Abstract
Flaviviruses are RNA viruses that constitute a worrisome threat to global human and animal health. Zika virus (ZIKV), which was initially reported to cause a mild disease, recently spread in the Americas, infecting millions of people. During this recent epidemic, ZIKV infection has been linked to serious neurological diseases and birth defects, specifically Guillain-Barrè syndrome (GBS) and microcephaly. Because information about ZIKV immunity remains scarce, we assessed the humoral response of immunocompetent mice to infection with three viral strains of diverse geographical origin (Africa, Asia and America). No infected animals showed any sign of disease or died after infection. However, specific neutralizing antibodies were elicited in all infected mice. Considering the rapid expansion of ZIKV throughout the American continent and its co-circulation with other medically relevant flaviviruses, such as West Nile virus (WNV), the induction of protective immunity between ZIKV and WNV was analyzed. Remarkably, protection after challenge with WNV was observed in mice previously infected with ZIKV, as survival rates were significantly higher than in control mice. Moreover, previous ZIKV infection enhanced the humoral immune response against WNV. These findings may be relevant in geographical areas where both ZIKV and WNV co-circulate, as well as for the future development of broad-spectrum flavivirus vaccines.
Emerging Microbes & Infections (2017) 6, e81; doi:10.1038/emi.2017.68; published online 20 September 2017
Introduction
Flaviviruses constitute a group of arboviruses that often represent a worrisome threat to global human and animal health. For example, since the introduction of West Nile virus (WNV) to the United States in 1999, the virus has spread across the country, where it is now considered endemic, and has caused thousands of human deaths. Similarly, WNV outbreaks are increasing in number, frequency, and severity in Europe, causing a considerable number of neuroinvasive cases in animals and humans, with hundreds of human and horse deaths across the continent.Citation1, Citation2
More recently, the introduction and explosive spread of Zika virus (ZIKV) in the Americas has resulted in the infection of millions of people.Citation3 ZIKV infection had initially been characterized as causing a mild disease, with sporadic reports of an association with Guillain-Barrè syndrome (GBS).Citation3, Citation4, Citation5 However, since the end of 2015, an increase in the number of GBS-associated cases and an astonishing number of microcephaly cases in fetuses and infants in Brazil have been linked to ZIKV infection, raising serious worldwide health and social concerns.Citation3, Citation4, Citation5, Citation6
Currently, information regarding the pathogenicity and cross-reactive immunity of ZIKV is limited, in part due to the lack of an accurate small animal model. Non-human primates can be used,Citation7, Citation8, Citation9, Citation10, Citation11 but in many instances, ethical and cost reasons discourage their utilization. Early ZIKV studies were based on the inoculation of mouse-adapted viral strains and were mostly conducted by direct intracranial inoculation of the virus and/or the use of juvenile animals.Citation12, Citation13, Citation14, Citation15 Hence, no accurate small animal model for ZIKV infection is currently available. However, both immunodeficientCitation16, Citation17, Citation18, Citation19, Citation20, Citation21, Citation22 and immunocompetent miceCitation11, Citation23, Citation24 are proving to be useful for the study of the pathogenesis and humoral responses elicited by ZIKV.
Antibody-mediated immunity is considered a major player in the protection against flavivirus infections,Citation25 including ZIKV infection.Citation26 Antibodies elicited against these viruses are often cross-reactive with other related flaviviruses; however, while they sometimes confer cross-protection, in other cases harmful consequences are observed due to an antibody-dependent enhancement (ADE) effect.Citation27 This, together with the high reported antibody prevalence in ZIKV-infected populations,Citation3, Citation28, Citation29 may have special relevance in areas where different flaviviruses co-circulate. Indeed, the relationships between the immune response after ZIKV and subsequent or previous Dengue virus (DENV) infection, endemic in areas of central and South America, are being now assessed.Citation30, Citation31, Citation32, Citation33 In this study, with the potential colonization of new territories by ZIKV, we explored the capability of this new invader to induce protection against WNV.
Materials and Methods
Ethics statement
All animals were handled in strict accordance with the guidelines of the European Community 86/609/CEE. The protocols were approved by the Committee on Ethics of animal experimentation of our Institution (INIA’s permit numbers 2016-006 and 2017-008). All experiments with infectious viruses were conducted in biosafety level 3 facilities.
Viruses
ZIKV strains of American (PA259459) and Asian (FSS13025) origin were kindly provided by Dr R. B. Tesh (World Reference Center for Emerging Viruses and Arboviruses, WRCEVA) and a strain of African origin (MR766) by Dr A. Vázquez (Instituto de Salud Carlos III, ISCIII). Vesicular stomatitis virus (VSV) Indiana strain was kindly provided by Dr Rafael Blasco (Department of Biotechnology, INIA). ZIKV strains, VSV, and a WNV NY99 strainCitation34 were propagated and titrated on Vero-81 cells (ATCC CCL-81, Manassas, VA, USA) as described.Citation35 ZIKV strains were partially sequenced (Macrogen Europe; Amsterdam, The Netherlands) using specific primers available upon request.
Mice
Groups (n=10–20) of 8-week-old Swiss albino CD1 male mice were intraperitoneally (i.p.) inoculated with 5 × 105 plaque-forming units (pfu)/mouse of the African, American and Asian ZIKV strains, or VSV, in 100 μL of Eagle’s minimal essential medium (EMEM) (BE12-125F, Lonza, Verviers, Belgium) as the vehicle. As a control, a group of CD1 mice was inoculated with vehicle alone. Mice were i.p. challenged with 104 pfu/mouse of a neurovirulent WNV NY99 strain 14 days after primary ZIKV or VSV infection. Viruses were back titrated to confirm inoculation doses. Animals were bled prior to infection, 5 and 13 days post-primary infection, and 5, 7 and 26 days post-WNV challenge (corresponding to 19, 21 and 40 days post-ZIKV infection, d.p.i.). Viral infections and sample collection were conducted as described.Citation36, Citation37, Citation38
During the experiments, all animals were monitored daily and received water and food ad libitum. Those mice showing signs of disease were anesthetized and killed, as were all surviving animals at the end of the experiment (40 days after ZIKV infection).
Immunological assays
Heat-inactivated sera (1:100 dilution) were assayed for anti-ZIKV antibodies by enzyme-linked immunosorbent assay (ELISA) as describedCitation38, Citation39, Citation40 using heat-inactivated viruses (WNV or ZIKV) produced in Vero cells as antigens. As positive controls, a WNV-specific mice sera poolCitation34 and a ZIKV-specific mouse monoclonal antibody (mAb T39627), kindly provided by Dr R. Tesh (WRCEVA), were included in the assay. Specific antibody induction was represented as the fold increase of each sample sera absorbance490 over the absorbance490 of control sera from uninfected mice.
Measurements of serum IFN-α level were performed using the VeriKine Mouse Interferon Alpha ELISA Kit (PBL Assays Science, Piscataway, NJ, USA) following the recommendations provided by the manufacturer.
Plaque reduction neutralization tests (PRNT) were conducted on Vero cells with WNV and ZIKV strains MR766, FSS13025 and PA259459, using twofold serial sera dilutions.Citation39, Citation41 Titers were calculated as the reciprocal of the serum dilution, diluted at least 1:20, which reduced plaque formation ≥90% (PRNT90) relative to samples incubated with negative control pooled sera.
Virological assays
Viral RNA was extracted from the processed tissues and fluids using a Speedtools RNA virus extraction kit (Biotools, Madrid, Spain). ZIKV-RNA was quantified by real time quantitative reverse transcription PCR (qRT-PCR) as described,Citation42 using a standard curve with previously titrated viruses.
Statistical analyses
Data were analyzed using GraphPad PRISM 6 (GraphPad Software, La Jolla, CA, USA). Two-way ANOVA with Bonferroni post t-test and Kaplan–Meier survival analysis were performed. Asterisks in the figures denote statistically significant differences *P<0.05, **P<0.01 and ***P<0.001.
Results
ZIKV infection protects mice against WNV challenge
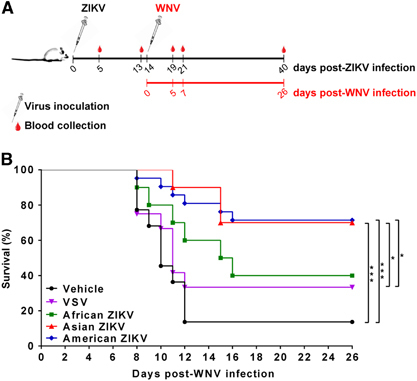
All mice survived i.p. ZIKV infection regardless of the origin of the infecting strain (African, Asian or American), and none showed signs of disease. Tissues (brains, spleens, and testicles) and fluids (urine and oral swabs) collected from ZIKV-infected, killed mice (six mice/group) at 5 d.p.i. were analyzed by qRT-PCR. Viral RNA was sporadically detected in a very limited number of samples (three brains, two spleens, one testicle and one urine sample), with titers ranging from 2 × 104 to 2 × 109 pfu/gram of tissue. To determine whether this infection was sufficient to elicit a protective immune response against other flaviviruses, mice infected with any of the ZIKV strains, with an unrelated virus (VSV), or with vehicle alone were challenged 14 days after infection with a highly virulent WNV strain (Figure 1A). Survival rates of 40%, 70% and 71% were recorded for WNV challenged mice initially infected with the African, Asian or American ZIKV strains, respectively, higher than the 33% and 14% recorded among VSV-inoculated and vehicle-inoculated animals, respectively (Figure 1B). Statistical analysis of survival curves confirmed that infection with the Asian or American strains conferred significant protection against a subsequent challenge with WNV compared to non-ZIKV-infected mice (vehicle and VSV-inoculated animals), confirming that the observed effect was specific for ZIKV. This was also observed when the mean survival times (MSTs) after WNV challenge were determined, as they were also higher in ZIKV-infected mice (>26 days for animals previously infected with Asian or American ZIKV, and 15.5 days for animals previously infected with African ZIKV) than in the control groups inoculated with vehicle alone (10 days) or with VSV (11 days).
Figure 1 Protection conferred by ZIKV infection against subsequent WNV infection. (A) Experimental schedule representing the immunization timeline. Eight-week-old Swiss albino CD1 male mice (n=10–20/group) were infected i.p. with 5 × 105 pfu/mouse of ZIKV (African, Asian or American strain), VSV, or culture medium (vehicle) as a negative control. Mice were subsequently infected intraperitoneally with WNV (104 pfu/mouse) at 14 days post-ZIKV infection. (B) Survival rates in mice previously infected with the African, American, and Asian ZIKV strains, VSV or vehicle and challenged with WNV at 14 d.p.i. Statistically significant differences are indicated with asterisks *P<0.05, **P<0.01, ***P<0.001.
ZIKV infection enhances anti-WNV antibody induction
Because protection against flavivirus infection is primarily based on humoral responses,Citation25 the antigenic relationships among the different viral strains were assessed. Cross-reactivity between ZIKV and WNV strains was addressed by testing a pool of sera from mice infected with the different viral isolates (13 days p.i.). For this, we utilized an in house developed ELISA based on inactivated whole-virus antigens produced from infected cell cultures. The ELISA was validated using a ZIKV-specific monoclonal antibody that showed good reactivity with the three ZIKV antigens, although it was higher with the African strain (Figure 2A), because this monoclonal antibody was produced against the African MR766 strain. Notably, whereas sera from mice infected with the Asian and American strains recognized the three ZIKV antigens in a similar manner, those from African infected mice mainly recognized their own specific antigen, as occurred with sera from WNV-infected mice (Figure 2A).
Figure 2 Induction of anti-ZIKV and anti-WNV IgGs in mice. (A) Assessment of reactivity and specificity of ELISAs for ZIKV and WNV. Plates were coated with ZIKV (African, American or Asian) or WNV antigens. Reactivity of sera pools of mice infected with the different ZIKV strains (13 d.p.i.) or control sera (WNV-specific sera pool or ZIKV-specific mAb) with the different antigens is represented as the fold increase of the sample OD490 over the OD490 of non-infected mice sera. Data are presented as the mean±SEM. (B) Anti-ZIKV or (C) anti-WNV IgGs in blood samples, collected at the days post-infection indicated in each case, were detected by an indirect ELISA using plates coated with heat-inactivated ZIKV or WNV, respectively. Solid lines represent the mean fold increase in absorbance of each group. Each point of the graph represents a single animal. Asterisks in panel B denote statistically significant differences among animals infected with the different ZIKV isolates. Asterisks in panel C denote statistically significant differences between animals previously infected with ZIKV and those only challenged with WNV (vehicle). *P<0.05, **P<0.01, ***P<0.001.
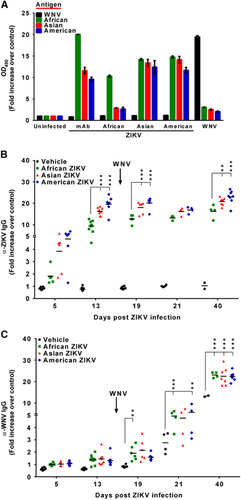
Therefore, individual mouse sera were assayed against their respective specific antigens. Some of the ZIKV-infected mice presented specific antibodies at 5 d.p.i., and all had detectable antibodies by day 13 p.i., which slightly increased after WNV challenge (Figure 2B). Remarkably, the African strain elicited a lower humoral immune response, as observed by a significantly lower level of antibodies in the sera of African ZIKV-infected mice compared to the other ZIKV-infected animals.
Interestingly, ZIKV infection enhanced specific WNV antibody production, because 5 days after WNV challenge (19 days post-ZIKV infection), specific anti-WNV antibodies were only observed in mice previously infected with ZIKV and not in those infected only with WNV (Figure 2C). Moreover, anti-WNV antibodies increased until the end of the experiment (40 days post-ZIKV infection, 26 days post-WNV infection), and were significantly higher in mice previously infected with ZIKV than in control mice infected only with WNV.
Considering the key protective role of neutralizing antibodies against flavivirus infections,Citation26, Citation27 the neutralization capability of seropositive samples was tested against ZIKV and WNV isolates using PRNT (Table ). Mice infected with the Asian or American strains neutralized the three ZIKV isolates, and those infected with the African strain only neutralized the homologous virus. Conversely, prior to WNV challenge, none of the sera nor the uninfected mouse sera neutralized WNV (Table ). After WNV challenge, the neutralization capability of each serum was assayed against both their homologous ZIKV strain and WNV (Table ). All tested sera were able to neutralize both viruses. None of the vehicle-inoculated mice infected with WNV presented neutralizing antibodies against ZIKV, and their PRNT90 titers against WNV were lower than those of ZIKV-infected mice (Table ). To further confirm that the antibodies induced by WNV infection do not neutralize ZIKV, the reactivity of a panel of WNV-inoculated mice sera was tested 26 d.p.i. Although its mean PRNT90 titer against WNV was 740±137, they did not neutralize ZIKV, confirming data recorded at 7 d.p.i. Taken together, these results suggest that previous ZIKV infection could prime the mice to a faster response, enhancing subsequent anti-WNV neutralizing antibody production.
Table 1 Induction of neutralizing antibodies in mice infected with ZIKV at 13 days post-ZIKV infection
Table 2 Induction of neutralizing antibodies in mice infected with ZIKV and challenged with WNV at 21 days post-ZIKV infection (7 days post-WNV challenge)
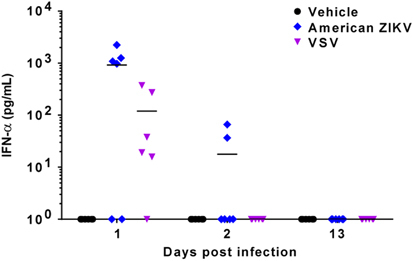
Because the innate immune response contributes to virus clearance and a more rapid and effective humoral response,Citation43 the production of interferon (IFN)-α in ZIKV-PA259459 infected mice was analyzed at early time points after ZIKV infection (1 and 2 d.p.i.), and prior to WNV challenge (13 d.p.i.). As a positive control, mice infected with VSV, in which the induction of IFN-α production has been documented,Citation44 were also analyzed. Although both viruses induced detectable levels of circulating IFN-α at early time points (1 and 2 d.p.i. for ZIKV, and 1 d.p.i. for VSV), no detectable levels of IFN-α were observed at later times (13 d.p.i.) just prior to WNV challenge (Figure 3). These results indicate that the observed protection against WNV challenge in ZIKV-infected mice was not due to a sustained IFN-α response.
Figure 3 Induction of IFN-α in mice infected with ZIKV. Eight-week-old Swiss albino CD1 male mice (n=18/group) were infected i.p. with 5 × 105 pfu/mouse of American ZIKV, VSV or culture medium (vehicle) as a negative control. A total of six mice per group were killed at 1, 2 and 13 days p.i., and IFN-α levels in serum were detected using a commercial assay. Solid lines represent the mean of each group. Each point of the graph represents a single animal.
Discussion
Flaviviruses are expanding to new geographical regions where they previously did not represent a health problem, in part due to vector colonization of new areas. A current example of flavivirus spread is ZIKV, responsible for a recent epidemic in the Americas that quickly raised worldwide social and health concerns because of its possible association with severe neurological pathologies, such as GBS and microcephaly.Citation3, Citation4, Citation5, Citation6
Currently, data about ZIKV pathogenesis and immunity remain scarce. We analyzed the development of a humoral response in immunocompetent mice infected with three different ZIKV isolates. In addition, considering a future eco-epidemiological scenario in which ZIKV will likely colonize new regions where WNV is already endemic, we studied the cross-reactivity of both flaviviruses. Because of the elevated seroprevalence of ZIKV during outbreaks (~75%)Citation45 due to its high human-to-human transmission, and the relatively reduced incidence of WNV, the infection of which in humans is sporadic (~1%–2%),Citation46, Citation47 we first infected mice with ZIKV and then challenged them with WNV. In this way, we recreated a possible eco-epidemiological scenario with rapid ZIKV spread and subsequent WNV infections. Our results showed that ZIKV-infected mice were protected against challenge with a neurovirulent WNV strain. Interestingly, survival rates of animals infected with the Asian or American strains were higher compared with animals inoculated with the African ZIKV. This effect was specific for ZIKV infection, because mice infected with the unrelated VSV were not significantly protected against WNV challenge. Because ZIKV strains are phylogenetically classified into two major lineages—one including the African strains, and the other the more recent Asian and American strainsCitation3, Citation48—our results suggest differences in immunogenicity between isolates from different lineages of ZIKV.
Antibody-mediated immunity is considered a major player in protection against flavivirus infections,Citation25 including ZIKV.Citation26 Moreover, cross-reactive antibodies can induce cross-protection against infection with related flaviviruses in some instances, even with quite low titers, but this is not always the case.Citation27 Conversely, cross-reactive immunity is also associated with enhanced infection and disease outcome in DENV infections, mainly due to the ADE effect.Citation27 Accordingly, there is evidence supporting ADE between DENV and ZIKV.Citation31, Citation32, Citation49, Citation50 By contrast, our results showed that WN disease did not cause exacerbation in animals previously infected with ZIKV. Furthermore, animals infected with American or Asian ZIKV strains were partly protected against WNV challenge. In this sense, recent observations in immunocompromised mice injected with immune plasma from WNV- and DENV-positive donors suggest that cross-reactive antibodies can also protect against a challenge with ZIKV and that an ADE phenomenon was only observed within a reduced range of plasma concentrations.Citation51 Although only one ZIKV serotype has been described,Citation52 our results showed that while specific anti-ZIKV Asian and American sera recognized all three ZIKV antigens, sera from mice infected with the African strain mainly reacted with their homologous antigen. These results indicate a differential pattern of antibody induction within animals infected with different ZIKV isolates. Therefore, the African strain seemed to be less immunogenic, as shown by the lower levels of antibodies induced compared with the other two strains. After WNV challenge, an enhancement in the production of anti-WNV antibodies, including neutralizing antibodies, was recorded in mice previously infected with ZIKV compared to control mice infected only with WNV.
Polyprotein sequence analysis predicts the presence of potential N-glycosylation sites in some of the ZIKV proteins, including the envelope (E) protein, which is a major target for neutralizing antibodies.Citation3, Citation5 The differences in immunization efficiency of the ZIKV strains used in this report may be due to distinct antigenicity or pathogenicity among isolates. For example, the African MR766 strain used in this study, representative of the African lineage, was derived from the original ZIKV isolated from a sentinel monkey in Uganda in 1947Citation13 and corresponds to a highly mouse-adapted virus.Citation12, Citation13 Consequently, sequence analysis of the MR766 strain used in this report confirmed that it exhibited a 4-amino acid deletion corresponding to the E protein 154 glycosylation motif found in many flavivirusesCitation48, Citation53, Citation54 and that seems to play a role in the biology of ZIKV.Citation55 By contrast, the two ZIKV strains from the Asian lineage (FSS13025 and PA259459) that correspond to non-mouse-adapted viruses isolated from infected humans in Cambodia (2010)Citation56 and Panama (2015), respectively, display an intact N-glycosylation motif (VNDT) at the E protein. Consistent with these observations, differences in tissue tropism, pathogenic behavior, infectivity and cellular response between the strains of the African and Asian lineages were previously reported both in vitro and in vivo by other authors.Citation57, Citation58 Thus, these differences may also contribute to the variation in the induction of the humoral response observed here.
Additionally, the activation of an adaptive immune response is related to active viral replication.Citation59 Here, qRT-PCR analysisCitation42 of the viral burden of tissues and fluids from ZIKV-infected mice killed 5 d.p.i. showed sporadic amplification in only a few of the infected mice (7/24), suggesting that viral replication is not a major player in the differences observed for protection between the ZIKV strains assayed. Although comparison between studies is difficult because mice strains, viral isolates, and time of sampling differ between them, these results are not very different from those described previously in other wild-type mice compared with immune compromised animals.Citation18, Citation19, Citation22
In brief, no mortality or clinical signs of disease were recorded in any of the immunocompetent mice after i.p. infection with three ZIKV isolates of different geographical origin. However, our results suggest antigenic and immunogenic differences. Indeed, we demonstrated that, contrary to recent findings for ZIKV and DENV,Citation31, Citation32, Citation50 WNV infection of ZIKV-infected mice did not induce ADE. Moreover, ZIKV infection elicited a protective immune response against WNV. It is worth mentioning that a study of protection against ZIKV by a previous WNV infection would be of interest. Herein, no cross-reactivity of WNV-induced antibodies against ZIKV was observed. However, considering that the cellular immune response could lead to some immunological cross-talk, resulting in protective immunity even in the absence of neutralizing antibodies, further studies should be performed. These findings may have implications in the eco-epidemiological scenario of regions not yet colonized by ZIKV where other flaviviruses circulate, and may be useful for the design of multi-flavivirus vaccines. However, further studies of the mechanism behind this protective response, including analysis of the cellular immune response, are required.
Acknowledgments
We are grateful to A Vázquez (National Center for Microbiology, ISCIII) for the MR766 ZIKV isolate, to Dr RB Tesh (World Reference Center for Emerging Viruses and Arboviruses, WRCEVA) for the FSS13025, PA259459 ZIKV isolates and ZIKV-specific monoclonal murine ascitic fluid, and to Dr R Blasco (Department of Biotechnology, INIA) for the VSV Indiana isolate. We also thank M Calvo (Department of Biotechnology, INIA) for technical assistance. This work was supported in part by grants E_RTA2013-00013-C04-2014 and ZIKA-BIO-2016-01 from INIA, PLATESA (P2013/ABI-2906) from the Comunidad Autónoma de Madrid, and AGL2014-56518-JIN from the Spanish Ministry of Economy and Competitiveness (MINECO). AV-C is a recipient of a ‘Contrato de formación postodoctoral’ from MINECO. TM-R is a recipient of a ‘Formación de Personal Investigador (FPI)’ pre-doctoral fellowship from INIA.
References
- Gray TJ, Webb CE.A review of the epidemiological and clinical aspects of West Nile virus. Int J Gen Med 2014;7: 193–203.
- Martin-Acebes MA, Saiz JC.West Nile virus: a re-emerging pathogen revisited. World J Virol 2012;1: 51–70.
- Saiz JC, Vazquez-Calvo A, Blazquez ABet al.Zika virus: the latest newcomer. Front Microbiol 2016;7: 496.
- Blazquez AB, Saiz JC.Neurological manifestations of Zika Virus infection. World J Virol 2016;5: 135–143.
- Lazear HM, Diamond MS.Zika virus: new clinical syndromes and its emergence in the western hemisphere. J Virol 2016;90: 4864–4875.
- Mlakar J, Korva M, Tul Net al.Zika virus associated with microcephaly. N Engl J Med 2016.
- Abbink P, Larocca RA, De La Barrera RAet al.Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016;353: 1129–1132.
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RPet al.Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 2016.
- Dudley DM, Aliota MT, Mohr ELet al.A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 2016;7: 12204.
- Li XF, Dong HL, Huang XYet al.Characterization of a 2016 clinical isolate of zika virus in non-human primates. EBioMedicine 2016 12: 170–177.
- Dowd KA, Ko SY, Morabito KMet al.Rapid development of a DNA vaccine for Zika virus. Science 2016;354: 237–240.
- Dick GW.Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952;46: 521–534.
- Dick GW, Kitchen SF, Haddow AJ.Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952;46: 509–520.
- Way JH, Bowen ET, Platt GS.Comparative studies of some African arboviruses in cell culture and in mice. J Gen Virol 1976;30: 123–130.
- Weinbren MP, Williams MC.Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 1958;52: 263–268.
- Aliota MT, Caine EA, Walker ECet al.Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl Trop Dis 2016;10: e0004682.
- Cugola FR, Fernandes IR, Russo FBet al.The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016;534: 267–271.
- Dowall SD, Graham VA, Rayner Eet al.A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis 2016;10: e0004658.
- Lazear HM, Govero J, Smith AMet al.A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016;19: 720–730.
- Miner JJ, Cao B, Govero Jet al.Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016;165: 1081–1091.
- Rossi SL, Vasilakis N.Modeling Zika virus infection in mice. Cell Stem Cell 2016;19: 4–6.
- Rossi SL, Tesh RB, Azar SRet al.Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg 2016;94: 1362–1369.
- Kim E, Erdos G, Huang Set al.Preventative vaccines for Zika virus outbreak: preliminary evaluation. EBioMedicine 2016.
- Larocca RA, Abbink P, Peron JPet al.Vaccine protection against Zika virus from Brazil. Nature 2016;536: 474–478.
- Vaughan AT, Roghanian A, Cragg MS.B cells—masters of the immunoverse. Int J Biochem Cell Biol 2011;43: 280–285.
- Sapparapu G, Fernandez E, Kose Net al.Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 2016.
- Lobigs M, Diamond MS.Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis serocomplex. Expert Rev Vaccines 2012;11: 177–187.
- Petersen E, Wilson ME, Touch Set al.Rapid spread of Zika virus in the Americas—implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic games. Int J Infect Dis 2016;44: 11–15.
- Younger DS.Epidemiology of Zika virus. Neurol Clin 2016;34: 1049–1056.
- Barba-Spaeth G, Dejnirattisai W, Rouvinski Aet al.Erratum: structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016.
- Dejnirattisai W, Supasa P, Wongwiwat Wet al.Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 2016;17: 1102–1108.
- Stettler K, Beltramello M, Espinosa DAet al.Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016;353: 823–826.
- Swanstrom JA, Plante JA, Plante KSet al.Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. MBio 2016;7.
- Merino-Ramos T, Blazquez AB, Escribano-Romero Eet al.Protection of a single dose west nile virus recombinant subviral particle vaccine against lineage 1 or 2 strains and analysis of the cross-reactivity with Usutu virus. PLoS One 2014;9: e108056.
- Martin-Acebes MA, Blazquez AB, Jimenez de Oya Net al.West Nile virus replication requires fatty acid synthesis but is independent on phosphatidylinositol-4-phosphate lipids. PLoS One 2011;6: e24970.
- Alonso-Padilla J, de Oya NJ, Blazquez ABet al.Recombinant West Nile virus envelope protein E and domain III expressed in insect larvae protects mice against West Nile disease. Vaccine 2011;29: 1830–1835.
- Blazquez AB, Saiz JC.West Nile virus (WNV) transmission routes in the murine model: intrauterine, by breastfeeding and after cannibal ingestion. Virus Res 2010;151: 240–243.
- Cordoba L, Escribano-Romero E, Garmendia Aet al.Pregnancy increases the risk of mortality in West Nile virus-infected mice. J Gen Virol 2007;88 (Pt 2): 476–480.
- Alonso-Padilla J, Loza-Rubio E, Escribano-Romero Eet al.The continuous spread of West Nile virus (WNV): seroprevalence in asymptomatic horses. Epidemiol Infect 2009;137: 1163–1168.
- Escribano-Romero E, Gamino V, Merino-Ramos Tet al.Protection of red-legged partridges (Alectoris rufa) against West Nile virus (WNV) infection after immunization with WNV recombinant envelope protein E (rE). Vaccine 2013;31: 4523–4527.
- Petrovic T, Blazquez AB, Lupulovic Det al.Monitoring West Nile virus (WNV) infection in wild birds in Serbia during 2012: first isolation and characterisation of WNV strains from Serbia. Euro Surveill 2013;18.
- Faye O, Diallo D, Diallo Met al.Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013;10: 311.
- McNab F, Mayer-Barber K, Sher Aet al.Type I interferons in infectious disease. Nat Rev Immunol 2015;15: 87–103.
- Fink K, Lang KS, Manjarrez-Orduno Net al.Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur J Immunol 2006;36: 2094–2105.
- Duffy MR, Chen TH, Hancock WTet al.Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360: 2536–2543.
- Hadjichristodoulou C, Pournaras S, Mavrouli Met al.West Nile virus seroprevalence in the Greek population in 2013: A Nationwide Cross-Sectional Survey. PLoS One 2015;10: e0143803.
- Cervantes DT, Chen S, Sutor LJet al.West Nile virus infection incidence based on donated blood samples and neuroinvasive disease reports, Northern Texas, USA, 2012. Emerg Infect Dis 2015;21: 681–683.
- Berthet N, Nakoune E, Kamgang Bet al.Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis 2014;14: 862–865.
- Charles AS, Christofferson RC.Utility of a Dengue-derived monoclonal antibody to enhance Zika infection in vitro. PLoS Curr 2016;8.
- Priyamvada L, Quicke KM, Hudson WHet al.Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci USA 2016;113: 7852–7857.
- Bardina SV, Bunduc P, Tripathi Set al.Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017;356: 175–180.
- Dowd KA, DeMaso CR, Pelc RSet al.Broadly neutralizing activity of Zika virus-immune sera identifies a single viral serotype. Cell Rep 2016;16: 1485–1491.
- Baronti C, Piorkowski G, Charrel RNet al.Complete coding sequence of Zika virus from a French polynesia outbreak in 2013. Genome Announc 2014;2.
- Kuno G, Chang GJ.Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol 2007;152: 687–696.
- Shirato K, Miyoshi H, Goto Aet al.Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol 2004;85 (Pt 12): 3637–3645.
- Haddow AD, Schuh AJ, Yasuda CYet al.Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012;6: e1477.
- Simonin Y, Loustalot F, Desmetz Cet al.Zika virus strains potentially display different infectious profiles in human neural cells. EBioMedicine 2016;12: 161–169.
- Tripathi S, Balasubramaniam VR, Brown JAet al.A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog 2017;13: e1006258.
- Honke N, Shaabani N, Cadeddu Get al.Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 2011;13: 51–57.
