Abstract
West Nile virus (WNV) is an arthropod-borne flavivirus of high medical and veterinary importance. The main vectors for WNV are mosquito species of the Culex genus that transmit WNV among birds, and occasionally to humans and horses, which are ‘dead-end’ hosts. Recently, several studies have been published that aimed to identify the mosquito species that serve as vectors for WNV in Europe. These studies provide insight in factors that can influence vector competence of European mosquito species for WNV. Here, we review the current knowledge on vector competence of European mosquitoes for WNV, and the molecular knowledge on physical barriers, anti-viral pathways and microbes that influence vector competence based on studies with other flaviviruses. By comparing the 12 available WNV vector competence studies with European mosquitoes we evaluate the effect of factors such as temperature, mosquito origin and mosquito biotype on vector competence. In addition, we propose a standardised methodology to allow for comparative studies across Europe. Finally, we identify knowledge gaps regarding vector competence that, once addressed, will provide important insights into WNV transmission and ultimately contribute to effective strategies to control WNV.
Emerging Microbes & Infections (2017) 6, e96; doi:10.1038/emi.2017.82; published online 8 November 2017
West Nile Virus in Europe
Natural transmission cycles of the arthropod-borne West Nile virus (WNV; family: Flaviviridae) can only be established if susceptible bird hosts and competent mosquito vectors are present in a certain area under suitable environmental conditions. Mammals, including humans and equines, can occasionally become infected with WNV, but they generally do not develop sufficient viraemia to sustain transmission (reviewed in Bowen and NemethCitation1). Thus, mammals are considered as ‘dead-end’ hosts. Most WNV infections in humans remain asymptomatic, although an estimated one in four cases develops into West Nile fever (reviewed in Petersen et al.Citation2). Less than one percent of human WNV infections results in neurological disease such as meningitis or encephalitis, which can be fatal. Thus far, cases of WNV infection in humans have only been reported from southern and central European countries (Figure ). Due to absence of human and equine cases in northern European countries, monitoring of WNV in these regions mainly depends on mosquito and bird surveillance programmes. Since routine surveillance programmes are not running in all European countries, and as most WNV infections in humans are asymptomatic, WNV circulation is likely to be underestimated.
Figure 1 European countries with human cases of West Nile fever reported to the European centre for disease prevention and control (ECDC). Colour gradient indicates the number of years during which cases of West Nile virus in humans have been reported since 2010. Reported cases are marked on the country level, except for the Balearic islands, Sicily, Sardinia, and Corsica, which are individually marked. Countries with no reported cases or no data available are marked in white. Data set provided by ECDC based on the data provided by WHO and Ministries of Health from the affected countries.
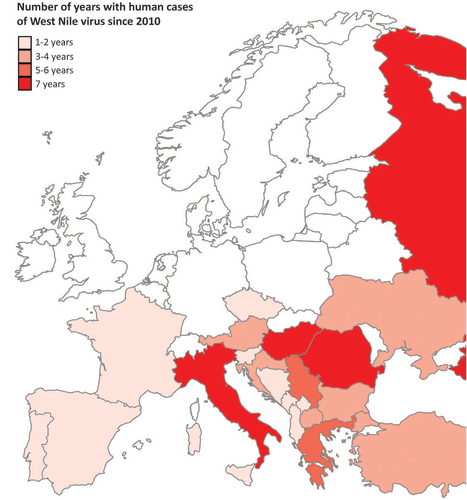
The differences in the number of reported WNV cases in humans between northern and southern Europe may be explained by two main hypotheses: (i) lower susceptibility of northern European bird populations and (ii) lower vectorial capacity of northern European mosquito species. Studies from Germany and the United Kingdom have shown serological evidence of WNV circulation among resident birds.Citation3, Citation4, Citation5 In addition, laboratory studies with carrion crows (Corvus corone),Citation6 and European jackdaws (Corvus monedula),Citation7 showed their susceptibility to infection with WNV. Viraemia of carrion crows (50 % tissue culture infectious dose: >105 TCID50/mL) was found to be sufficient to support WNV transmission, but this was not the case for most of the jackdaws (⩽105 TCID50/mL).Citation6, Citation7 The combined evidence of both field and laboratory studies on northern European birds, indicates that bird hosts are likely not a limiting factor for WNV transmission in northern Europe. Although corvid birds are highly susceptible to WNV infection, studies in North America have shown that other species such as the American robin (Turdus migratorius), contribute proportionally more to the WNV transmission cycle.Citation8, Citation9, Citation10 The larger contribution of American robins is mainly due to the high feeding preference of the main WNV mosquito vectors for this bird species. Although several studies determined feeding preferences of European mosquitoes,Citation11, Citation12, Citation13, Citation14, Citation15 in-depth studies on spatial and temporal host-feeding dynamics of specific mosquito species remain scarce. A recent study on Culex (Cx.) pipiens mosquitoes originating from Italy showed that blackbirds (Turdus merula) and Eurasian magpies (Pica pica) were highly preferred by these mosquitoes.Citation14 More studies focussing on the identification of host-feeding preferences of mosquitoes are needed to make a thorough comparison between mosquito–bird contact rates across a larger European scale. Such a comparison will provide important insights in key mosquito vector species and key bird host species, which should be tested for their susceptibility to WNV.
Lowered vectorial capacity of northern European mosquitoes can be an alternative explanation for the differences in WNV outbreaks across Europe. Vectorial capacity can be defined as the efficiency of a vector to transmit a pathogen.Citation16 Vectorial capacity relies on several factors, such as the abovementioned feeding behaviour of mosquitoes, mosquito abundance, mosquito survival and environmental conditions. One of the key components of vectorial capacity is vector competence, which is defined as the ability of a vector to acquire, maintain and transmit a pathogen.Citation17 Only competent vectors can contribute to the transmission of WNV, which makes vector competence an important determinant of vectorial capacity. However, a highly competent mosquito species may not efficiently contribute to WNV transmission if it only feeds on mammals and not on birds. Nevertheless, vector competence provides important insights in species that should be considered as important contributors to WNV transmission. This is necessary for targeted control of highly competent mosquitoes to reduce the potential of WNV transmission. In this review we aim to provide a complete and detailed overview of factors that influence vector competence of mosquitoes in Europe.
Thus far, most vector competence studies on WNV have been carried out with North-American mosquito species.Citation18, Citation19, Citation20, Citation21 However, differences in vector species and environment make it difficult to extrapolate results from studies with North-American mosquitoes to understand the European situation. Until 2014, only two published studies had evaluated the vector competence of Culex species originating from Europe for WNV.Citation22, Citation23 Although these studies provided insight into the possibility of WNV transmission by European mosquito species, they could not explain why WNV outbreaks seem limited to southern and central Europe. As the European Commission was concerned about further spread of WNV across Europe, several studies were initiated to investigate vector competence of European mosquitoes for WNV. As a result, several new vector competence studies on European mosquito species have been published during the past 3 years.
This review shows the results of vector competence studies on European mosquito species in order to identify key factors that influence vector competence for WNV. The concept of vector competence is explained, including the barriers to arbovirus infection of mosquitoes. Studies on vector competence of European mosquito species for WNV are being evaluated based on their methodology and outcomes to provide recommendations for future vector competence studies. The outcomes of the vector competence studies are linked to the available literature on mosquito barriers, immune pathways and interactions with other microbes that together determine vector competence. Finally, recommendations for WNV surveillance in Europe and perspectives for future research are discussed.
Mosquito Barriers to Arbovirus Infection and Transmission
The outcome of the interaction between mosquito and WNV is largely dependent on the specific combination of the mosquito species, mosquito origin, WNV lineage and WNV strain. For a virus-exposed mosquito to become infectious, the virus has to overcome various barriers within the mosquito body: the peritrophic membrane, the midgut barrier and the salivary gland barrier. The midgut and salivary gland barriers are both further divided into an infection and an escape barrier (Figure ).Citation17 These barriers can limit virus infection both mechanically and through a range of antiviral immune responses, thereby determining the vector competence of the mosquito to transmit a certain arbovirus.
Figure 2 Schematic overview of the mosquito barriers to arbovirus infection. Schematic longitudinal cross-section of a mosquito. Arrows indicate the passage of virions through the midgut (MG) and salivary gland (SG) barriers. The dashed circle in the midgut represents the peritrophic membrane that is formed after ingestion of blood. Right inset: (i) Infection of midgut epithelial cells via binding to a putative receptor protein. (ii) Virus replication in midgut epithelial cells. (iii) Release of virus via budding from midgut epithelial cells and direct passage through the basal lamina into the haemocoel. (iv) Direct virus passage into the haemocoel through a ‘leaky’ midgut. (v) Virus infection of trachea after budding from midgut epithelial cells. (vi) Budding of virus from the trachea into the haemocoel. Left inset: (i) Infection of the salivary gland epithelial cells after passage through the basal lamina. (ii) Virus replication in the salivary gland cells. (iii) Virus release via budding from salivary gland cells into the salivary gland lumen. (iv) Virus release from the salivary gland cells into the salivary gland lumen via apoptosis.
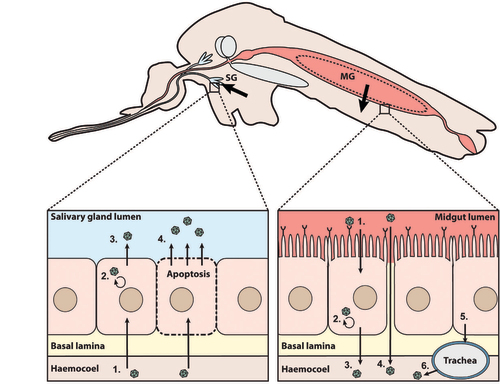
After ingestion of an infectious blood meal, virus particles travel through the foregut, cardia (proventriculus; foregut-midgut junction), and eventually end up in the midgut. Although infection of the foregut and cardia has been described for some arboviruses,Citation24 the majority of virus infections occur in the midgut epithelial cells (Figure ; right inset). The first potential mosquito barrier that arboviruses encounter is the peritrophic membrane. The peritrophic membrane is a sac-like structure composed of chitin, proteins and glycoproteins that form a filamentous matrix surrounding the blood meal in the midgut.Citation25 The peritrophic membrane is not constantly present in adult mosquitoes, but forms within a few hours after uptake of a blood meal. In Culex species, formation of the peritrophic membrane can be readily observed at 2–8 h post blood feeding, and reaches its peak thickness of ~100 μm after 24 h.Citation26 The ~20 nm pores of the peritrophic membrane,Citation27 are smaller than the ~50 nm diameter of WNV virions.Citation28 This makes it necessary for WNV to infect the midgut epithelial cells within 8 h after ingestion of the viraemic blood meal.Citation27, Citation28 Indeed, flaviviruses can infect the midgut epithelial cells within 8 h after a blood meal, and knock-down of genes required for the formation of the peritrophic membrane does not affect flavivirus infection in mosquitoes.Citation29, Citation30 Thus, despite the limited literature available, the peritrophic membrane does not seem to pose a strong barrier against WNV and other flaviviruses in mosquitoes.
Entry of flaviviruses into the midgut epithelial cells most likely occurs through interaction with cellular membrane-associated receptors (Figure ; right inset). Flavivirus receptors in mosquitoes have not yet been identified, although some studies have suggested the involvement of cellular proteins for dengue virus (DENV) entry in C6/36 mosquito cells.Citation31, Citation32 These proteins are required for the binding and uptake of virions during DENV infection and are either membrane-bound or membrane-associated intracellular proteins.32 Studies with WNV in Culex mosquitoes indicated that infection of the midgut can be observed by the formation of irregular virus positive clusters of midgut epithelial cells at 3 days post initial midgut infection (dpi).33 At 5 dpi the virus spreads more widely throughout the midgut and fully infects the midgut at 7 dpi. Studies with WNV virus-like-particles showed that initial infections occur randomly across the luminal posterior midgut epithelium, suggesting that most cells in the posterior midgut are susceptible for virus entry.Citation34
After infection and replication in the midgut epithelial cells, virus particles need to disseminate through the haemocoel in order to reach the salivary glands. The haemocoel can be reached by passing through the basal lamina with or without a virus replication cycle in the midgut epithelial cells, through infection of the midgut tracheae or through direct budding into the haemolymph (Figure ). The basal lamina is a dense matrix of proteins that blocks large entities such as bacteria, fungi, and larger viruses. However, the basal lamina is permeable to small molecules, potentially forming a barrier for flavivirus dissemination to the haemocoel.35 The thickness of the midgut basal lamina differs between different mosquito species and strains, and can affect the ability of virions to pass to the haemocoel. Differences in thickness of the basal lamina of Aedes (Ae.) albopictus did not affect the dissemination of DENV-1.Citation36 In addition, WNV particles can be observed within the intact basal lamina which suggests that WNV virions move freely within the lamina.Citation28 These observations suggest that the basal lamina does not pose a strong barrier to flaviviruses. An alternative way of reaching the haemolymph is via infection of the tracheae. These form a network of tubular structures that reach from the midgut epithelial cells to the outer layer of the basal lamina. This network can sustain virus passage through the basal lamina.Citation37 Indeed, DENV-2 can infect the tracheal system and infection of the tracheae is positively correlated with virus dissemination.Citation38 Furthermore, it has been shown that the midgut tracheae of Cx. pipiens can serve as a conduit for Rift Valley fever virus passage through the basal lamina.39 Finally, it has been shown that a small proportion of mosquitoes can have a ‘leaky’ midgut, which refers to the direct passage of virions into the haemolymph immediately after an infectious blood meal, without a replication cycle in the midgut epithelial cells.Citation26, Citation40 Although leaky midguts have not been shown as a characteristic of flavivirus infections, it is quite possible that in a small proportion of mosquitoes an infectious blood meal leads to direct infection of the haemolymph. To summarize, while most WNV infections pass through the midgut epithelial cells, some might pass this barrier by infection of the tracheae or directly through the leaky midgut.
After infection of the haemolymph, virions disseminate towards and infect secondary tissues including the fat body, haemocytes, muscles and ultimately the salivary glands. After infection of the salivary glands, viral titers increase in the saliva, and eventually reach plateau levels.27 Female Culex mosquitoes have two salivary glands that each consist of one median and two lateral lobes.Citation41 The salivary gland lobes are surrounded by the salivary gland basal lamina, which forms a physical barrier to virus infection of the salivary gland epithelial cells.35,39,41 Although less profound than the midgut barrier, the presence of a salivary gland entry barrier has been reported for several flaviviruses in both Aedes and Culex mosquitoes.Citation42–Citation44 Both the mechanical and the molecular nature of the salivary gland infection and escape barriers have not been defined completely. Presumably, the combination of basal lamina and organization of the tissue surrounding the salivary glands can prevent viruses from infecting the salivary glands, while antiviral immune pathways can restrict virus replication intracellularly.Citation35, Citation45 In addition, some studies have shown that the release of transmissible virus into the salivary gland lumen might require the induction of apoptosis.Citation39, Citation46
European Vector Competence Studies on Wnv
Methodology of European vector competence studies for WNV
From the >60 mosquito species that have been implicated as potential WNV vector species in the United States of America (USA),Citation47 seven species that also occur in Europe have been experimentally tested for WNV susceptibility (Table ). From Table it becomes clear that there is large variation in the methodology of different vector competence studies. Vector competence studies have been conducted with mosquitoes collected in the field, or mosquitoes that were reared for many generations in the laboratory. Laboratory colonization can affect vector competence of mosquitoes for viruses.Citation58, Citation59 Laboratory colonies, which generate high numbers of mosquitoes that readily feed via artificial membrane feeders, can provide a useful model system to test for underlying mechanisms of vector competence. However, one should be careful in the extrapolation of results obtained with laboratory colonies to the situation in the field. Thus, use of field-derived mosquitoes is necessary to reliably estimate vector competence of mosquito populations in the field.
Table 1 Overview of vector competence studies on West Nile virus with European mosquito species
Another factor that affects the vector competence is the method of virus stock production, such as the passage number, cell type, and multiplicity of infection. These parameters can affect the composition of the virus population and either reduce or increase virus fitness.Citation60 While most studies grow WNV on C6/36 mosquito cells, others generate viral stocks on mammalian Vero cells. Cell lines lack the viral bottlenecks that are present in the vector and host, and therefore affect the quasi-species composition of the viral stock. Moreover, extensive virus passage on a specific cell line may accumulate mutations that have an advantage for viral replication in that specific cell line. Consequently, viral passage on cells can lead to both an increase or decrease in viral infectiousness to mosquito hosts, depending on the type of cells used. For instance, WNV grown on mammalian cells loses fitness in mosquito cells.Citation61 It is therefore important that WNV stocks used for vector competence studies have a low number of passages on cells.
Various methods of blood feeding, such as the use of artificial membrane feeders or cotton sticks, have proven to be successful for infection of mosquitoes (Table ). Such oral exposure techniques are necessary to obtain a reliable proxy for vector competence, because mosquitoes are exposed to WNV via an infectious blood meal. Comparison of blood-feeding methods for other virus–vector combinations has indicated that there is an effect on the infection rate depending on the feeding method used.Citation62 However, the effect of the feeding technique on the infection rate of mosquitoes for WNV remains to be investigated. Nevertheless, it is important to use a standardized infection protocol that resembles the natural situation. As most laboratories use membrane feeding as a standard technique, we propose to set membrane-based feeding as the standard method for oral infection of Culex mosquitoes with WNV. More artificial infection techniques, such as microinjection, are not suitable for the assessment of vector competence, because they circumvent the midgut barrier. However, comparisons between oral exposure and injection techniques can provide important insights in the role of mosquito barriers in WNV infection, dissemination and transmission.
A large diversity of (un)natural blood sources (for example, rabbit blood or washed erythrocytes) is being used to expose mosquitoes to WNV. It has been shown for several arbovirus-vector combinations that the blood source used in an artificial blood meal can affect the vector competence of mosquitoes.Citation63 Under natural conditions, transmission of WNV occurs via the bite of a mosquito on an infectious bird. Thus, ideally an avian blood source, such as chicken blood, should be used for studies with WNV. Only four out of the twelve assessed vector competence studies used an avian (chicken) blood source to infect mosquitoes with WNV (Table ). As the blood source can have consequences for the initial stages of virus infectivity in the midgut cells, it is important to standardize the blood source for vector competence studies. As chicken blood is widely available and most comparable to the blood of natural bird hosts, we propose the use of chicken blood as a standard blood source for vector competence studies with WNV.
Another parameter that affects the outcome of vector competence studies is the viral dose in the infectious blood meal. Generally, a higher infectious dose results in a higher infection rate, and in some cases also a higher transmission rate.Citation18, Citation19 In order to compare the outcomes of different vector competence studies, it is important to standardize the infectious dose of the blood meal. The maximal viral dose of WNV in viraemic birds is highly species-specific and can reach peak titers up to 1.0 × 1012 TCID50/mL.Citation64 However, many bird species will not reach peak viraemia higher than 1.0 × 108 TCID50/mL.Citation64, Citation65 As a proxy for natural bird vireamia, we propose to include an infectious dose of 1.0 × 107 TCID50/mL in all future vector competence studies of Culex mosquitoes for WNV.
Quantification of virus is routinely done by determining the number of viral genomic RNA copies with quantitative reverse-transcription (qRT)-PCR or through determining the amount of infectious virus as either the 50 % tissue culture infectious dose (TCID50) or numbers of plaque forming units (PFU). Although there is a correlation between the number of viral RNA copies and infectious virus particles, the viral genome copies can be 100–5000 times higher than the number of truly infectious virus particles.Citation66, Citation67 This can be due to the presence of defective-interfering particles, that can be detected by qRT-PCR but not with an infectivity assay as they are non-infectious. It is therefore highly important to determine the number of infectious virus particles, as only these virus particles play a role in infection. This can be done by either the use of cell infectivity assays or proper validation of qRT-PCR results by means of a standard curve against the number of infectious particles per Ct value.
Most of the vector competence studies report a combination of infection, dissemination, and transmission rates which provide insight in the different stages of viral dissemination linked to the two main barriers inside the mosquito: the midgut and the salivary glands. Infection rates are determined based on the entire mosquito body, and represent successful infection of the midgut cells. Dissemination rates can be determined by testing peripheral mosquito body parts, such as the head or legs, and provides insight in the proportion of mosquitoes in which WNV managed to escape from the midgut cells and disseminate to the tested body part. Transmission rates are determined by testing saliva for the presence of virus, which provides insight in the proportion of mosquitoes that can actually transmit WNV. This is the most relevant parameter for vector competence, as only mosquitoes with infectious saliva will contribute to WNV transmission.
Vector competence studies on WNV with European mosquito species
WNV transmission rates have only been determined for five European mosquito species: Ae. albopictus, Ae. caspius, Ae. detritus, Cx. modestus and Cx. pipiens (Table ). Seven out of nine studies reporting WNV transmission rates were conducted with the species Cx. pipiens, which has been identified as one of the most important WNV vectors in the USA and Europe.68,69 In this review, we will focus on results of those studies reporting transmission rates, because only transmission rates can provide a reliable proxy for the capability of a mosquito to transmit WNV. Overall, WNV transmission rates of European Cx. pipiens mosquitoes ranged between 0 and 60 % (Table ).22,Citation49, Citation53, Citation54, Citation55, Citation56 Maximum transmission rates of northern European populations were 33 %,Citation54, Citation56 compared to up to 60 % for southern European populations.Citation49 Part of this variation may be explained by intrinsic differences in vector competence of geographically distinct populations, or may be due to differences in study protocols (for example, viral strain, virus dose, incubation time or incubation temperature). Distinct populations of mosquitoes may differ in the effectiveness of their midgut and salivary gland barriers or may impose a stronger antiviral immune response, resulting in variation in vector competence. However, in a systematic attempt to directly compare transmission rates of a northern and southern European population, thereby excluding all variation due to experimental design, no actual difference was observed in the transmission rates of both populations.Citation57 This suggests that there is no intrinsic difference in vector competence between northern and southern European Cx. pipiens populations. However, follow-up studies are needed to verify these results on a broader European scale.******
Table 2 Transmission rates of European mosquito species for West Nile virus
Temperature is another important factor that can influence vector competence. The positive effect of temperature on vector competence has been well established by previous American studies on WNV, and other arboviruses (reviewed in Kenney and BraultCitation17). Several studies confirmed that vector competence of European mosquitoes for WNV increases with temperature.Citation54, Citation55, Citation56 In the temperature range from 18 °C to 28 °C, transmission rates of northern European Cx. pipiens increased from 0 % to 33 %.Citation54, Citation56 Thus, average northern European summer temperatures of 18 °C appear to be an important limiting factor for WNV transmission.Citation54, Citation55, Citation56, Citation57
The species Cx. pipiens comprises two behaviorally different biotypes, pipiens and molestus, which can form hybrids. The pipiens biotype has a preference for birds as hosts,Citation70, Citation71 and is thus important for the natural WNV transmission cycle. Biotype molestus and hybrids are thought to play a more important role in the spill over of WNV from birds to humans, due to the preference of the molestus biotype for mammals and the more opportunistic feeding behaviour of hybrids.Citation70, Citation71 Thus far, two studies reported transmission rates of Cx. pipiens mosquitoes which were identified to the biotype level.Citation56, Citation57 One of these studies revealed that overall there is no difference in vector competence among the two biotypes in the Netherlands.Citation56 However, the two biotypes did respond differentially to temperature. Higher temperatures increased the transmission rates of biotype pipiens and hybrids, but not of biotype molestus.Citation56 This shows the importance of identifying Cx. pipiens to the biotype level and suggests that closely related mosquitoes may have different vector competence. Thus, the Cx. pipiens biotypes provide an interesting model system for comparative studies on the underlying genetic and molecular mechanisms of temperature-dependent vector competence.
Transmission rates have also been determined for Cx. modestus, originating from France. Relatively high transmission rates of 40–55 % were found, which indicates that Cx. modestus is an efficient vector of WNV.Citation22, Citation23 However, Cx. modestus was exposed to high WNV titers (1010 PFU/mL) in both studies, which can explain the relatively high transmission rates compared to other mosquito species which were exposed to lower WNV titers.
Vector competence for WNV has only been determined for a few European Aedes species. An Italian population of the invasive mosquito species Ae. albopictus shows variation in transmission rates ranging between 0 % and 40 %, which can be explained by different incubation periods.Citation49 Although Ae. albopictus mosquitoes are competent vectors for WNV, their contribution to WNV transmission in the field is expected to be low due to their strong feeding-preference for human hosts.Citation11 An Ae. detritus population from the United Kingdom had moderate transmission rates of 21 %.Citation50 Dissemination and transmission rates of Ae. caspius were 1 % and 0 %,Citation22 respectively, and infection rates of Ae. japonicus japonicus were 0 %,Citation51 which indicates that these European populations are not competent. This contrasts to the relatively high transmission rates found for Ae. j. japonicus in the USA.Citation72, Citation73 To summarize, Aedes mosquitoes do not seem to contribute significantly to WNV circulation in Europe.
Underlying Mechanisms of Vector Competence of Culex Mosquitoes for Flaviviruses
Vector competence of a given mosquito vector depends on the ability of the virus to overcome the mosquito barriers to transmission. It is therefore important to understand the nature of these barriers and how they interfere with virus dissemination. One way to study the role of the midgut barrier is to compare infection rates between orally exposed and intrathoracically injected mosquitoes. Several studies have shown that intrathoracic injections of WNV into northern European Cx. pipiens mosquitoes results in infection rates of ~100 %, as compared to up to 63 % after an infectious blood meal where the virus has to escape from the midgut lumen by infecting midgut epithelial cells.Citation54, Citation55, Citation56, Citation74 This indicates that Cx. pipiens mosquitoes have a midgut infection barrier for WNV.
The same studies show that WNV transmission rates (WNV presence in saliva) of European Cx. pipiens are lower compared to WNV infection rates (WNV presence in body) after oral exposure. This suggests the presence of either a midgut escape barrier, a salivary gland barrier, or both. The presence of a salivary gland barrier can be assessed by comparing transmission rates between orally exposed and injected mosquitoes. In the absence of a salivary gland barrier, mosquitoes intrathoracically injected with WNV would have transmission rates of ~100 %. Indeed, it has been repeatedly shown that intrathoracic injections result in transmission rates of ~100 % in Cx. pipiens mosquitoes, indicating the absence of a salivary gland barrier for WNV transmission.Citation54, Citation55, Citation56, Citation74 However, studies on Cx. modestus suggest the presence of a salivary gland barrier, as they reach only 40–50 % transmission rates whereas dissemination rates were 89–91 %.Citation22, Citation23 As the vector competence of European mosquitoes is dependent on the ability of the virus to overcome the midgut and salivary gland barriers, it is important to understand the underlying mechanism of these barriers.
Innate immune responses
As a defence to incoming pathogens, mosquitoes have evolved several antiviral responses, such as the RNA interference (RNAi), Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT), Toll, immune deficiency (IMD) and apoptosis pathways.Citation75 In addition, experimental evidence suggests that heat-shock proteins and reactive oxygen species also have antiviral properties (reviewed in Saraiva et al.Citation76), but due to the limited knowledge available on these responses they will not be discussed here. The expression of genes that encode proteins and factors that function in the mosquito innate immune pathways can be upregulated or activated after ingestion of an infectious blood meal. In this way, these immune pathways play an important role in the midgut and possibly the salivary gland barriers that in turn contribute to the vector competence of a mosquito for certain arboviruses. Different mosquito species can have variation in the effectiveness of their antiviral immune responses against certain arboviruses. Such variation might explain differences in vector competence of different mosquito species for certain viral strains. Most knowledge on the invertebrate immunity is derived from research on Drosophila, although there is an increasing number of studies on immunity against alphaviruses and flaviviruses, mostly conducted on Aedes mosquitoes. Here we review the literature concerning flavivirus infections and their role in triggering the mosquito antiviral immune responses in both Aedes and Culex species mosquitoes.
RNAi is one of the most important immune responses of invertebrate vectors.Citation77 The RNAi response is an antiviral response of insects that is induced upon recognition of non-self double-stranded (ds)RNA. Even though flaviviruses are single stranded (ss)RNA viruses, dsRNA intermediates are produced during viral RNA replication that serve as substrates for the endoribonuclease Dicer-2 to activate the RNAi response. In addition, the genomic RNA can fold into dsRNA secondary structures that can be recognized by the RNAi machinery of the mosquito. Activation of the RNAi response leads to degradation of viral genomic RNA through cleavage by Argonaute-2, resulting in decreased virus replication. Various studies have shown that viral small-interfering RNAs are produced during infection of Usutu virus, DENV-2, and WNV in mosquitoes.Citation54, Citation74, Citation78 This indicates that the RNAi response is initiated upon infection of arboviruses in mosquitoes, and can influence the susceptibility of these mosquitoes for arbovirus infection. RNAi is capable of controlling DENV infection in Ae. aegypti mosquitoes and results in decreased viral titers and transmission rates.Citation79 In addition, silencing of important effector proteins in the RNAi pathway increases DENV-2 infection rates and titers in Ae. aegypti.Citation80 Interestingly, in Cx. quinquefasciatus RNAi targets the WNV genomic RNA in the mosquito midguts.Citation81
As RNAi is a potent antiviral response that restricts flavivirus transmission, it is not surprising that several RNAi suppressors have been identified in flavivirus genomes. For example, yellow fever virus capsid proteins can bind to double-stranded RNA and thereby could suppress RNA silencing, while for DENV the NS4B protein was identified as an RNAi suppressor.Citation82, Citation83 WNV encodes for an RNAi suppressor in the form of a highly structured subgenomic RNA, that acts as a decoy substrate for Dicer and thereby prevents induction of the RNAi response.Citation74 Correspondingly, a mutant WNV that lacks a potential viral suppressor of RNAi (based on in vitro studies) has decreased transmission rates after an infectious blood meal, but not after intrathoracic injection.Citation74 This observation suggests that WNV requires this potential RNAi suppressor to suppress the RNAi response in the mosquito midgut and thereby overcome the midgut barrier.
The invertebrate JAK/STAT pathway is the insect innate immune pathway that resembles the mammalian interferon response.Citation84 The JAK/STAT pathway is initiated upon virus recognition by a transmembrane receptor that activates a signaling cascade, which upregulates the expression of antiviral response genes. These antiviral genes act by recognizing virus infection, suppressing virus replication, and by triggering nearby cells to enter an antiviral state. Although strongly established as an antiviral defense system in Drosophila (reviewed in Arbouzova et alCitation85), the influence of the JAK/STAT pathway on flavivirus infection in mosquitoes remains to be resolved. One study with DENV-2 in Ae. aegypti mosquitoes showed that silencing either components or effectors of the JAK/STAT pathway increases DENV-2 titers, while silencing of a JAK/STAT antagonist decreases DENV-2 titers.Citation86 This suggests that under natural conditions, the Ae. aegypti JAK/STAT pathway restricts DENV-2 and influences the vector competence. In Ae. aegypti, infection with WNV and DENV has been shown to modulate the expression of JAK/STAT pathway-related genes.Citation87, Citation88 WNV infection in Cx. quinquefasciatus activates the JAK/STAT transcription factor STAT1A, suggesting that the JAK/STAT pathway is involved in controlling WNV infection. However, this activation has not been shown to restrict WNV dissemination or transmission.Citation89 In Cx. quinquefasciatus, activation of the RNAi pathway increases expression of the cytokine Vago, which is secreted from infected cells, to subsequently activate the JAK/STAT signaling cascade in neighbouring cells. Through this indirect mechanism, Vago thus decreases WNV titers in a JAK/STAT-dependent manner,Citation90 supporting the role of the JAK/STAT pathway in controlling flavivirus infections in Culex mosquitoes.
Another conserved immune response in mosquitoes is the Toll pathway. The Toll pathway is activated after recognition of virus infection by Toll-like receptors. This eventually results in the expression of antiviral effector genes (reviewed in Sim et al.Citation91). DENV-2 infection of Ae. aegypti mosquitoes activates the main components of the Toll pathway by increasing their transcriptional level.Citation88 Depletion of Toll regulators results in increased DENV-2 and DENV-4 titers, whereas silencing of a Toll pathway inhibitor results in decreased DENV titers.Citation88, Citation92 This indicates that the Toll pathway restricts flavivirus replication in mosquitoes and could influence the vector competence of mosquitoes. DENV infection of salivary glands in Ae. aegypti results in upregulation of Toll pathway-related genes in the salivary glands, indicating that the salivary glands can mount an antiviral response against flaviviruses.Citation45 Transcriptomic approaches have shown that components of the Toll pathway are also differentially expressed during yellow fever virus, but not WNV infection.Citation87 In another study, infection of Cx. quinquefasciatus with WNV alters the regulation of various genes, but Späetzle-like genes were the only modulated Toll pathway-related genes.Citation89 Future studies should identify whether the Toll pathway truly restricts flavivirus transmission by mosquitoes and whether the Toll pathway is also involved in restriction of WNV by Culex mosquitoes.
The IMD pathway is activated upon recognition of virus infection by the adaptor IMD protein, subsequently resulting in the transcription of IMD effector genes (reviewed in Sim et al.Citation91). Studies with DENV-2 have shown that infected Ae. aegypti have increased expression of IMD pathway components.Citation88, Citation93 However, silencing of the IMD antagonist Caspar does not affect DENV-2 titers, suggesting that the IMD pathway is not involved in controlling DENV-2 infection in Ae. aegypti.Citation88 Interestingly, silencing of the IMD pathway in a DENV refractory Ae. aegypti population resulted in increased DENV-2 replication suggesting that the IMD pathway contributes to the refractoriness of this mosquito population.Citation94 WNV infection in Cx.quinquefasciatus does not affect the regulation of IMD related genes,Citation89 although more studies are required to understand the role of the IMD pathway during WNV infections in Culex.
Apoptosis (programmed cell death) is considered an antiviral response by the host to eliminate viral infection by killing infected and neighboring cells. Many viruses inhibit apoptosis in order to facilitate their replication and to prevent clearance of the infected cells.Citation46 Interestingly, some arboviruses utilize cellular caspases during replication or use apoptosis as a measure to release virions from infected cells. WNV infection in Cx. pipiens causes apoptosis in the midgut cells, mainly in the anterior region.Citation95 Infection of Ae. aegypti mosquitoes with DENV results in upregulation of apoptosis-related genes. For some genes that function in the apoptosis pathway, silencing results in increased DENV replication.Citation96 In addition, DENV infection of both a refractory and susceptible strain of Ae. aegypti induces the expression of the apoptotic gene mx as soon as 3 h post blood meal in the refractory, but not in the susceptible strain.Citation97 These observations suggest that apoptosis acts as an antiviral response in the midgut. Also, in the salivary glands, WNV infection causes apoptotic-like cell death, most likely due to the triggering of cellular apoptotic pathways as a result of active virus replication.Citation28, Citation98 In addition, infection of Cx. quinquefasciatus salivary glands with WNV results in a decrease in a Culex apoptosis inhibitor.Citation99 Although apoptosis is, thus, induced in the salivary glands, virus titers do not rapidly decline over time, suggesting that apoptosis is not effectively clearing the virus-infected cells in the salivary glands.Citation98 Alternatively, virus-induced apoptosis in the salivary glands can perhaps increase the release of transmissible virus into the saliva thereby actually facilitating virus transmission. Future studies should elucidate the effect of apoptosis on WNV infection of the midgut and release of WNV into the saliva.
The mosquito microbiome
Mosquitoes live in symbiosis with a wide variety of bacteria and fungi, which are abundantly present in the mosquito gut and are referred to as the mosquito gut microbiome.Citation100 These microorganisms interact with the mosquito gut and have a strong impact on the mosquito’s metabolism, but also affect interactions with pathogens.Citation101 The microbiome of Ae. aegypti is important for successful infection of flaviviruses, as has been shown for DENV-2.Citation100 The introduction of specific bacterial strains into the mosquito gut microbiome can affect the infection or replication of flaviviruses in mosquitoes either in a positive or negative way, which in turn may affect their vector competence for certain viruses.Citation100 In addition, mosquitoes can be persistently infected with insect-specific viruses that normally do not affect the mosquito life cycle, but can have an effect on the ability of the mosquito to become infected with other viruses.Citation102
Apart from bacteria in the mosquito midgut, some bacteria are present inside the cells of mosquitoes and are transmitted both horizontally between species and vertically to the mosquito offspring.Citation103 The best documented intracellular bacterium of mosquitoes is Wolbachia. Wolbachia is an endosymbiotic bacteria that can affect vector competence for arboviruses (reviewed in JohnsonCitation104). Although there is a large body of literature describing the interactions between flaviviruses and Wolbachia, we will here discuss those studies addressing the effect of Wolbachia on transmission and replication of WNV only. Wolbachia infection increased WNV replication, but resulted in >100-fold less infectious virus production, suggesting that Wolbachia interferes with virion assembly within or secretion of virus from mosquito cells.Citation105 Consequently, Ae. aegypti mosquitoes infected with Wolbachia have ~50 % reduction in WNV infection and 100 % reduction in transmission rates. Infection of Cx. quinquefasciatus with Wolbachia results in increased resistance to WNV infection via a blood meal. Dissemination rates and transmission rates were significantly lower (2–3-fold) as compared to mosquitoes cleared from Wolbachia with tetracycline.Citation106 However, Cx. tarsalis mosquitoes infected with Wolbachia showed 1.5–2-fold enhanced WNV infection rates due to downregulation of Toll pathway-related genes by Wolbachia.Citation107 Accordingly, a study on Wolbachia in field-caught Cx. quinquefasciatus populations showed that densities (that is, the bacterial load) of Wolbachia were too low to inhibit WNV infection and did not affect vector competence of these same mosquito colonies. Thus, for Cx. quinquefasciatus the virus inhibitory effect depends on the density of Wolbachia in the mosquito.Citation108 The effect of Wolbachia on vector competence of mosquitoes is further dependent on the strain of Wolbachia in combination with the species of mosquito used.Citation104 Taken together infection with Wolbachia can have a strong effect on vector competence of mosquitoes for WNV, although the mechanism remains unclear.
Discussion and Conclusions
Until now, four European mosquito species, Ae. albopictus,Citation49 Ae. detritus,Citation50 Cx. modestusCitation22, Citation23 and Cx. pipiens,Citation49, Citation53, Citation54, Citation55, Citation56, Citation57 have been confirmed to be competent to transmit WNV. In contrast, Ae. caspius and Ae. japonicus japonicus are not competent as vector for WNV.Citation22, Citation51 Highest transmission rates were found for Cx. pipiens, which also have high WNV infection rates in the field,Citation109 and are highly abundant during summer.Citation110 Therefore, Cx. pipiens is considered to be the most important vector for WNV in Europe.
No intrinsic differences in vector competence were found in a direct comparison between a northern and southern European Cx. pipiens population.Citation57 Importantly, increased WNV transmission was observed at higher temperatures for both northern and southern European Cx. pipiens.Citation57 This confirms that temperature is likely the most important factor to explain why WNV outbreaks have thus far been limited to southern and central Europe.Citation55 Considering the presence of intrinsically competent mosquito vectors,Citation22, Citation23, Citation49, Citation50, Citation53, Citation54, Citation55 susceptible bird hostsCitation6, Citation7 and predictions of more frequent and prolonged temperature anomalies, there are no limiting factors for future WNV circulation in northern Europe. In fact, the recent circulation of Usutu virus in northern Europe during the autumn of 2016,Citation111 exemplifies that there are no evident restrictions for Culex-borne flaviviruses to become endemic in northern Europe. Because of the similar transmission cycle and comparable transmission rates of both Usutu and West Nile virus with their main vector Cx. pipiens, the circulation of Usutu virus can be considered as a prelude to WNV transmission.Citation54
Implications for WNV surveillance
Insights in vector competence allow for targeted surveillance and control of highly competent mosquito species which pose a high risk for WNV transmission. Several European countries such as Italy and Greece, have implemented WNV surveillance programmes by monitoring mosquitoes, (sentinel-)birds, (sentinel-)equines, or humans for presence of virus, seroconversion, or disease symptoms. With the knowledge presented in this review, we recommend to implement WNV surveillance programmes throughout Europe. Examples of such surveillance programmes for both northern (for example, United Kingdom) and southern European countries (for example, Italy and Greece) were reviewed in (Engler et al.Citation109 and Gossner et al.Citation112). We favour integrating national surveillance initiatives into a Europe-wide surveillance network, as reviewed in (Engler et al.Citation109 and Gossner et al.Citation112). This should result in the use of general protocols, which allows for comparisons across Europe. However, selection of appropriate surveillance tools should still be based on the specific needs of individual countries.
Surveillance programmes can either be active through the screening for virus in living organisms or passive, involving the screening of dead birds or based on serology.Citation109 Such screening techniques would rely on the detection of WNV by PCR, or detection of WNV-specific antibodies (serology) in humans, equines and birds. Active screening of mosquitoes or sentinel-birds allows for detection early in the season, whereas passive screening of dead birds or cases of neurological disease in equines or humans is most useful to provide insight in emergence of WNV into new areas. Detection early in the season allows for awareness, targeted mosquito control and personal protection which can reduce WNV outbreaks in humans later in the season. This form of surveillance is, therefore, highly recommended for countries, which encounter yearly cases of WNV in humans. However, it is less essential for countries in which no human cases have yet emerged. Active mosquito surveillance is costly and WNV is usually only detected in a few percent of mosquito pools,Citation109 even during outbreaks. This makes passive surveillance of suspected cases more suitable for countries without known cases of WNV infection in humans.
Recommendations for vector competence studies
Considering the vector competence studies of European mosquito species for WNV, it becomes clear that the large variation in study protocols makes it difficult to directly compare studies. Variation in techniques, and choice of mosquito populations and viral strains, have an effect on the outcomes of vector competence studies. To enable comparison between vector competence studies, detailed information on the mosquito vector, virus strain and applied techniques need to be reported. Such information should at the minimum include accurate identification of the mosquito vector, exact origin of mosquito populations (coordinates), mosquito generation, mosquito rearing protocols, incubation temperature and incubation time. Information regarding the virus isolate should include the viral strain used (including GenBank reference), virus passage number, cell types used to grow virus stocks and technical details on how the virus stock was generated. Technical details should include detailed descriptions of blood feeding methods (including the blood source) and infectivity assays. Studies should at the minimum report summary statistics that include number of replicates, sample sizes, mean or median and variation in data. In addition, raw data should be made publicly available, to allow for quantitative meta-analyses. Future research consortia should discuss and adjust research protocols in order to increase comparability between studies from different laboratories. Inclusion of more detailed information and consistency in research protocols allows for better comparison between studies and makes it easier to draw conclusions on observed results.
Choice of incubation time, incubation temperature, and virus dose can strongly influence the outcome of vector competence studies. Studies that focused on the effect of incubation time,Citation49 incubation temperatureCitation54, Citation55, Citation56, Citation57 and WNV dose confirmed their effects on transmission rates.Citation113 For studies aiming to determine vector competence of a certain mosquito species, we recommend to use fixed incubation periods and temperatures in order to increase comparability between studies. On the basis of previous studies, we suggest a standardised incubation period of 14 days, which was shown to be sufficiently long for temperatures of 18 °C and higher.Citation114 Furthermore, we suggest a standardised incubation period of 27±1 °C. Additional incubation temperatures that represent average summer temperatures of the region the mosquito population originates from are also advised.Importantly, when assessing relatively low incubation temperatures of below 18 °C, the minimum incubation period needs to be increased. Finally, we suggest a standardised WNV dose of 107 TCID50/mL. Standardizing vector competence protocols will largely increase the ability to make comparisons between studies, which is essential to increase the understanding on WNV transmission in Europe.
Vector competence studies can provide information such as infection, dissemination and transmission rates, which provides insights in the likelihood of the vector to transmit the virus in the field. Some studies also include the rate at which a model organism can become infected by a viraemic mosquito, which gives a better proxy for transmission in the field. However, these studies are labour intensive, and can often only be performed with a small number of individuals due to ethical constraints and costs. Therefore, assessing the presence of virus in the saliva is the best alternative to estimate transmission rates. In addition, vector competence studies should focus on using cell culture to assess infectivity instead of relying on qRT-PCR methods. Finally, we strongly recommend that future vector competence studies determine transmission rates by measuring virus in the saliva in order to better understand the state of WNV transmission in Europe.
Future directions for understanding vector competence
In this review, we have shown that European mosquito species vary in their level of competence for WNV, and that some species are not competent. Although this indicates that surveillance for WNV in European mosquitoes is necessary, it does not answer the question why some European vectors are more competent than others. In order to fully understand arbovirus transmission dynamics, it is important to identify the determining factors of vector competence. Especially the influence of the mosquito barriers, immune responses (specifically against flaviviruses), and microbes on vector competence are still poorly understood. Studying the underlying mechanisms of how these barriers and responses influence vector competence in mosquitoes is therefore important to understand variation in vector competence.
As the most important vector species for WNV transmission in Europe is Cx. pipiens, more research on virus-vector interactions in Cx. pipiens is required to overcome the challenges related to virus control. Fortunately, the genome sequence of Cx. quinquefasciatus, the first sequenced genome of a Culex species, is available and allows for genetic studies with Culex mosquitoes. However, as the genome sequence of Cx. pipiens is not available, genetic studies should be performed with Cx. quinquefasciatus mosquitoes. Therefore, sequencing of the Cx. pipiens genome is an important research goal. Alternatively, attempts can be made to perform de novo transcriptomics in Cx. pipiens and compare the outcomes with the annotation of the Cx. quinquefasciatus genome, although such an approach is likely to miss some candidate genes due to genetic variations.
Thus far, most studies on mosquito immune pathways involved in flavivirus infections have been done with Aedes mosquitoes. The limited research on mosquito immune pathways in Culex has confirmed the presence of the RNAi, JAK/STAT, Toll, and IMD pathway, as putative antiviral responses towards flavivirus infections. Yet, the relative contribution and effectiveness of these pathways in controlling transmission of WNV and other flaviviruses by Culex mosquitoes is still unclear and requires further investigation. Potentially, such studies can indicate novel antiviral responses that are unique for Culex species such as the antiviral Vago response that has thus far only been observed in Culex mosquitoes.Citation90 Studies that aim to understand the molecular interactions between the vector and the virus often rely on controlled experiments in cell lines. Culex cell lines are available for the species Cx. quinquefasciatus (Hsu) and Cx. tarsalis(CxT), but a cell line of Cx. pipiens is not available. This limits studies on immune responses and virus replication in Cx. pipiens mosquitoes to extrapolations from cell lines of other Culex species. Because Cx. pipiens is the predominant vector species for WNV in Europe, a Cx. pipiens cell line would greatly contribute to research that aims to understand the basis of vector competence for WNV in European mosquitoes.
Studies that investigated the effect of temperature on the transmission of WNV by European mosquitoes have shown that increasing temperatures result in higher transmission rates (Table ). Indeed, higher temperatures can lead to higher virus replication rates. Alternatively, temperature can have an effect on immune regulatory pathways or the microbiome, resulting in altered vector competence.Citation115 Interestingly, it has been shown that at low rearing temperatures of 18 °C, the RNAi pathway is less effective in Ae. aegypti resulting in increased susceptibility to arbovirus infection.Citation116 Similar studies in Culex mosquitoes could highlight why the vector competence changes with alternating temperature or whether it is simply the lowered virus replication at lower temperatures. Apart from temperature also the viraemic dose and genetic differences between mosquito populations might affect the induction of innate immune responses. Although it seems natural that a higher viral dose in the initial infection would lead to a stronger induction of immune responses, research supporting this hypothesis is currently lacking. Furthermore, mosquito populations that are more susceptible to WNV infection could have a lower induction of antiviral immune responses upon viral infection. Future studies should try to answer these questions in order to increase our understanding of WNV transmission by mosquitoes. Finally, it has been shown that the composition of the larval environment such as availability of nutrition, larval density, and temperature during development can influence the vector competence of mosquitoes.Citation117 The effect of nutrition appeared to be negligible, while competition in the larval environment increases the vector competence. Interestingly, warm rearing temperatures decrease the susceptibility of mosquitoes for infection, in contrary to the influence of temperature during the extrinsic incubation period. Whether differences in larval environment also influence innate immune responses, and thereby alter the vector competence in mosquitoes requires further research.
Understanding the molecular mechanisms of virus transmission allows for more directed control approaches by for example, targeting barrier specific genes or application of microorganisms that reduce vector competence. Various vector control strategies have been proposed based on our current understanding of the mosquito’s immune system such as (i) the development of transmission blocking vaccines, (ii) the generation of refractory mosquitoes through genetic modification of either the mosquito or (iii) the mosquito’s microbiome and (iv) infection of mosquitoes with intracellular bacteria such as Wolbachia.Citation104, Citation118, Citation119 Although no attempts have been made to employ the mosquito’s immune system for the control of arboviral disease, promising results have been made in field studies with Wolbachia-infected mosquitoes. Studies with Wolbachia-infected Aedes mosquitoes in Australia and Vietnam have already shown the potential of Wolbachia to invade the local mosquito population.Citation120 While the effect on virus-transmission in the field is yet to be determined, this study indicates the feasibility of releasing Wolbachia-infected mosquitoes as an arboviral control strategy. Potentially, similar release strategies could be performed with genetically modified mosquitoes that employ the mosquito’s immune system to further prevent the transmission of arboviruses.
Acknowledgments
We thank Marcel Dicke, Laboratory of Entomology, Wageningen University & Research, and Monique van Oers, Laboratory of Virology, Wageningen University & Research, for feedback on an earlier version of the manuscript. This work is supported by the Strategic Fund of the graduate school Production Ecology & Resource Conservation, The Netherlands.
References
- Bowen RA, Nemeth NM.Experimental infections with West Nile virus. Curr Opin Infect Dis 2007;20: 293–297.
- Petersen LR, Brault AC, Nasci RS.West Nile virus: review of the literature. JAMA 2013;310: 308–315.
- Buckley A, Dawson A, Gould EA.Detection of seroconversion to West Nile virus, Usutu virus and Sindbis virus in UK sentinel chickens. Virol J 2006;3: 71.
- Buckley A, Dawson A, Moss SRet al.Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J Gen Virol 2003;84: 2807–2817.
- Linke S, Niedrig M, Kaiser Aet al.Serologic evidence of West Nile virus infections in wild birds captured in Germany. Am J Trop Med Hyg 2007;77: 358–364.
- Lim SM, Brault AC, van Amerongen Get al.Susceptibility of carrion crows to experimental infection with lineage 1 and 2 West Nile Viruses. Emerg Infect Dis 2015;21: 1357–1365.
- Lim SM, Brault AC, van Amerongen Get al.Susceptibility of European jackdaws (Corvus monedula to experimental infection with lineage 1 and 2 West Nile viruses. J Gen Virol 2014;95: 1320–1329.
- Hamer GL, Kitron UD, Goldberg TLet al.Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg 2009;80: 268–278.
- Kent R, Juliusson L, Weissmann Met al.Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol 2009;46: 380–390.
- Kilpatrick AM, Daszak P, Jones MJet al.Host heterogeneity dominates West Nile virus transmission. Proc R Soc Lond B Biol Sci 2006;273: 2327–2333.
- Muñoz J, Eritja R, Alcaide Met al.Host-feeding patterns of native Culex pipiens and invasive Aedes albopictus mosquitoes (Diptera: Culicidae) in urban zones from Barcelona, Spain. J Med Entomol 2011;48: 956–960.
- Muñoz J, Ruiz S, Soriguer Ret al.Feeding patterns of potential West Nile virus vectors in South-West Spain. PLoS ONE 2012;7: e39549.
- Osório HC, Zé-Zé L, Alves MJ.Host-feeding patterns of Culex pipiens and other potential mosquito vectors (Diptera: Culicidae) of West Nile virus (Flaviviridae) collected in Portugal. J Med Entomol 2012;49: 717–721.
- Rizzoli A, Bolzoni L, Chadwick EAet al.Understanding West Nile virus ecology in Europe: Culex pipiens host feeding preference in a hotspot of virus emergence. Parasit Vectors 2015;8: 213.
- Brugman VA, Hernández-Triana LM, England MEet al.Blood-feeding patterns of native mosquitoes and insights into their potential role as pathogen vectors in the Thames estuary region of the United Kingdom. Parasit Vectors 2017;10: 163.
- Garrett-Jones C.Prognosis for interruption of malaria transmission through assessment of the mosquito's vectorial capacity. Nature 1964;204: 1173–1175.
- Kenney J, Brault A.The role of environmental, virological and vector interactions in dictating biological transmission of arthropod-borne viruses by mosquitoes. In: Maramorosch K, Murphy FAAdvances in Virus Research vol. 89.Waltham: Academic Press.2014 pp 39–83.
- Goddard LB, Roth AE, Reisen WKet al.Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis 2002;8: 1385–1391.
- Sardelis MR, Turell MJ, Dohm DJet al.Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis 2001;7: 1018.
- Turell MJ, Dohm DJ, Sardelis MRet al.An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol 2005;42: 57–62.
- Turell MJ, Sardelis MR, Dohm DJet al.Potential North American vectors of West Nile virus. Ann N Y Acad Sci 2001;951: 317–324.
- Balenghien T, Vazeille M, Grandadam Met al.Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis 2008;8: 589–595.
- Balenghien T, Vazeille M, Reiter Pet al.Evidence of laboratory vector competence of Culex modestus for West Nile virus. J Am Mosq Control Assoc 2007;23: 233–236.
- Lerdthusnee K, Romoser WS, Faran MEet al.Rift Valley Fever virus in the cardia of Culex pipiens: An immunocytochemical and ultrastructural study. Am J Trop Med Hyg 1995;53: 331–337.
- Terra WR.The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol 2001;47: 47–61.
- Houk EJ, Obie F, Hardy JL.Peritrophic membrane formation and the midgut barrier to arboviral infection in the mosquito, Culex tarsalis Coquillett (Insecta, Diptera). Acta Trop 1979;36: 39–45.
- Hardy JL, Houk EJ, Kramer LDet al.Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol 1983;28: 229–262.
- Girard YA, Popov V, Wen Jet al.Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae). J Med Entomol 2005;42: 429–444.
- Whitfield SG, Murphy FA, Sudia WD.St. Louis encephalitis virus: an ultrastructural study of infection in a mosquito vector. Virology 1973;56: 70–87.
- Kato N, Mueller CR, Fuchs JFet al.Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti. Vector Borne Zoonotic Dis 2008;8: 701–712.
- Mendoza MY, Salas-Benito JS, Lanz-Mendoza Het al.A putative receptor for dengue virus in mosquito tissues: localization of a 45-kDa glycoprotein. Am J Trop Med Hyg 2002;67: 76–84.
- Vega-Almeida TO, Salas-Benito M, De Nova-Ocampo MAet al.Surface proteins of C6/36 cells involved in dengue virus 4 binding and entry. Arch Virol 2013;158: 1189–1207.
- McGee CE, Shustov AV, Tsetsarkin Ket al.Infection, dissemination, and transmission of a West Nile virus green fluorescent protein infectious clone by Culex pipiens quinquefasciatus mosquitoes. Vector Borne Zoonotic Dis 2010;10: 267–274.
- Scholle F, Girard YA, Zhao Qet al.trans-Packaged West Nile virus-like particles: infectious properties in vitro and in infected mosquito vectors. J Virol 2004;78: 11605–11614.
- Franz A, Kantor A, Passarelli Aet al.Tissue barriers to arbovirus infection in mosquitoes. Viruses 2015;7: 3741–3767.
- Thomas RE, Wu W-K, Verleye Det al.Midgut basal lamina thickness and dengue-1 virus dissemination rates in laboratory strains of Aedes albopictus (Diptera: Culicidae). J Med Entomol 1993;30: 326–331.
- Romoser WS, Wasieloski LP, Pushko Pet al.Evidence for arbovirus dissemination conduits from the mosquito (Diptera: Culicidae) midgut. J Med Entomol 2004;41: 467–475.
- Salazar MI, Richardson JH, Sánchez-Vargas Iet al.Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol 2007;7: 1.
- Romoser WS, Turell MJ, Lerdthusnee Ket al.Pathogenesis of Rift Valley fever virus in mosquitoes—tracheal conduits & the basal lamina as an extra-cellular barrier. Arch Virol 2005;19: 89–100.
- Weaver SC, Scott TW, Lorenz LHet al.Detection of Eastern equine encephalomyelitis virus deposition in Culiseta melanura following ingestion of radiolabeled virus in blood meals. Am J Trop Med Hyg 1991;44: 250–259.
- da Cunha Sais T, de Moraes RM, Ribolla PEet al.Morphological aspects of Culex quinquefasciatus salivary glands. Arthropod Structure & Development 2003;32: 219–226.
- Göertz GP, Vogels CBF, Geertsema Cet al.Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLoS Negl Trop Dis 2017;11: e0005654.
- Turell MJ, Mores CN, Dohm DJet al.Laboratory transmission of Japanese encephalitis and West Nile viruses by molestus form of Culex pipiens (Diptera: Culicidae) collected in Uzbekistan in 2004. J Med Entomol 2006;43: 296–300.
- Vazeille M, Yébakima A, Lourenço-de-Oliveira Ret al.Oral receptivity of Aedes aegypti from Cape Verde for yellow fever, dengue, and chikungunya viruses. Vector Borne Zoonotic Dis 2013;13: 37–40.
- Sim S, Ramirez JL, Dimopoulos G.Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS Pathog 2012;8: e1002631.
- Clem RJ.Arboviruses and apoptosis: the role of cell death in determining vector competence. J Gen Virol 2016;97: 1033–1036.
- Hayes EB, Komar N, Nasci RSet al.Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis 2005;11: 1167–1173.
- Brustolin M, Talavera S, Santamaría Cet al.Culex pipiens and Stegomyia albopicta (=Aedes albopictus populations as vectors for lineage 1 and 2 West Nile virus in Europe. Med Vet Entomol 2016;30: 166–173.
- Fortuna C, Remoli ME, Severini Fet al.Evaluation of vector competence for West Nile virus in Italian Stegomyia albopicta (=Aedes albopictus mosquitoes. Med Vet Entomol 2015;29: 430–433.
- Blagrove MSC, Sherlock K, Chapman GEet al.Evaluation of the vector competence of a native UK mosquito Ochlerotatus detritusAedes detritus for dengue, chikungunya and West Nile viruses. Parasit Vectors 2016;9: 1–6.
- Huber K, Jansen S, Leggewie Met al.Aedes japonicus japonicus (Diptera: Culicidae) from Germany have vector competence for Japan encephalitis virus but are refractory to infection with West Nile virus. Parasitol Res 2014;113: 3195–3199.
- Leggewie M, Badusche M, Rudolf Met al.Culex pipiens and Culex torrentium populations from Central Europe are susceptible to West Nile virus infection. One Health 2016;2: 88–94.
- Fortuna C, Remoli ME, Di Luca Met al.Experimental studies on comparison of the vector competence of four Italian Culex pipiens populations for West Nile virus. Parasit Vectors 2015;8: 463.
- Fros JJ, Miesen P, Vogels CBet al.Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health 2015;1: 31–36.
- Fros JJ, Geertsema C, Vogels CBet al.West Nile Virus: High transmission rate in north-western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoS Negl Trop Dis 2015;9: e0003956.
- Vogels CBF, Fros JJ, Göertz GPet al.Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasit Vectors 2016;9: 393.
- Vogels CBF, Göertz GP, Pijlman GPet al.Vector competence of northern and southern European Culex pipiens pipiens mosquitoes for West Nile virus across a gradient of temperatures. Med Vet Entomol 2017;31: 358–364.
- Gargan TP, Bailey CL, Higbee GAet al.The effect of laboratory colonization on the vector-pathogen interactions of Egyptian Culex pipiens and Rift Valley Fever Virus. Am J Trop Med Hyg 1983;32: 1154–1163.
- Grimstad PR, Craig GB Jr, Ross QEet al.Aedes triseriatus and La Crosse virus: geographic variation in vector susceptibility and ability to transmit. Am J Trop Med Hyg 1977;26: 990–996.
- Domingo E, Sheldon J, Perales C.Viral quasispecies evolution. Microbiol Mol Biol Rev 2012;76: 159–216.
- Ciota AT, Lovelace AO, Ngo KAet al.Cell-specific adaptation of two flaviviruses following serial passage in mosquito cell culture. Virology 2007;357: 165–174.
- Pesko K, Westbrook CJ, Mores CNet al.Effects of infectious virus dose and blood meal delivery method on susceptibility of Aedes aegypti and Aedes albopictus to chikungunya virus. J Med Entomol 2009;46: 395–399.
- Mahmood F, Chiles RE, Fang Yet al.Methods for studying the vector competence of Culex tarsalis for western equine encephalomyelitis virus. J Am Mosq Control Assoc 2004;20: 277–282.
- Komar N, Langevin S, Hinten Set al.Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 2003;9: 311.
- Reisen WK, Fang Y, Martinez VM.Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol 2005;42: 367–375.
- Bae H-G, Nitsche A, Teichmann Aet al.Detection of yellow fever virus: a comparison of quantitative real-time PCR and plaque assay. J Virol Methods 2003;110: 185–191.
- Choy MM, Ellis BR, Ellis EMet al.Comparison of the mosquito inoculation technique and quantitative real time polymerase chain reaction to measure dengue virus concentration. Am J Trop Med Hyg 2013;89: 1001–1005.
- Kilpatrick AM, Kramer LD, Campbell SRet al.West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis 2005;11: 425–429.
- Papa A, Xanthopoulou K, Tsioka Aet al.West Nile virus in mosquitoes in Greece. Parasitol Res 2013;112: 1551–1555.
- Fritz ML, Walker ED, Miller JRet al.Divergent host preferences of above- and below-ground Culex pipiens mosquitoes and their hybrid offspring. Med Vet Entomol 2015;29: 115–123.
- Osório HC, Zé-Zé L, Amaro Fet al.Sympatric occurrence of Culex pipiens (Diptera, Culicidae) biotypes pipiensmolestus and their hybrids in Portugal, Western Europe: feeding patterns and habitat determinants. Med Vet Entomol 2014;28: 103–109.
- Sardelis MR, Turell MJ.Ochlerotatus j. japonicus in Frederick County, Maryland: discovery, distribution, and vector competence for West Nile virus. J Am Mosq Control Assoc 2001;17: 137–141.
- Turell MJ, O'Guinn ML, Dohm DJet al.Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol 2001;38: 130–134.
- Göertz GP, Fros JJ, Miesen Pet al.Non-coding subgenomic flavivirus RNA is processed by the mosquito RNAi machinery and determines West Nile virus transmission by Culex pipiens mosquitoes. J Virol 2016;90: 10145–10159.
- Prasad AN, Brackney D, Ebel G.The role of innate immunity in conditioning mosquito susceptibility to West Nile virus. Viruses 2013;5: 3142.
- Saraiva RG, Kang S, Simões MLet al.Mosquito gut antiparasitic and antiviral immunity. Dev Comp Immunol 2016;64: 53–64.
- Blair CD.Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol 2011;6: 265–277.
- Hess AM, Prasad AN, Ptitsyn Aet al.Small RNA profiling of dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC Microbiol 2011;11: 45.
- Franz AWE, Sanchez-Vargas I, Adelman ZNet al.Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA 2006;103: 4198–4203.
- Sánchez-Vargas I, Scott JC, Poole-Smith BKet al.Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito's RNA interference pathway. PLoS Pathog 2009;5: e1000299.
- Brackney DE, Beane JE, Ebel GD.RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog 2009;5: e1000502.
- Kakumani PK, Ponia SS, KS Rajgokulet al.Role of RNA interference (RNAi) in dengue virus replication and identification of NS4B as an RNAi suppressor. J Virol 2013;87: 8870–8883.
- Samuel GH, Wiley MR, Badawi Aet al.Yellow fever virus capsid protein is a potent suppressor of RNA silencing that binds double-stranded RNA. Proc Natl Acad Sci USA 2016;113: 13863–13868.
- Fragkoudis R, Attarzadeh-Yazdi G, Nash AAet al.Advances in dissecting mosquito innate immune responses to arbovirus infection. J Gen Virol 2009;90: 2061–2072.
- Arbouzova NI, Zeidler MP.JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 2006;133: 2605–2616.
- Souza-Neto JA, Sim S, Dimopoulos G.An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA 2009;106: 17841–17846.
- Colpitts TM, Cox J, Vanlandingham DLet al.Alterations in the Aedes aegypti transcriptome during infection with West Nile, dengue and yellow fever viruses. PLoS Pathog 2011;7: e1002189.
- Xi Z, Ramirez JL, Dimopoulos G.The Aedes aegypti Toll pathway controls dengue virus infection. PLoS Pathog 2008;4: e1000098.
- Bartholomay LC, Waterhouse RM, Mayhew GFet al.Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 2010;330: 88–90.
- Paradkar PN, Trinidad L, Voysey Ret al.Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc Natl Acad Sci USA 2012;109: 18915–18920.
- Sim S, Jupatanakul N, Dimopoulos G.Mosquito immunity against arboviruses. Viruses 2014;6: 4479–4504.
- Ramirez JL, Dimopoulos G.The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev Comp Immunol 2010;34: 625–629.
- Luplertlop N, Surasombatpattana P, Patramool Set al.Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following infection with dengue virus. PLoS Pathog 2011;7: e1001252.
- Sim S, Jupatanakul N, Ramirez JLet al.Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl Trop Dis 2013;7: e2295.
- Vaidyanathan R, Scott TW.Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis 2006;11: 1643–1651.
- Barón OL, Ursic-Bedoya RJ, Lowenberger CAet al.Differential gene expression from midguts of refractory and susceptible lines of the mosquito, Aedes aegypti, infected with Dengue-2 virus. J Insect Sci 2010;10: 41.
- Liu B, Behura SK, Clem RJet al.P53-mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster. PLoS Pathog 2013;9: e1003137.
- Girard YA, Schneider BS, McGee CEet al.Salivary gland morphology and virus transmission during long-term cytopathologic West Nile virus infection in Culex mosquitoes. Am J Trop Med Hyg 2007;76: 118–128.
- Girard YA, Mayhew GF, Fuchs JFet al.Transcriptome changes in Culex quinquefasciatus (Diptera: Culicidae) salivary glands during West Nile virus infection. J Med Entomol 2010;47: 421–435.
- Hegde S, Rasgon JL, Hughes GL.The microbiome modulates arbovirus transmission in mosquitoes. Curr Opin Virol 2015;15: 97–102.
- Jupatanakul N, Sim S, Dimopoulos G.The insect microbiome modulates vector competence for arboviruses. Viruses 2014;6: 4294.
- Salas-Benito JS, De Nova-Ocampo M.Viral interference and persistence in mosquito-borne flaviviruses. J Immunol Res 2015;2015: 14.
- O'Neill SL, Hoffmann AA, Werren JH. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction.Oxford, UK: Oxford University Press.1997.
- Johnson KN.The impact of Wolbachia on virus infection in mosquitoes. Viruses 2015;7: 5705–5717.
- Hussain M, Lu G, Torres Set al.Effect of Wolbachia on replication of West Nile virus in a mosquito cell line and adult mosquitoes. J Virol 2013;87: 851–858.
- Glaser RL, Meola MA.The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 2010;5: e11977.
- Dodson BL, Hughes GL, Paul Oet al.Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl Trop Dis 2014;8: e2965.
- Micieli MV, Glaser RL.Somatic Wolbachia (Rickettsiales: Rickettsiaceae) levels in Culex quinquefasciatus and Culex pipiens (Diptera: Culicidae) and resistance to West Nile virus infection. J Med Entomol 2014;51: 189–199.
- Engler O, Savini G, Papa Aet al.European surveillance for West Nile virus in mosquito populations. Int J Env Res Public Health 2013;10: 4869–4895.
- Chaskopoulou A, L’Ambert G, Petric Det al.Ecology of West Nile virus across four European countries: review of weather profiles, vector population dynamics and vector control response. Parasit Vectors 2016;9: 482.
- Cadar D, Lühken R, van der Jeugd Het al.Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill 2017;22: 30452.
- Gossner CM, Marrama L, Carson Met al.West Nile virus surveillance in Europe: moving towards an integrated animal-human-vector approach. Euro Surveill 2017;22.
- Anderson SL, Richards SL, Tabachnick WJet al.Effects of West Nile virus dose and extrinsic incubation temperature on temporal progression of vector competence in Culex pipiens quinquefasciatus. J Am Mosq Control Assoc 2010;26: 103–107.
- Kilpatrick AM, Meola MA, Moudy RMet al.Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog 2008;4: e1000092.
- Murdock CC, Paaijmans KP, Cox-Foster Det al.Rethinking vector immunology: the role of environmental temperature in shaping resistance. Nat Rev Microbiol 2012;10: 869–876.
- Adelman ZN, Anderson MAE, Wiley MRet al.Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl Trop Dis 2013;7: e2239.
- Alto BW, Lounibos LPVector competence for arboviruses in relation to the larval environment of mosquitoes. In: Takken W, Koenraadt CJMEcology of Parasite-Vector Interactions.Wageningen: Wageningen Academic Publishers.2013 pp 81–101.
- Gonçalves D, Hunziker P.Transmission-blocking strategies: the roadmap from laboratory bench to the community. Malar J 2016;15: 95.
- Wang S, Jacobs-Lorena M.Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol 2013;31: 185–193.
- Nguyen TH, Nguyen HL, Nguyen TYet al.Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors 2015;8: 563.
