Abstract
Emerging Microbes & Infections (2017)6, e93; doi:10.1038/emi.2017.80; published online 1 November 2017
Dear Editor,
Zika virus (ZIKV) was first isolated from a rhesus monkey in Uganda in 1947.Citation1 Human infection with ZIKV often causes self-limiting symptoms with fever. A ZIKV outbreak in humans occurred in 2007 on Yap Island, in Micronesia, in the western Pacific,Citation2 and large outbreaks have swept through Brazil, Columbia, and other countries in South America since 2015, resulting in hundreds of thousands of infectious cases. Infectious cases of ZIKV and imported cases have been reported in dozens of countries around the world.Citation3 Moreover, many cases of microcephaly and Guillain-Barre syndrome associated with ZIKV infection have been identified. The World Health Organization (WHO) has declared these large-scale ZIKV infections to be a Public Health Emergency of International Concern, due to the huge public health burden they cause.Citation4 Previous studies have suggested that Aedes mosquitoes are the major vectors of ZIKV. ZIKV in South America was spread by Aedes aegypti.Citation5 In this study, a strain of ZIKV (GZDJ1685) was isolated from specimens of Culex quinquefasciatus collected in the southern China. This is not only the first report of ZIKV isolation in mainland China in nature, but also the first time that ZIKV has been isolated from this species in East Asia.
In mid-August 2016, a survey of mosquitoes and mosquito-borne viruses was carried out in Dejiang County, Guizhou Province, in southwestern China (108° 02′24″ E, 28° 15′19″ N latitude). Mosquito specimens were classified by morphology and stored in liquid nitrogen, with 50 mosquitoes per pool. Each pool of mosquito samples was ground and centrifuged at 13 000 r/min at 4 °C for 30 min. The supernatant was inoculated into tissue culture cells in parallel, and cytopathic effects (CPEs) were observed daily, using a microscope.Citation6 Virus RNA was extracted from cultures that were positive for CPE to prepare viral cDNA. Then the cDNA was used as a template to detect the genes of Alphavirus, Flavivirus and Bunyavirus.Citation6, Citation7 Sequences of positive products were assembled using SeqMan in the DNASTAR software package and then aligned with the relevant sequences downloaded from GenBank. A phylogenetic analysis of viral genes was conducted using Mega software version 6.0.Citation6 A total of 5795 mosquitoes were collected, of which 2540 were Anopheles sinensis, 1700 were Culex quinquefasciatus, 1530 were Armigeres subbalbeatus and 25 were Culex fuscanus. After grinding samples, inoculating supernatants into cells, four virus isolates (GZDJ1608, GZDJ1609, GZDJ1648 and GZDJ168585) that were stable in C6/36 and BHK cells were obtained. They were all isolated from Culex quinquefasciatus. Molecular identification showed negative results with Bunyavirus and Alphavirus-specific primers, but positive products were obtained with Flavivirus-specific amplification primers. Sequencing and molecular analyses of PCR-positive products indicated that GZDJ1608, GZDJ1609 and GZDJ1648 were Japanese encephalitis virus (JEV). Phylogenetic analyses based on the JEV E gene showed that these three isolates were genotype 1 (data not shown).
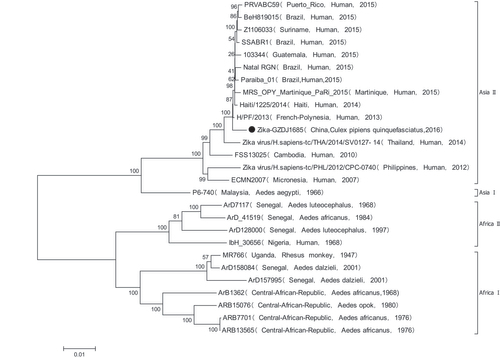
GZDJ1685 had CPEs in BHK and C6/36 cells at 48 h. The nucleotide sequence of the amplified product of GZDJ1685 extracted using Flavivirus-specific primers was highly homologous with ZIKV. The sequence of the coding region of GZDJ1685 (GenBank No. MF099651) was obtained using an amplification primer of the whole ZIKV genome sequence.Citation7 Nucleotide and amino acid homology was greatest between GZDJ1685 and H/PF-2013, isolated from ZIKV-infected patients in French Polynesia in 2013 (99.5% and 99.5%, respectively). Phylogenetic analyses (Figure ) showed that GZDJ1685 was located in the Asian branch of ZIKV on the phylogenetic tree, along with ZIKV strains isolated from Brazil (2015), Puerto Rico (2015) and Yap Island (2007).
Figure 1 Phylogenetic analysis based on the coding region sequences of ZIKV (GZDJ1685) isolated from Culex quinquefasciatus.
Dejiang County is located in the southwestern area of China’s Yunnan-Guizhou Plateau, and it has an average elevation of 1000–1500 m. It has a warm and humid climate; the annual average temperature ranges from 13 °C to 17 °C, and it has a frost-free period of 295 days with ample sunshine and rainfall. The collection points were distributed in mountainous areas where residents build houses and terraces on the hillside. Corn and sorghum are the main agricultural products. The local farmers keep livestock such as pigs, sheep, chickens and ducks near their dwellings. Dejiang County is traditionally an endemic region for JE,Citation8 but no ZIKA case and Aedes aegypti have been reported in the local region.
Phylogenetic analyses of the viral genome sequence revealed that the ZIKV (GZDJ1685) isolated from Culex quinquefasciatus in China belongs to genotype 2 in the Asian evolutionary branch of ZIKV (Figure ), as do ZIKVs isolated from Brazil, Haiti, Puerto Rico, French Polynesia and Thailand during the period of 2013 to 2016, suggesting that the strain isolated from Culex quinquefasciatus was derived from the Asian population of ZIKV.
Although ZIKV is an Aedes mosquito-transmitted virus, it has been detected or isolated from a variety of mosquito species in nature. There were 31 ZIKVs isolated from mosquitoes collected in Seychelles, 28 of which were isolated from 10 species of Aedes and the other three were isolated from Ma. uniformis, Cx. perfuscus and An. coustani, respectively.Citation9 Early in the 1950s, a laboratory study has found that Culex quinquefasciatus was a potential vector for ZIKV.Citation1 Recent studies further revealed that ZIKV can be detected in the salivary glands of Culex quinquefasciatus 7–15 days after its infection with ZIKV in the laboratory.Citation10, Citation11 In addition, PCR-positive ZIKV has been identified in the brain tissues of mice bitten by ZIKV-infected mosquitoes of Culex quinquefasciatus.Citation11 Furthermore, a total of 1496 Culex quinquefasciatus mosquitoes and 408 Ae. aegypti mosquitoes were collected from different sites in Brazil during the ZIKV epidemic period (February to May) in 2016. From 270 pools of Culex quinquefasciatus and 117 pools of Ae. aegypti assayed by RT-qPCR, three pools of Culex quinquefasciatus and two pools of Ae. aegypti were detected as PCR-positive for ZIKV. And two ZIKVs were isolated from Culex quinquefasciatus and were stable in Vero cells.Citation12 Taken together, these results suggest that Culex quinquefasciatus has the ability to not only help replicate ZIKV but also spread ZIKV to other animals through bites.
During the outbreaks of ZIKV spread in Brazil and other countries in Americas since 2015, imported cases infected with ZIKV have also been reported in China, but there were no secondary cases discovered.Citation13 Previous field and laboratory studies on mosquito-borne arbovirus in China did not detect and isolate ZIKV in China in last two decades either.Citation14 Thus, the result of this study is the first report of ZIKV isolation in nature not only in mainland China, but also in East Asia. And it will bring new challenges for the prevention and control of ZIKV in China. For example, will ZIKV infection exist in local wild animals, especially in non-human primates at the place where this Culex quinquefasciatus carried ZIKV was isolated? Are there any ZIKVs carried by Aedes mosquitoes, especially the domestic form of Ae. aegypti, spread in that area? Recent studies have suggested the domestic form of Ae. aegypti played an important role in the emergence of Aedes transmitted arboviruses and associated arboviral diseases.Citation15 What is the infection status of the local human population? To answer these questions and evaluate the burden of disease and public health impact, additional detection and monitoring of ZIKV and its infection in China are necessary to be carried out thoroughly, including the use of seroprevalence studies for evidence of exposure to ZIKV.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81290342 and 81501757); the Special National Project on Research and Development of Key Biosafety Technologies (2016YFC1201904); the National Key Plan for Scientific Research and Development of China (2016YFD0500300); the development grants of State Key Laboratory of Infectious Disease Prevention and Control (2014SKLID103 and 2015SKLID505); the National Key Research and Development Program of China (2017YFC1200202); the Open Research Fund Program of Wuhan National Bio-Safety Level 4 Lab of CAS (2017SPCAS003).
References
- Karabatsos N. International Catalogue of Arthropod-borne Viruses.San Antonio (TX): American Society for Tropical Medicine and Hygiene.1985.
- Duffy MR, Chen TH, Hancock WTet al.Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360: 2536–2543.
- Franca RF, Neves MH, Ayres CFet al.First International Workshop on Zika Virus Held by Oswaldo Cruz Foundation FIOCRUZ in Northeast Brazil March 2016—A Meeting Report. PLoS Negl Trop Dis 2016;10: e0004760.
- WHO. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome.Available at http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/(accessed 1 February 2016).
- Haddow AD, Nasar F, Guzman Het al.Genetic characterization of Spondweni and Zika viruses and susceptibility of geographically distinct strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni virus. PLoS Negl Trop Dis 2016;10: e0005083.
- Wang J, Zhang H, Sun Xet al.Distribution of mosquitoes and mosquito-borne arboviruses in Yunnan Province near the China-Myanmar-Laos border. Am J Trop Med Hyg. 2011;84: 738–746.
- Li S, Shi Y, Zheng Ket al.Morphologic and molecular characterization of a strain of Zika virus imported into Guangdong, China. PLoS One 2017;12: e0169256.
- Ye X, Wang H, Fu Set al.Etiological spectrum of clinically diagnosed Japanese encephalitis cases reported in Guizhou Province, China, in 2006. J Clin Microbiol 2010;48: 1343–1349.
- Diawo D, Sall AA, Diagne CTet al.Weaver and Mawlouth Diallo; Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014;9: e109442.
- Lourenço-de-Oliveira R, Failloux AB.Lessons learned on Zika virus vectors. PLoS Negl Trop Dis 2017;11: e0005511.
- Guo XX, Li CX, Deng YQet al.Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect 2016;5: e102.
- Guedes DR, Paiva MH, Donato MMet al.Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect 2017;6: e69.
- Li J, Xiong Y, Wu Wet al.Zika virus in a traveler returning to China from Caracas, Venezuela. Emerg Infect Dis 2016;22: 1133–1136.
- Liu H, Gao X, Liang G.Newly recognized mosquito-associated viruses in mainland China in the last two decades. Virol J 2011;8: 68.
- Gould E, Pettersson J, Higgs Set al.Emerging arboviruses: why today? One Health 2017;4: 1–13.
