. RF2125, Candidatus Rickettsia asemboensis, Rickettsia sp. California 2, R. felis URRWXCal2, R. tamurae, Rickettsia sp. Kagoshima6, R. honei, R. conorii, R. heilongjiangensis, R. japonica and R. typhi were retrieved from the GenBank database. Rickettsiae reported in previous Malaysian studies, including Rickettsia sp. clone HL2a, and clone HL15c, derived from cat fleas, Rickettsia sp. strain Mal from a febrile patient, and Rickettsia sp. 0095 from infected monkeys, were also included in the dendrogram.
Results
Table presents the rickettsial seropositivity rates of different study groups based on gender and age. The median ages of 87 farm workers, 61 blood donors and 102 indigenous people were 38 years (range, 23 to 59 years), 31 years (range, 19 to 54 years) and 27 years (range, 8 to 78 years), respectively. The male-to-female ratio was 1.34 (143:107). The indigenous people had the highest seropositivity rates towards R. conorii (50.0%, 95% CI: 40.1%–59.9%) and R. felis (22.5%, 95% CI: 14.3%–30.8%). A total of 13.8% (95% CI: 6.4%–21.2%) and 16.1% (95% CI: 8.2%–24.0%) of the farm workers were seropositive for R. conorii and R. felis, respectively. The seropositivity rate for rickettsiae was the lowest among the urban blood donors, given that only 3.3% (95% CI: 0.0%–7.9%) were seropositive to R. conorii and none was seropositive for R. felis. Pairwise comparison within the study groups (using Games–Howell post hoc test) demonstrated a significantly higher R. conorii-seropositivity rate (50.0%±50.2%) in the indigenous people, compared with the animal farm workers (13.8%±34.7%, P<0.001) and the urban blood donors (3.3%±18.0%, P<0.001). The R. felis-seropositivity rate of the indigenous people (22.5%±42.0%) was also significantly higher compared with urban blood donors (0.0%, P<0.001), but not animal farm workers (16.1%±37.0%, P=0.500). Seropositivity against both R. conorii and R. felis was detected in 23 individuals (0 (0.0%) in urban blood donors, including 5 (5.7%) for farm workers and 18 (17.6%) for the indigenous community) in this study.
No significant differences in the seropositivity rates for R. conorii and R. felis were noted in any of the study groups based on gender (P=0.072 and P=0.509 for R. conorii and R. felis, respectively; Table ). Significant differences were noted in the seropositivity rates for both R. conorii (P=0.011) and R. felis (P=0.001) within different age groups. The participants in the age group of ≥51 years old demonstrated the highest seropositivity rates to both R. conorii and R. felis (Table ). Games–Howell post hoc tests revealed significantly higher R. conorii-seropositivity rate among participants over 50 years old (43.6%±50.2%) compared with those 41–50 years old (7.7%±27.0%, P=0.002). R. felis-seropositivity rate was also significantly higher among participants over 50 years old of age (33.3%±47.8%), compared with those ≤20 years old (5.4%±22.9%, P=0.015) and 31–40 years old (5.0%±22.0%, P=0.009).
Table 1 Seropositivity of R. conorii and R. felis with respect to different category of the participants investigated in this study
The majority of the animal farm workers and urban blood donors were of the Malay ethnic group, which is the largest ethnic group in Malaysia, followed by Chinese and Indians. The indigenous people comprised different tribes including Temiar, Semoq Beri, Semai, Temuan, Jakun, Jah Hut, Kensui and others. Therefore, comparison of rickettsial-seropositivity rates between ethnic groups was not possible as each study group was composed of different ethnic group.
The tick/flea samples investigated in this study included:
270 ticks (70 Rhipicephalus microplus and 200 Haemaphysalis bispinosa) collected from cattle and sheep from eight animal farms. Each tick was processed individually.
186 ticks collected from 47 peri-domestic animals (which are cats, chickens, cattle, dogs and goats) from rural villages. The ticks (majority identified as Haemaphysalis spp.) were segregated into 64 pools (one to ten individuals) prior to DNA extraction. A total of 33 Rh. sanguineus collected from two animal shelters in urban area were also included.
153 fleas (42 C. felis and 111 C. orientis) infesting cats and dogs in rural villages and 210 fleas collected from stray cats in urban area (Kuala Lumpur).
Tables and summarize the results of PCR screening for rickettsial DNA from the ticks and fleas collected in this study. Rickettsial DNA was detected in 25 (9.3%) cattle ticks (21 H. bispinosa and 4 Rh. microplus) from four farms, with detection rates ranging from 2.1% to 27.7% (Table ). BLAST analyzes were performed for 42 rickettsial sequences (20 gltA, 7 ompA and 15 ompB) obtained in this study (Supplementary Table S1). Sequence analyzes of the gltA fragments (375 bp) from 20 ticks reveal the identification of rickettsiae closely related to R. raoultii (n=15), R. heilongjiangensis (n=2), Rickettsia sp. RF2125 (n=1), R. tamurae (n=1) and Rickettsia sp. TCM1 (n=1). BLAST analysis of ompA (518 bp) in seven ticks (H. bispinosa from cattle) indicate the identification of a rickettsia closely related to R. heilongjiangensis. Nine and six of the ompB sequences (774–826 bp) matched those of R. raoultii and Rickettsia sp. RF2125, respectively (Supplementary Table S1).
Table 2 Seroprevalences of R. conorii and R. felis among animal farm workers, indigenous people and blood donors in different localities; information on the tick species and detection rates of rickettsiae in each locality are also included
The gltA sequences for the Malaysian R. raoultii strains demonstrated high sequence similarity (98%) to that of R. raoultii (GenBank accession NO: JQ 697956) reported from H. hystricis ticks in Japan. The ompB sequences demonstrated the highest identities (93%) to that of R. raoultii strain Khabarovsk (DQ365798), whereas the rickettsial ompA gene was not amplifiable. Further characterization is required to determine whether it represents a novel species of rickettsial species.
Of 186 ticks collected from peri-domestic animals in the rural villages (Table ), rickettsial DNA was amplified from 40.6% (26/64) of the ticks (23 pools Haemaphysalis spp., one Rh. sanguineus and two Rh. microplus). Rickettsial-positive ticks were identified from nine rural villages, with the detection rates ranging from 14.3% to 66.7% in each village. A total of 43 sequences (13 gltA, 11 ompA and 19 ompB) were analyzed, and the BLAST results are presented in Supplementary Table S1. On the basis of sequence analysis, rickettsiae closely related to those of R. tamurae (98%), R. felis URRWXCal2 (99%), Rickettsia sp. RF2125 (98%–100%), R. raoultii (98%–99%) and R. heilongjiangensis (99%) were identified. In addition, a rickettsia identified from a Haemaphysalis cat tick from Kelantan shared 100% sequence similarity with the gltA sequence of Rickettsia sp. LON-13 (AB516964),Citation32 whereas the ompB sequence derived from the tick resembled that of R. hulinensis (AY260452).Citation33
Rickettsial DNA (either gltA, ompA and ompB gene fragments) was amplified from 39.4% (13/33) of Rh. sanguineus dog ticks in two animal shelters in Kuala Lumpur. The BLAST result reveals the identification of rickettsiae closely related to Rickettsia sp. RF2125, R. conorii type strains/R. raoultii strain Khabarovsk and R. heilongjiangensis (Supplementary Table S1).
A total of 60.1% (92/153) fleas collected from rural villages were positive for rickettsial DNA using both gltA and ompB PCR assays. Due to the large number of positive samples, only 22 amplified gltA and ompB fragments from different hosts and geographical locations were selected for sequence determination (Supplementary Table S2). The sequences were differentiated into two distinct types, of which one was more closely related to R. felis strain URRWXCal2 (99%, 373/375 bp) for gltA and 100% (808/808 bp) for ompB sequences, GenBank accession no.: CP000053) and another one was more closely related to Rickettsia sp. RF2125 (99%, 373/374 bp) for gltA (GenBank accession no.: AF516333) and 100% (756/756 bp) for ompB sequences (GenBank accession no.: JX183538). Only 8.1% of C. felis collected from the urban area were positive in the rickettsial gltA PCR assays; however, the sequences were not determined due to insufficient amounts of amplified fragments.
The overall distribution of rickettsiae detected in the tick and flea samples and their animal hosts are summarized in Table . The detection rates of rickettsial DNA in animal ectoparasites varied from 0.0% to 66.7% for ticks and 0.0% to 96.9% for fleas in different locations (Tables and ). A dendrogram was constructed based on 19 gltA sequence types (375 bp) derived from ticks and fleas from different geographical regions in Peninsular Malaysia. The sequences were differentiated into two distinct groups: one closely related to the type strain of R. felis and another with the type strains of SFG rickettsiae.
Table 4 Overall distribution of rickettsiae detected in ticks/fleas and their animal hosts in each location
In this study, eight gltA sequence variants were identified in the R. felis group (exhibiting 2–13 nucleotide differences), as compared to that of R. felis strain URRWXCal2 (CP000053; ). One matched 99% to that of R. felis strain URRWXCal2 and the remaining seven sequence variants matched 97%–99% with the Rickettsia sp. RF2125 (AF516333). One matched 100% with the uncultured Rickettsia sp. clone-4-G/JP-10-2 reported in dog flea in Guatemala and Costa Rica (JN982949).Citation34 Owing to the close sequence similarity between the SFG rickettsiae, low bootstrap values were noted between branches in the dendrogram (). The gltA sequences derived from this study have been deposited in the GenBank database: KU948226 – KU948246.
Figure 1 Phylogenetic placement of rickettsial gltA sequences (375 bp) amplified from ticks and fleas from different locations. The origins and details of the rickettsiae are presented in extended data (Supplementary Tables S1 and S2) and Table . Bootstrap analysis was performed with 1000 replications. The scale bar indicates the nucleotide substitutions per site. * indicates rickettsiae detected in this study.
Discussion
This study provides an updates on the exposure of Malaysian indigenous community and animal farm workers to rickettsioses through serological assessment against R. conorii and R. felis. Molecular detection of rickettsiae was also conducted in ticks and fleas infesting domestic animals in the respective surveyed areas to identify possible rickettsial agents that were circulating in our environment. Antigenic cross-reactivity has been reported among SFG rickettsiae.Citation2, Citation35 The cross-reactivity between members of SFG rickettsiae, including R. conorii, R. rickettsii, R. helvetica, R. slovaca, R. massiliae, R. africae and others has been highlighted by the manufacturer. Hence, it is possible that IgG for other SFG rickettsiae members to be present in the participants of this study. Similarly, cross-reactivity between R. felis and TG Rickettsia, and R. akari and R. australis was also stated by the manufacturer in the brochure. Znazen et al.Citation36 hypothesized that many reactions due to R. conorii could be caused by R. felis. In this study, individuals seropositive for both rickettsial species were noted of the 5.7% of farm workers, 17.6% of the indigenous community and none of the urban blood donors. However, it is difficult to differentiate R. felis and other SFG rickettsia without the use of further serological assays, such as cross-absorption techniques and western blot.Citation35
Our findings indicate that SFG rickettsioses are prevalent in the indigenous community. Up to 50% of the individuals exhibit seropositivity against R. conorii, whereas approximately one-fourth of the population was previously exposed to R. felis. The seroprevalence of rickettsioses is affected by the geographical differences, lifestyle and occupation of subjects investigated.Citation9 According to a recent survey by Chandren et al.,Citation17 a majority of the indigenous people in Malaysia lived in wooden houses or simple cement homes in close proximity to jungle and plantation areas, which expose them to infected animal ectoparasites such as ticks and fleas. Their close contact with animals and work environment enhances the risk of contracting tick- and flea-borne diseases. In addition, there is a lack of awareness about rickettsioses that hampers the prevention practices in the community. The seropositivity to R. conorii in urban blood donors was relatively low (3.3%) compared with that obtained from a previous serosurvey (1.7%).Citation4
Farm workers may be subjected to increasing risk of tick- and flea-borne diseases.Citation37 For instance, exposures to several tick- and flea-borne pathogens have been reported among farm workers in Tianjin, China.Citation11 The presence of various SFG rickettsiae and R. felis in cattle ticks (H. bispinosa and Rh. microplus) was demonstrated in the vector surveillance in this study (Table ). Although the vectorial capacity of the infected ticks is yet to be established, the results in this study highlight the potential exposure of farm workers to rickettsioses. Higher seropositivity rates against R. conorii and R. felis were observed in older age group (>50 years old), compared with younger age groups (Table ) in this study. This result could be due to long-term persistence of antibody, as also noted for scrub typhus in Malaysia.Citation9 A low seroprevalence (3.3% and 0.0% for R. conorii and R. felis, respectively) was noticed in urban blood donors, and this finding could be due to low exposure of the urban population or different species of ticks found compared with those in the rural areas. In addition, the occurrence of Rickettsia spp. in fleas collected from urban areas is significantly lower compared with the fleas collected from rural areas (Table ) and this result may be an explanation for the relatively lower seropositivity observed in urban blood donors.
Table 3 The detection rates of rickettsiae from fleas collected in each locality
Recent investigations have demonstrated the prevalence of SFG rickettsioses in Southeast Asia. A relatively high seropositivity rate of SFG rickettsial infection has been reported in Thai patients from Chiangrai (33.0%) and Mae Sot (27.3%), who presented with undifferentiated febrile illness.Citation38 A seroprevalence of 20.4% towards R. conorii has also been reported in healthy rural residents from Gag Island, Indonesia.Citation39 Sequence analysis of amplified fragments of gltA, ompA and ompB genes from ticks and fleas collected in this study shows the identification of a number of rickettsiae that had been previously reported, including R. raoultii, R. tamurae, Rickettsia sp. TCM1 and Rickettsia sp. RF2125 (Table ). In addition to these rickettsial species, a R. heilongjiangensis-like organism was detected for the first time from Haemaphysalis ticks in cattle, cats, chickens, dogs and Rh. sanguineus from dogs in urban area. This rickettsia is distributed in the Russian Far East and Northern China. Recently, a phylogenetically related strain (PMK94) was isolated from a patient with septic shock in Thailand.Citation40, Citation41
Since the first report of R. felis infection among rural residents of the central Thai Myanmar border,Citation42 R. felis has been identified in febrile patients in several Asian countries, including Korea, Thailand, and Laos.Citation35 A R. felis-like organism (RFLO) was detected in a febrile patient,Citation43 cat fleas and cynomolgus monkeys in recent Malaysian studies.Citation44, Citation45, Citation46 The findings in this study indicate that 22.5% of the indigenous populations and 16.1% of farm workers were previously been exposed to R. felis. In contrast, none of the urban blood donors tested was seropositive to R. felis (Table ). The R. felis-seropositivity rate (16.1%) in our farm workers was similar to that reported for healthy individuals in Jiangsu province, China.Citation11 In a recent study in Spain, higher seroprevalence of R. felis in rural areas (7.1%) compared with urban (3.5%) and semirural area (1.7%) was reported.Citation47 In this study, the higher R. felis seropositivity in the indigenous community correlates with higher detection rates of R. felis/RFLO in fleas collected from rural areas as compared with urban areas (Table ). The detection of R. felis/RFLO from fleas (C. felis and C. orientis), and various tick species (Haemaphysalis spp., Rh. microplus and Rh. sanguineus) collected from cattle, sheep, chickens, cats and dogs from different study sites in Peninsular Malaysia (Table ), suggests the widespread existence of the rickettsial organism.
Several studies reported the presence of rickettsiae in cattle ticks in the Asia-Pacific region. Uncharacterized Rickettsia sp. has been reported in H. longicornis (12.4%) from grazing cattle in Korea.Citation48 Rickettsiae exhibited high sequence similarities with R. heilongjiangensis, and Rickettsia sp. LON-13 was identified in Rh. microplus in Laos.Citation49 In northeastern China, rickettsiae exhibiting a close phylogenetic relationship with R. raoultii (0.6%) and R. japonica (3.3%) was reported in H. longicornis ticks collected from domestic animals (sheep and cattle).Citation50 In this study, rickettsiae closely related to R. raoultii, R. heilongjiangensis and R. felis/RFLO were identified from some cattle ticks (Table ). All these findings suggest that cattle ticks could be a potential maintenance host for rickettsiae; however, further investigation is required to determine the vectorial capability of the ticks.
In a recent Malaysian study, a rickettsia closely related to R. raoultii has been implicated as the aetiological agent for rickettsioses in two febrile patients.Citation43 R. raoultii, the causative agent for tick-borne lymphadenopathy, was reported in Haemaphysalis ticks from Thailand and is widely distributed in Dermacentor ticks in northern China.Citation51, Citation52, Citation53 In addition to cattle ticks, this study also reports the identification of closely related strains of R. raoultii in Rh. sanguineus and Haemaphysalis ticks infesting peri-domestic animals in the rural villages (Table ). Previously, closely related strains of R. raoultii were reported in Malaysian wild ratsCitation45 and Amblyomma spp. parasitizing wild snakes.Citation54
Rickettsia sp. LON-13 (closely related to R. japonica)Citation55 was reported for the first time from a Haemaphysalis cat tick in this study. A mixed rickettsial infection was suspected in the Haemaphysalis cat tick as R. hulinensis (first isolated from H. concinna ticks collected in Hulin Country, China),Citation56 was also detected from the same tick through sequence analysis of the ompB sequence. In fact, based on BLAST analyses of rickettsial gltA, ompA and ompB gene sequences in this study, the presence of more than one rickettsial organism in a single tick was noted in this study.
Rh. sanguineus, a three-host tick mainly infesting dogs, is the main reservoir of R. conorii.Citation57 The vector surveillance in this study indicates that Rh. sanguineus was the only tick species recovered from urban dogs in this study. The detection of a rickettsia closely related to RFLO (resembling Rickettsia sp. RF2125) in Rh. sanguineus dog ticks, has also been reported in a Chinese study.Citation58
Taken together, this study provides a glimpse of the serological status of Malaysian indigenous people and farm workers against rickettsioses. Some potential limitations of the vector surveillance study are addressed here. For instance, as rickettsiae were detected mainly by PCR approach, it is possible that some might have remained undetected due to the bias of PCR assays in amplifying certain rickettsiae.Citation59 Hence, other microbial detection methods should be used to complement the findings, especially when more than one type of rickettsial species is present in the tick or flea samples. The species status of rickettsiae should be confirmed by isolation of the rickettsiae. Extensive studies should be conducted on a larger sample sizes in multi-locations to assess the correlation between the rickettsia in ticks/fleas and human seropositivity in urban area. Determination of the vectorial capacity of ticks and fleas is necessary to illustrate the involvement of these ectoparasites in the transmission of rickettsioses.
On the basis of serological data obtained in this study, infections due to R. conorii and R. felis appear to be a health concern to the Malaysian indigenous community and farm workers. The data obtained from vector surveillance in this study would be helpful for the public health authority in formulating prevention and control strategies for rickettsioses.
Supplementary Figure 1
Download PDF (252.9 KB)Supplementary Figure 2
Download PDF (312.1 KB)Supplementary Table 1
Download MS Word (19.8 KB)Supplementary Table 2
Download MS Word (13.3 KB)Acknowledgments
We thank all the participants in this study. We also thank Professor Yvonne Lim, Department of Parasitology, University of Malaya, Mr Saidon, Department of Orang Asli Development (JAKOA), Department of Veterinary Services, Ministry of Agriculture and Agro-Based Industry, Malaysia for assistance in the samples collection. We also thank Mdm Harvinder Kaur (Department of Medical Microbiology, Faculty of Medicine, University of Malaya) and Miss Meeta Devi Nurkunasegran (Department of Medical Microbiology, Faculty of Medicine, University of Malaya) for their technical assistance in this project. This study was funded by University of Malaya Research Grants (RP013-2012A/E), Ministry of Science, Technology and Innovation E-Science Fund (SF014-2015), and Postgraduate Research Fund (PG026-2012B).
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
References
- Renvoisé A, Mediannikov O, Raoult D.Old and new tick-borne rickettsioses. Int Health 2009;1: 17–25.
- Parola P, Paddock CD, Socolovschi Cet al.Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013;26: 657–702.
- McQuiston J. Infectious Diseases Related to Travel - Rickettsial (Spotted & Typhus Fevers) and Related Infections (Anaplasmosis & Ehrlichiosis). In: Brunette GW (ed). CDC Health Information for International Travel 2016. USA: Oxford University Press, 2015; 294–298.
- Tay ST, Kamalanathan M, Rohani MY.Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among Malaysian blood donors and febrile patients in the urban areas. Southeast Asian J Trop Med Public Health 2003;34: 165–170.
- Tay ST, Ho TM, Rohani MYet al.Antibodies to Orientia tsutsugamushiRickettsia typhi and spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans R Soc Trop Med Hyg 2000;94: 280–284.
- Tay ST, Kamalanathan M, Suan KAet al.Seroepidemiologic survey of Orientia tsutsugamushiRickettsia typhi, and TT118 spotted fever group rickettsiae in rubber estate workers in Malaysia. Am J Trop Med Hyg 1999;61: 73–77.
- Heinrich N, Dill T, Dobler Get al.High seroprevalence for spotted fever group rickettsiae, is associated with higher temperatures and rural environment in Mbeya region, Southwestern Tanzania. PLoS Negl Trop Dis 2015;9: e0003626.
- Masron T, Masami F, Ismail N.Orang asli in Peninsular Malaysia: population, spatial distribution and socio-economic condition. J Ritsumeikan Soc Sci Hum 2013;6: 75–115.
- Tay ST, Mohamed Zan HA, Lim YALet al.Antibody prevalence and factors associated with exposure to Orientia tsutsugamushi in different aboriginal subgroups in West Malaysia. PLoS Negl Trop Dis 2013;7: e2341.
- Chmielewska-Badora J, Moniuszko A, Zukiewicz-Sobczak Wet al.Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferiAnaplasma phagocytophilumBartonella spp. and Babesia microti. Ann Agric Environ Med 2012;19: 271–274.
- Zhang L, Shan A, Mathew Bet al.Rickettsial Seroepidemiology among farm workers, Tianjin, People's Republic of China. Emerg Infect Dis 2008;14: 938–940.
- Wu XB, Na RH, Wei SSet al.Distribution of tick-borne diseases in China. Parasit Vectors 2013;6: 119.
- Yeon-Joo C, Won-Jong J, Jong-Hyun Ket al.Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis 2005;11: 237.
- Phongmany S, Rolain JM, Phetsouvanh Ret al.Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis 2006;12: 256–262.
- Lai CH, Chang LL, Lin JNet al.Human spotted fever group rickettsioses are underappreciated in southern Taiwan, particularly for the species closely-related to Rickettsia felis. PLoS One 2014;9: e95810.
- Sophie E, Saithip B, Scott FDet al.Two human cases of Rickettsia felis infection, Thailand. Emerg Infect Dis 2014;20: 1780.
- Chandren JR, Wong LP, AbuBakar S.Practices of dengue fever prevention and the associated factors among the orang asli in Peninsular Malaysia. PLoS Negl Trop Dis 2015;9: e0003954.
- Lani R, Mohd Rahim NF, Hassan Het al.First report on the seroprevalence of the Crimean-Congo haemorrhagic fever virus, a tick-borne virus, in Malaysia's Orang Asli population. Eur Rev Med Pharmacol Sci 2015;19: 461–466.
- Mohd Shukri M, Ling Kho K, Ghane Kisomi Met al.Seroprevalence report on tick-borne encephalitis virus and Crimean-Congo hemorrhagic fever virus among Malaysian’s farm workers. BMC Public Health 2015;15: 704.
- Walker AR, Bouattour A, Camicas J-Let al.Ticks of Domestic Animals in Africa: a Guide to Identification of Species. Edinburgh, UK: Bioscience Reports, 2003;221.
- Geevarghese G, Mishra AC. Haemaphysalis Ticks of India.London, UK: Elsevier Science, 2011.
- Black WC 4th, Piesman J.Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA 1994;91: 10034–10038.
- Menier K, Beaucournu JC.Taxonomic study of the genus Ctenocephalides Stiles and Collins, 1930 (Insecta: Siphonaptera: Pulicidae) by using aedeagus characters. J Med Entomol 1998;35: 883–890.
- Duh D, Punda-Polic V, Avsic-Zupanc Tet al.Rickettsia hoogstraalii sp. nov., isolated from hard- and soft-bodied ticks. Int J Syst Evol Microbiol 2010;60: 977–984.
- Labruna MB, Whitworth T, Horta MCet al.Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 2004;42: 90–98.
- Regnery RL, Spruill CL, Plikaytis BD.Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 1991;173: 1576–1589.
- Roux V, Raoult D.Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB. Int J Syst Evol Microbiol 2000;50: 1449–1455.
- Ramírez-Hernández A, Montoya V, Martínez Aet al.Molecular detection of Rickettsia felis in different flea species from Caldas, Colombia. Am J Trop Med Hyg 2013;89: 453–459.
- Lopes MG, Junior JM, Foster RJet al.Ticks and rickettsiae from wildlife in Belize, Central America. Parasit Vectors 2016;9: 1–7.
- Krawczak FS, Nieri-Bastos FA, Nunes FPet al.Rickettsial infection in Amblyomma cajennense ticks and capybaras (Hydrochoerus hydrochaeris in a Brazilian spotted fever-endemic area. Parasit Vectors 2014;7: 1–7.
- Tamura K, Stecher G, Peterson Det al.MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30: 2725–2729.
- Fujita H.Cell culture system for isolation of disease agents: 15 years of experience in Ohara Research Laboratory. Annu Rep Ohara Hosp 2008;48: 21–42.
- Mediannikov OY, Sidelnikov Y, Ivanov Let al.Acute tick-borne rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerg Infect Dis 2004;10: 810–817.
- Troyo A, Alvarez D, Taylor Let al.Rickettsia felis in Ctenocephalides felis from Guatemala and Costa Rica. Am J Trop Med Hyg 2012;86: 1054–1056.
- Hun L, Troyo A.An update on the detection and treatment of Rickettsia felis. Res Rep Trop Med 2012;3: 47–55.
- Znazen A, Rolain JM, Hammami Aet al.Rickettsia felis infection, Tunisia. Emerg Infect Dis 2006;12: 138–140.
- Centers for Disease Control and Prevention.Tick-Borne Diseases - NIOSH Workplace Safety and Health Topic. 2011. Available at http://www.cdc.gov/niosh/topics/tick-borne/ (accessed April 2016).
- Blacksell SD, Kantipong P, Watthanaworawit Wet al.Underrecognized arthropod-borne and zoonotic pathogens in northern and northwestern Thailand: serological evidence and opportunities for awareness. Vector Borne Zoonotic Dis 2015;15: 285–290.
- Richards AL, Ratiwayanto S, Rahardjo Eet al.Serologic evidence of infection with ehrlichiae and spotted fever group rickettsiae among residents of Gag Island, Indonesia. Am J Trop Med Hyg 2003;68: 480–484.
- Gaywee J, Sunyakumthorn P, Rodkvamtook Wet al.Human infection with Rickettsia sp. related to R. japonica, Thailand. Emerg Infect Dis 2007;13: 671–673.
- Takada N, Fujita H, Kawabata Het al.Spotted fever group Rickettsia sp. closely related to Rickettsia japonica, Thailand. Emerg Infect Dis 2009;15: 610–611.
- Parola P, Sanogo OY, Lerdthusnee Ket al.Identification of Rickettsia spp. and Bartonella spp. in from the Thai-Myanmar border. Ann NY Acad Sci 2003;990: 173–181.
- Kho KL, Koh FX, Lakhbeer Singh HKet al.Spotted fever group rickettsioses and murine typhus in a Malaysian teaching hospital. Am J Trop Med Hyg 2016;95: 765–768.
- Mokhtar AS, Tay ST.Molecular detection of Rickettsia felisBartonella henselae, and B. clarridgeiae in fleas from domestic dogs and cats in Malaysia. Am J Trop Med Hyg 2011;85: 931–933.
- Tay ST, Mokhtar AS, Low KCet al.Identification of rickettsiae from wild rats and cat fleas in Malaysia. Med Vet Entomol 2014;28: 104–108.
- Tay ST, Koh FX, Kho KLet al.Rickettsial infections in monkeys, Malaysia. Emerg Infect Dis 2015;21: 545.
- Nogueras MM, Cardenosa N, Sanfeliu Iet al.Serological evidence of infection with Rickettsia typhi and Rickettsia felis among the human population of Catalonia, in the northeast of Spain. Am J Trop Med Hyg 2006;74: 123–126.
- Kang SW, Doan HT, Choe SEet al.Molecular investigation of tick-borne pathogens in ticks from grazing cattle in Korea. Parasitol Int 2013;62: 276–282.
- Kernif T, Socolovschi C, Wells Ket al.Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp Immunol Microbiol Infect Dis 2012;35: 51–57.
- Dong X, Chen XP, Liu Net al.Co-circulation of multiple species of Rickettsiales bacteria in one single species of hard ticks in Shenyang, China. Ticks Tick Borne Dis 2014;5: 727–733.
- Parola P, Rovery C, Rolain JMet al.Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg Infect Dis 2009;15: 1105–1108.
- Ahantarig A, Malaisri P, Hirunkanokpun Set al.Detection of Rickettsia and a novel Haemaphysalis shimoga symbiont bacterium in ticks in Thailand. Curr Microbiol 2011;62: 1496–1502.
- Tian ZC, Liu GY, Shen Het al.First report on the occurrence of Rickettsia slovaca and Rickettsia raoultii in Dermacentor silvarum in China. Parasit Vectors 2012;5: 19.
- Kho KL, Koh FX, Tay ST.Molecular evidence of potential novel spotted fever group rickettsiae, Anaplasma and Ehrlichia species in Amblyomma ticks parasitizing wild snakes. Parasit Vectors 2015;8: 112.
- Hanaoka N, Matsutani M, Kawabata Het al.Diagnostic assay for Rickettsia japonica. Emerg Infect Dis 2009;15: 1994–1997.
- Yu X, Jin Y, Fan Met al.Genotypic and antigenic identification of two new strains of spotted fever group rickettsiae isolated from China. J Clin Microbiol 1993;31: 83–88.
- Levin ML, Killmaster LF, Zemtsova GE.Domestic dogs (Canis familiaris as reservoir hosts for Rickettsia conorii. Vector Borne Zoonotic Dis 2012;12: 28–33.
- Zhang J, Lu G, Kelly Pet al.First report of Rickettsia felis in China. BMC Infect Dis 2014;14: 682.
- Kanagawa T.Bias and artifacts in multitemplate polymerase chain reactions (PCR). J Biosci Bioeng 2003;96: 317–323.
