Abstract
Central to the One Health approach and any disease eradication program is the question of whether a pathogen has a non-human reservoir. Despite well-established conceptual frameworks that define a reservoir of infection, empirical characterization of reservoirs often remains controversial, challenging and sometimes misleading. What is essentially missing are applicable requirements that standardize the use of the term ‘reservoir of infection’ across multiple disciplines. We propose an empirical framework, considering maintenance and feasible transmission of a pathogen, to standardize the acceptance of a disease reservoir across multiple disciplines. We demonstrate the intended use of these requirements by applying them to different diseases that are known to infect both humans and animals.
Emerging Microbes & Infections (2017) 6, e79; doi:https://dx.doi.org/10.1038/emi.2017.65; published online 6 September 2017
A reservoir needs to maintain the pathogen and have a feasible transmission route
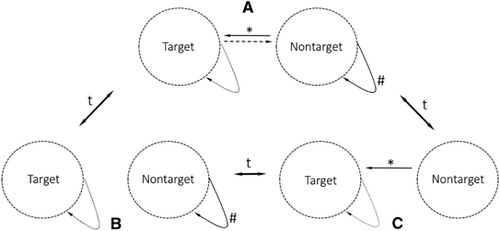
The high prevalence of infectious agents of zoonotic and anthropozoonotic origin pose a major health threat to both human and animal populations. A conceptual framework for understanding a reservoir of infection has been established through various studies that have emphasized different aspects of zoonotic diseases.Citation1, Citation2, Citation3, Citation4 However, empirical characterization of reservoirs often remains controversial and challenging. The most applicable and accepted way to investigate and define a reservoir emphasizes the annotation of a target group (Figure 1), which is an explicitly defined population of interest in a dynamic and heterogeneous landscape (for example, humans at the livestock–wildlife–human interface).Citation4, Citation5 According to Haydon et al.,Citation4 the target group is a matter of definition and may therefore be disconnected from the ecological reality. The target group provides a directionality to the study of a reservoir system. All other susceptible populations (non-target populations), which directly or indirectly connect epidemiologically to the target (Figures 1 and 2), can be part of the potential reservoir.Citation4 For a non-target population to be considered an accepted functional reservoir, maintenance of a single pathogen in the population needs to be shown in combination with a feasible transmission route between the target and non-target populations.Citation4
Figure 1 Three scenarios describing the dynamics of a simple reservoir system. (A) Pathogen maintenance in the non-target population and feasible transmission route towards the target population. Only this constellation fulfills the requirements of a functional reservoir system. (B) Pathogen maintenance in the non-target but no feasible transmission route towards the target population. This is a likely situation whether contact rates between the non-target and target populations are below the threshold. (C) No pathogen maintenance in the non-target, but a feasible transmission route exists. An example of the effect of a vaccination strategy in the non-target population. The dynamic of the system is indicated by arrows associated with a ‘t’ (time factor). #Maintenance, *feasible transmission, solid arrows=obligatory, broken line=optional.
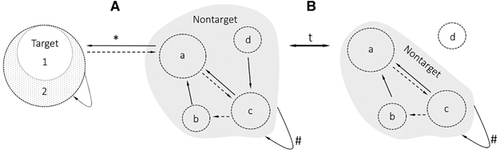
Although the conceptual framework of a disease reservoir is already well-defined, applicable requirements for an evidence-based rejection or acceptance of a reservoir are currently missing. In particular, interdisciplinary standards on genetic and functional similarities of reservoir and human isolates of pathogens are nonexistent. Considering the increase in interdisciplinary research, we see the need to critically discuss and standardize the use of the term ‘reservoir of infection’ across different research fields to oppose the tendency of published scientific data to exaggerate positive results and hype certain areas of science.Citation6, Citation7 Although we do not claim absolute standardization of empirical requirements to accept a reservoir across disciplines, we present a framework to serve as a basis for a pending discussion in the growing One Health community. The simplicity and functional orientation of the presented framework allows for straightforward application but does not negate more complex populations, as the same principles can be applied to multi-species systems and metapopulations (Figure 2).
Figure 2 The simplicity and functional orientation of the presented framework allows for straightforward application but does not negate more complex populations. The same principles apply to multi-species systems and metapopulations. The defined target group may be adjusted based on interest and may therefore include metapopulations (targets 1 and 2). The non-target group increases in complexity due to the inclusion of multiple populations (a–d). (A) Similarly, to a simple reservoir system, all susceptible populations that connect to the target either (a) directly or (b–d) indirectly are part of the non-target population. (B) Temporal shifts in the ecological landscape of the non-target population may lead to the (d) exclusion of populations either due to lack of connectivity or susceptibility. The dynamic of the system is indicated by arrows associated with a ‘t’ (time factor). #Maintenance, *feasible transmission, solid arrows=obligatory, broken line=optional.
According to the accepted definition of a reservoir proposed by Haydon et al.,Citation4 we discuss the requirements in two parts: the pathogen’s maintenance in a potential population or community followed by a discussion on proof of a feasible transmission route. Although the two components are addressed separately, only together they demonstrate the existence of a functional reservoir.
Proof of pathogen maintenance in a potential reservoir
Increases in technological advancements (for example, next-generation sequencing) and vast quantities of available data have not led to concrete applicable criteria when examining the capacity of a pathogen to be maintained in a population. Recognizing both the ethical limitations in regards to animal testingCitation8 and the advances in the molecular detection of pathogens, we propose the following criteria to demonstrate the maintenance of a pathogen in a population: (i) a high-genetic similarity of the pathogen found in the reservoir system, (ii) a high degree of functional similarity (infectivity and viability), and (iii) a longitudinal approach that considers the factor of time (Table ). Owing to the functional orientation of the requirements and for simplicity, all entities involved in the biological lifecycles of a parasite (for example, primary and intermediate hosts) should be considered a single functional unit. Appropriate sequence and functional analysis of a pathogen isolated multiple times from a potential reservoir should be required to prove that a pathogen is maintained in a population. The ability to quickly and cheaply sequence whole genomes has allowed for better genetic resolution.Citation49, Citation50 Sequence data can be used to examine similarity in the pathogen between a potential reservoir and a target. However, mutation rates vary significantly between pathogensCitation51, Citation52 and the threshold for sequence and functional similarity must be individually defined and accepted by the scientific community. A single-nucleotide difference can potentially result in a loss of infectivity, for example, when important invasion mechanisms are affected (receptor affinity). In bacteria, investigations can be further complicated by plasmids that can be exchanged and mutated over time.Citation53 A high amount of phylogenetic relatedness of pathogens isolated from the non-target and target populations does not provide sufficient evidence for the involvement of a pathogen and its ability to infect both groups. Importantly, DNA-based analyses only provide information on the functional potential of a pathogen and must not reflect the gene-expression within a host.Citation54 For example, the bacterium Treponema paraluiscuniculi (which causes syphilis in rabbits), is over 99% identical on the basis of the whole genome to the human pathogen T. pallidum (which causes human treponematosis), but does not infect humans.Citation55 As phylogenetic information fails to reflect the downstream effects of mutations, proof that a pathogen can proliferate in the potential reservoir is required.Citation56 Information on the transcriptome and proteome of bacteria or the phenotype of viruses are necessary to see the effect of mutations on pathogen viability.Citation57 There are different ways to test for the functional ability of a pathogen in different species. Owing to the ethical concerns, cell and tissue assays have been increasingly used in therapeutic research instead of animal models.Citation8 Although these assays are limited in their conclusiveness, they can provide important insight into the molecular mechanisms involved. For example, the failure to infect primary tissue culture from rhesus macaques with human immunodeficiency virus 1 (HIV-1) demonstrates that non-human primates were unlikely to act as a maintenance population (Table ).Citation58 In some instances, for example, with uncultivable bacteria such as Treponema pallidum, it may be necessary to use animal models to examine the functionality of a pathogen within a potential reservoir species. Knowledge of the biology of the pathogen is essential to properly define a sequence and functional similarity threshold for a particular reservoir system.
Table 1 Applicable requirements that need to be fulfilled for the acceptance of a disease reservoir and their exemplary use in selected diseases that are known to infect humans and animals
When examining pathogen maintenance, a longitudinal approach is required to consider the dynamics of a potential reservoir system, including the influence of genetic variation in any given population. Defining a population that was infected at a single time point as a maintenance population for a pathogen is based on assumptions and is therefore speculative. Sero-prevalence surveys are an attractive way to detect the presence of pathogens in a population, as it indicates that an immunocompetent subject was in contact with the pathogen.Citation1 However, only longitudinal studies with adequate sampling regimes (multiple sampling) to test for antibodies against a pathogen can provide information on the timing or frequency of infection, both of which are important for reservoir studies.Citation59 Furthermore, cross-reactivity and erroneous assays can lead to false-positive results. For more diffuse reservoir systems, including multi-species compositions where the diversity of host susceptibility (at the individual, species or population level) protects against widespread infection (dilution effect),Citation60 a longer time frame must be applied. This guarantees a more accurate understanding of the maintenance within a population (for example, EbolaCitation36).
Proof of feasible transmission route
Maintenance of a pathogen in a population alone does not provide sufficient proof that a functional reservoir exists. A connection between the target and the non-target populations must be established;Citation4 otherwise the non-target population remains a maintenance population with the potential to be a reservoir. Therefore, the determination of a feasible and somewhat permanent transmission route between the non-target and target populations is key to identifying a reservoir system (Figure 1). For multi-species reservoir systems, the transmission route between the target and non-target populations may be indirect (Figure 2, connection between b and target), possibly incorporating different hierarchical levels of a non-target community.Citation4, Citation61 The type of transmission route dictates the form of evidence needed to prove that a feasible transmission route exists between the reservoir and target. For simplicity, we define vectors as part of the transmission route, although under certain circumstances (for example, permanency or substantial amplification in the vector), they may act as part of the non-target community.Citation61 Four basic requirements need to be met to make a compelling argument for the existence of a feasible transmission route: (i) spatial (direct or indirect) and temporal connectivity between the reservoir system and the target population, (ii) pathogen involvement in this feasible transmission route, (iii) proof of viability of the pathogen during the proposed transmission route and (iv) a longitudinal approach that requires the isolation of a pathogen multiple times in a given transmission route (Table ).
To prove the feasibility of a transmission route, direct or indirect spatial connectivity as well as temporal overlap between the non-target and target populations must be present. Connectivity measurements depend on the type of transmission route; for example, direct contact transmission requires overlapping territory. Computational tools can help determine the necessary overlap in a population by modeling the transmission across an affected population.Citation62 In addition to spatial and temporal overlap, the involvement of the pathogen in the particular transmission route needs to be shown, which again requires long-term field projects. In the case of Lyme disease caused by Borrelia burgdorferi, nucleic acids from the bacterium were detected in ticks using PCR.Citation44 However, the detection of DNA does not directly prove that transmission occurs. To gain further confidence that the transmission is feasible, it is therefore essential to show that the infectious organism remains viable during the proposed transmission route.Citation45 This means that in addition to PCR detection, the viable pathogen needs to be isolated during a transmission event, where the measure of viability depends on the type of pathogen. In airborne transmission, for example, environmental factors such as size of droplets, UV light and humidity can greatly influence the transmissibility of a virus (as reviewed in TangCitation63). If the amount of viable and therefore infectious organisms is below the infectious dose, the particular transmission route is unfeasible. Without a feasible transmission route between target and non-target populations, no functional reservoir exists. Furthermore, to include all parts of a reservoir population, long-term investigations must focus on the transmission between the non-target and target groups as well as feasible transmission within the non-target community.Citation61 Unconnected maintenance host populations may become a future reservoir through temporal shifts of the ecosystem.
Challenges of implementation
Biological systems are dynamic and can change over time (Figure 1). Single transmission events do not confirm a reservoir of infection (for example, HIV,Citation20 Table ). It is therefore important to show continuity and persistence in both maintenance and transmission, which can only be achieved through multiple and adequately timed (field) investigations. Well-designed intervention studies can be used as quasi-experiments to study a reservoir of infection but should not be used as a stand-alone test for the existence of a reservoir.Citation1 Despite sufficient planning, the cause and effect of intervention studies are often difficult to determineCitation1, Citation64 and the removal of a pathogen from a particular ecosystem may cause unanticipated effects. A negative outcome does not necessary indicate the lack of a reservoir or transmission route.Citation64, Citation65 Instead, it can show that the intervention may have been incomplete or that the complexity of a reservoir is not entirely understood.
Pathogens must be studied in the context of natural ecosystems. The complexity of reservoir systems increases as multiple non-target populations interact as an ecological entity, which is influenced by factors such as competition, co-existence or predation.Citation66 Furthermore, the artificial environment in a laboratory, which is often used to study the susceptibility of a species, differs substantially from a natural setting.Citation67 The use of laboratory animals or cell- and tissue-based assays can be advantageous when studying pathogenicity, but it cannot solely contribute to the understanding of the epidemiology of a pathogen, which is largely impacted by variables such as genetic diversity, co-infection, cross-protective immunity and spatial connectivity. As a consequence, any epidemiological model requires additional information on the geographic range and the ecological landscape.Citation68 This includes population densities and functional profiles of species that are involved in the reservoir system.Citation60, Citation69 The importance of sample size in field studies and animal experiments cannot be stressed enough as it greatly affects the efficacy of analysis, especially in reservoirs with low-frequency crossover events.
Neither laboratory experiments, nor intervention studies, nor epidemiological models alone can provide a full understanding of a natural reservoir of infection. Only the combination of methods that are based on established and validated species-specific assays and technically sound field investigations can provide confidence that the pathogen is maintained in a non-target population and that a feasible transmission route exists. This, however, requires the political will and financial support to conduct long-term One Health studies to explore diseases in their natural context.
Conclusion
The term ‘disease reservoir’ should be used carefully and only if there is convincing evidence demonstrating the maintenance and a feasible transmission route of a particular pathogen (Figure 1). We propose overarching requirements that must be fulfilled to provide ample proof that a reservoir exists (Table ). Classical reservoir systems (for example, Lyme disease caused by Borrelia burgdorferi) fulfill all of the requirements proposed in this study, whereas some well-known diseases, such as Ebola, need further research until a reservoir system can be accepted (Table ). For the pathogens without an accepted reservoir, the framework introduced in this study also indicates the outstanding questions that future research should focus on to investigate the presence of a reservoir system. A broader expert-based multidisciplinary discussion is needed to develop standards for the diversity of pathogens.
Acknowledgments
VJM is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). SK received funding from the German Research Foundation (DFG): KN1097–3-1 and KN1097–4-1. We would like to thank the reviewer for addressing important aspects that clearly improved the proposed framework.
References
- Viana M, Mancy R, Biek Ret al.Assembling evidence for identifying reservoirs of infection. Trends Ecol Evol 2014;29: 270–279.
- Drexler JF, Corman VM, Müller MAet al.Bats host major mammalian paramyxoviruses. Nat Commun 2012;3: 796.
- Ashford R.When is a reservoir not a reservoir? Emerg Infect Dis 2003;9: 1495–1496.
- Haydon DT, Cleaveland S, Taylor LHet al.Identifying reservoirs of infection: a conceptual and practical challenge. Emerg Infect Dis 2002;8: 1468–1473.
- Dunning JB, Stewart DJ, Danielson BJet al.Spatially explicit population models: current forms and future uses. Ecol Appl 1995;5: 3–11.
- Caulfield T, Sipp D, Murry CEet al.Confronting stem cell hype. Science 2016;352: 776–777.
- Vinkers CH, Tijdink JK, Otte WM.Use of positive and negative words in scientific PubMed abstracts between 1974 and 2014: retrospective analysis. BMJ 2015;351: h6467–h6777.
- Ferdowsian HR, Beck N.Ethical and scientific considerations regarding animal testing and research. PLoS One 2011;6: e24059.
- Simon G, Larsen LE, Dürrwald Ret al.European surveillance network for influenza in pigs: surveillance programs, diagnostic tools and Swine influenza virus subtypes identified in 14 European countries from 2010 to 2013. PLoS One 2014;9: e115815.
- Brookes SM, Núñez A, Choudhury Bet al.Replication, pathogenesis and transmission of pandemic (H1N1) 2009 virus in non-immune pigs. PLoS One 2010;5: e9068.
- Steel J, Staeheli P, Mubareka Set al.Transmission of pandemic H1N1 influenza virus and impact of prior exposure to seasonal strains or interferon treatment. J Virol 2010;84: 21–26.
- Maines TR, Jayaraman A, Belser JAet al.Transmission and pathogenesis of swine-origin 2009A (H1N1) influenza viruses in ferrets and mice. Science 2009;325: 484–487.
- Müller MA, Meyer B, Corman VMet al.Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis 2015;15: 559–564.
- Mohd HA, Al-Tawfiq JA, Memish ZA.Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol J 2016;13: 87.
- Van Doremalen N, Bushmaker T, Munster V.Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill 2013;18: 20590.
- Haagmans BL, van den Brand JM, Raj VSet al.An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2015;351: 77–81.
- Dean AS, Crump L, Greter Het al.Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis 2012;6: e1865.
- de Figueiredo P, Ficht TA, Rice-Ficht Aet al.Pathogenesis and immunobiology of brucellosis: review of Brucella–Host Interactions. Am J Pathol 2015;185: 1505–1517.
- Paixão TA, Roux CM, Den Hartigh ABet al.Establishment of systemic Brucella melitensis infection through the digestive tract requires urease, the type IV secretion system, and lipopolysaccharide O antigen. Infect Immun 2009;77: 4197–4208.
- Gao F, Bailes E, Robertson DLet al.Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 1999;397: 436–441.
- Knauf S, Dahlmann F, Batamuzi EKet al.Validation of serological tests for the detection of antibodies against Treponema pallidum in nonhuman primates. PLoS Negl Trop Dis 2015;9: e0003637.
- Knauf S, Raphael J, Mitjà Oet al.Isolation of Treponema DNA from necrophagous flies in a natural ecosystem. EBioMedicine 2016;11: 85–90.
- Harper KN, Knauf S. Treponema pallidum infection in primates: clinical manifestations, epidemiology, and evolution of a stealthy pathogen. In: Brinkworth JF, Pechenkina K (eds). Primates, Pathogens, and Evolution. New York, NY: Springer, 2013: 189–219.
- Walker TM, Lalor MK, Broda Aet al.Assessment of Mycobacterium tuberculosis transmission in Oxfordshire, UK, 2007–12, with whole pathogen genome sequences: an observational study. Lancet Respir Med 2014;2: 285–292.
- Fitzgerald S, Kaneene J.Wildlife reservoirs of bovine tuberculosis worldwide: hosts, pathology, surveillance, and control. Vet Pathol 2013;50: 488–499.
- Michel AL, Müller B, van Helden PD.Mycobacterium bovis at the animal-human interface: a problem, or not? Vet Microbiol 2010;140: 371–381.
- Sidwa TJ, Wilson PJ, Moore GMet al.Evaluation of oral rabies vaccination programs for control of rabies epizootics in coyotes and gray foxes: 1995–2003. J Am Vet Med Assoc 2005;227: 785–792.
- Rupprecht CE, Hanlon CA, Hemachudha T.Rabies re-examined. Lancet Infect Dis 2002;2: 327–343.
- Hemachudha T, Laothamatas J, Rupprecht CE.Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol 2002;1: 101–109.
- Chautan M, Pontier D, Artois M.Role of rabies in recent demographic changes in red fox (Vulpes vulpes populations in Europe. Mammalia 2000;64: 391–410.
- Schweiger A, Ammann RW, Candinas Det al.Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis 2007;13: 878–882.
- Veit P, Bilger B, Schad Vet al.Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology 1995;110: 79–86.
- Craig P.Echinococcus multilocularis. Curr Opin Infect Dis 2003;16: 437–444.
- Kuenzi AJ, Douglass RJ, Bond CWet al.Long-term dynamics of Sin Nombre viral RNA and antibody in deer mice in Montana. J Wildl Dis 2005;41: 473–481.
- Bi Z, Formenty PB, Roth CE.Hantavirus infection: a review and global update. J Infect Dev Ctries 2008;2: 3–23.
- Leroy EM, Kumulungui B, Pourrut Xet al.Fruit bats as reservoirs of Ebola virus. Nature 2005;438: 575–576.
- Pourrut X, Kumulungui B, Wittmann Tet al.The natural history of Ebola virus in Africa. Microb Infect 2005;7: 1005–1014.
- Weingartl HM, Embury-Hyatt C, Nfon Cet al.Transmission of Ebola virus from pigs to non-human primates. Sci Rep 2012;2: 811.
- Roth A, Mercier A, Lepers Cet al.Concurrent outbreaks of dengue, chikungunya and Zika virus infections-an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill 2014;19: 20929.
- Favoretto S, Araujo D, Oliveira Det al.First detection of Zika virus in neotropical primates in Brazil: a possible new reservoir. BioRxiv 2016, doi: http://www.biorxiv.org/content/early/2016/04/20/049395.
- Dick G.Zika virus (II). Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952;46: 521–534.
- Marano G, Pupella S, Vaglio Set al.Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2016;14: 95–100.
- Ogden NH, Bouchard C, Kurtenbach Ket al.Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ Health Perspect 2010;118: 909–914.
- Barbour AG, Maupin GO, Teltow GJet al.Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis 1996;173: 403–409.
- Moriarty TJ, Norman MU, Colarusso Pet al.Real-time high resolution 3D imaging of the lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog 2008;4: e1000090.
- Tilly K, Rosa PA, Stewart PE.Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am 2008;22: 217–234.
- Almeida MA, Cardoso JDC, dos Santos Eet al.Surveillance for yellow fever virus in non-human primates in Southern Brazil, 2001–2011: a tool for prioritizing human populations for vaccination. PLoS Negl Trop Dis 2014;8: e2741.
- Monath TP.Yellow fever: an update. Lancet Infect Dis 2001;1: 11–20.
- Roetzer A, Diel R, Kohl TAet al.Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLoS Med 2013;10: e1001387.
- Sanger F, Nicklen S, Coulson AR.DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci 1977;74: 5463–5467.
- Dapp MJ, Heineman RH, Mansky LM.Interrelationship between HIV-1 fitness and mutation rate. J Mol Biol 2013;425: 41–53.
- Taylor LH, Latham SM, Mark E.Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 2001;356: 983–989.
- Okinaka R, Cloud K, Hampton Oet al.Sequence, assembly and analysis of pX01 and pX02. J Appl Microbiol 1999;87: 261–262.
- Miosge LA, Field MA, Sontani Yet al.Comparison of predicted and actual consequences of missense mutations. Proc Natl Acad Sci 2015;112: 5189–5198.
- Šmajs D, Zobaníková M, Strouhal Met al.Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS One 2011;6: e20415.
- Morozova O, Marra MA.Applications of next-generation sequencing technologies in functional genomics. Genomics 2008;92: 255–264.
- Prakash T, Taylor TD.Functional assignment of metagenomic data: challenges and applications. Brief Bioinform 2012;13: 711–727.
- Hofmann W, Schubert D, LaBonte Jet al.Species-specific, postentry barriers to primate immunodeficiency virus infection. J Virol 1999;73: 10020–10028.
- Hoye BJ, Munster VJ, Nishiura Het al.Reconstructing an annual cycle of interaction: natural infection and antibody dynamics to avian influenza along a migratory flyway. Oikos 2011;120: 748–755.
- Roche B, Rohani P, Dobson APet al.The impact of community organization on vector-borne pathogens. Am Nat 2012;181: 1–11.
- Zeppelini CG, de Almeida AMP, Cordeiro-Estrela P.Zoonoses as ecological entities: a case review of plague. PLoS Negl Trop Dis 2016;10: e0004949.
- Kao RR, Haydon DT, Lycett SJet al.Supersize me: how whole-genome sequencing and big data are transforming epidemiology. Trends Microbiol 2014;22: 282–291.
- Tang JW.The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface 2009;6: 737–746.
- Dietze R, Barros GB, Teixeira Let al.Effect of eliminating seropositive canines on the transmission of visceral leishmaniasis in Brazil. Clin Infect Dis 1997;25: 1240–1242.
- Ezenwa VO, Jolles AE.Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science 2015;347: 175–177.
- Menge BA, Sutherland JP.Species diversity gradients: synthesis of the roles of predation, competition, and temporal heterogeneity. Am Nat 1976;110: 351–369.
- McCafferty J, Mühlbauer M, Gharaibeh RZet al.Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J 2013;7: 2116–2125.
- Lindenfors P, Nunn CL, Jones KEet al.Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Glob Ecol Biogeogr 2007;16: 496–509.
- Giardina AR, Schmidt KA, Schauber EMet al.Modeling the role of songbirds and rodents in the ecology of Lyme disease. Can J Zool 2000;78: 2184–2197.
