Abstract
There has been limited research on the therapeutic efficacy of molecular diagnosis of spinal tuberculosis. We attempted to determine whether the utilization of molecular diagnosis to detect multidrug-resistant spinal tuberculosis can improve clinical outcomes. A multicenter retrospective study was conducted from February 2009 to June 2015. Ninety-two consecutive culture-confirmed multidrug-resistant tuberculosis (MDR-TB) patients with spinal tuberculosis who were diagnosed clinically and by imaging were enrolled in the study. The initial time to treatment for MDR-TB, the method of infection control, the erythrocyte sedimentation rate (ESR) and the occurrence of complications in patients who were diagnosed using molecular methods were compared with those of patients diagnosed using standard culture and drug susceptibility test methods. Of 92 MDR-TB patients with spinal tuberculosis, 41 (45%) were diagnosed by standard culture and drug susceptibility test methods (Group A), and 51 (55%) were diagnosed following implementation of detection using molecular diagnosis (Group B). The patients in Group B began the rational use of second-line drugs earlier than patients in Group A (5 days vs 73 days, P<0.05). Among patients who were admitted to a general tuberculosis ward, those in Group B spent less time in the ward than those in Group A (4 days vs 33 days, P<0.05). At the one-month follow-up, the ESR was significantly lower in Group B. In patients who completed 6 months of follow-up (n=92), the incidence of complications was significantly lower in Group B. The use of molecular diagnosis resulted in noteworthy clinical advances, including earlier initiation of MDR-TB treatment, improved infection control, better clinical outcome, a more rapid decrease in ESR and fewer complications.
Emerging Microbes & Infections (2017) 6, e97; doi:10.1038/emi.2017.83; published online 8 November 2017
Introduction
The number of new tuberculosis (TB) cases in China was 980 000 in 2013; multidrug-resistant tuberculosis (MDR-TB) accounted for ~5.7% of these cases, and the proportion of MDR-TB in patients undergoing retreatment was as high as 26%. There remains a large gap in the detection and treatment of global MDR-TB. China is currently ranked first among the 27 countries with the highest burden of MDR-TB and extensively drug-resistant tuberculosis in the world.Citation1 Spinal TB, almost all cases of which originate from pulmonary TB, is one of the most common extrapulmonary TB pathologies. It can result in serious complications and is also subject to the challenge of drug resistance.
Anti-TB drug regimens are the cornerstone of spinal TB treatment. Effective drug regimens are fundamentally required to kill Mycobacterium tuberculosis and cure the disease. Early diagnosis of drug resistance and drug susceptibility testing (DST)-guided individualized chemotherapy are crucial for the optimal management of this disease. However, conventional DST for M. tuberculosis still relies on culture of the bacilli and requires a minimum of several weeks.Citation2 The use of ineffective anti-TB regimens during the testing period may result in acquired drug resistance and local recurrence.Citation3 Moreover, DST for TB spondylitis is not performed routinely in most resource-poor hospitals in China due to biosafety concerns and inadequate infrastructure, and this presents a major hindrance to the treatment of the disease. Therefore, rapid, accurate detection and early diagnosis are necessary for the successful management of MDR-TB in China.Citation4
In recent years, a number of molecular DST kits, such as INNO-LiPA, Genotype MDR-TBplus, DNA microarrays and Xpert MTB/RIF, have been developed for the detection of mutations associated with resistance to Rifampin (RMP) and Isoniazide (INH).Citation5, Citation6, Citation7, Citation8, Citation9, Citation10 A number of studies have verified that these molecular testing methods are extremely effective in detecting TB and relevant drug resistance, particularly in acid-fast bacilli smear-positive samples.Citation11, Citation12 Nevertheless, there is little relevant information on the clinical outcomes of patients who have undergone rapid molecular diagnosis of MDR-TB, especially spinal TB.
Previous research in this area has resulted in the development of a TB DST chip. The gene chip is a DNA microarray chip, the function of which is based on the principle of hybridization of DNA to oligonucleotides immobilized on the chip surface. To create the chip used in our study, oligonucleotide probes were printed onto OPAldehydeSlidealdehyde-activated slides (CapitalBio Corporation, Beijing, China) and were covalently immobilized on the slides via amino groups at their 5′ ends. Each array contained 16 oligonucleotide probes designed (CapitalBio Corporation, Beijing, China) to detect mutations of rpoB (codons 531, 526, 513, 516, 511 and 533), inhA (nucleotide 15 within the promoter) and katG (codon 315). In addition, 17 oligonucleotide probes for several species-specific sequence regions of the 16S rRNA gene were chosen for identification of different Mycobacterium species (Figures, , ).Citation13
Figure 1 Schematic diagram of the DNA probe array for different Mycobacterium species. Seventeen oligonucleotide probes that hybridize to species-specific sequence regions of the 16S rRNA gene were chosen for identification of different Mycobacterium species. All probes were immobilized horizontally five times.
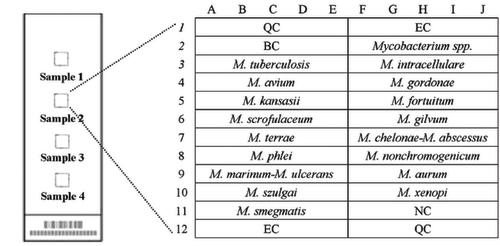
Figure 2 Schematic diagram of the DNA probe array for rpoB, katG and inhA detection. (A) The biochip contains two microarrays, and two specimens can be analyzed in parallel; in each array, one subarray is for RMP, and the other is for INH. (B) Six rpoB wild-type probes and thirteen mutation-type probes were designed for the detection of RMP resistance. (C) For the detection of INH resistance, one probe covers the wild-type codon 315 of katG and two mutation-type probes for the same region, and one wild-type probe and one mutation-type probe are used to detect the inhA promoter region. All probes were immobilized horizontally five times. QC, quality controls; EC, external controls; BC, blank controls; NC, negative controls; IC, internal controls; WT, wild type.
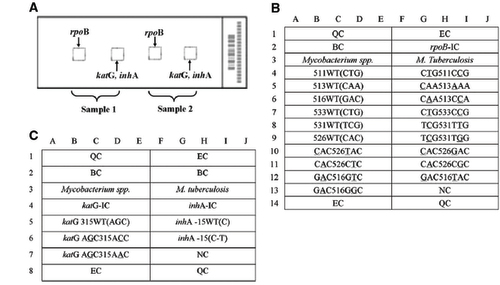
Figure 3 Hybridization pattern of spinal tuberculosis specimens obtained with the DNA probe. (A) Hybridization patterns on the gene chip used to identify M. tuberculosis strains (white frame). (B) RMP resistance detected by the presence of the rpoB 531 (TCG->TTG) mutation; the hybridization signal generated by probe TCG531TTG (solid rectangle) is more intense than that generated by the corresponding wild-type probe (dashed rectangle). (C) RMP resistance detected by the presence of the rpoB 516 (GAC->GTC) mutation; the hybridization signal generated by probe GAC516GTC (solid rectangle) is more intense than that generated by the corresponding wild-type probe (dashed rectangle). (D and E) INH resistance tested by the presence of the katG 315 (AGC->ACC) mutation; the hybridization signals generated by the katG probe AGC315ACC (solid rectangles) are more intense than those generated by the corresponding wild-type probe (dashed rectangles). (E) INH resistance detected by the presence of the inhA-15 C-T mutation; the hybridization signal generated by the inhA 15C→T probe (solid rectangle is higher than that generated by the corresponding wild-type probe (dashed rectangle). (Figures, , were from a previous publication by our team that appeared in BMC Infectious Diseases 2012).

The molecular diagnosis methods used in three of the hospitals included Genotype MDR-TBplus and Xpert MTB/RIF. We performed these tests according to the standard test procedures for each.Citation2, Citation4, Citation11
In this research, we attempted to clarify whether the use of molecular diagnostic methods (CapitalBio™ DNA Microarray assay, Genotype MDR-TBplus and Xpert MTB/RIF) to detect multidrug-resistant spinal TB can improve clinical outcomes.
Materials and methods
Study patients and research design
We conducted a multicenter retrospective study of patients with MDR spinal TB in four hospitals.Citation14 All of the study participants were diagnosed using culture methods for MDR, and the diagnosis was confirmed by conventional DST. Forty-one patients were diagnosed solely by conventional culture and DST methods, and 51 patients were diagnosed following implementation of detection using molecular diagnosis. These hospitals serve areas in which there is a high incidence of TB. A sequential series of patients were initially identified using molecular diagnosis; these patients were then diagnosed clinically and by imaging and compared with similar patients who had been identified by conventional culture and DST. We reviewed the ESR at 1, 3 and 6 months. The study was approved by the Ethics Committee of the Southwest Hospital.
Treatment
In accordance with the recommendations of the MDR-TB treatment association in China, a standard chemotherapy regimen was implemented for each patient before his or her second-line drug sensitivity test results were available. After receiving the final DST data, an individualized therapeutic regimen was implemented for each patient in accordance with the test results and as recommended by the World Health Organization (WHO).Citation1 The treatments were designed to include 4–5 drugs to which the patient’s M. tuberculosis isolate was susceptible. All therapeutic protocols included fluoroquinolone, an injectable agent (streptomycin or aminoglycosides), isoniazide and pyrazinamide. All TB patients received therapy through directly observed treatment. On beginning MDR-TB therapy, patients were started on primary treatment and were not required to remain in the hospital until they had acquired good body condition and normal laboratory indicators. Whether or not patients with drug-susceptible TB were hospitalized in a center for patients with infectious disease depended on the physician’s judgment. Patients whose diagnosis was uncertain or who were awaiting DST results were commonly admitted to a ward housing patients with drug-susceptible infections. Once a patient was diagnosed with MDR-TB, the patient was required to enter an isolation ward for patients with drug-resistant infections. Patients who required individualized surgical procedures due to their general condition underwent focal debridement, fusion and instrumentation. The absolute surgical indications included severe deformity, spinal instability and nerve dysfunction. Many patients in whom spinal lesions are reduced by drug therapy can be treated conservatively or with percutaneous catheter drainage and local chemotherapy.
Statistical analysis
The χ2 test or Fisher’s exact test was used to examine categorical variables; for continuous variables, the t-test was applied. P-values of <0.05 were considered to indicate statistical significance.
Results
Demographic and clinical characteristics
We enrolled a total of 92 patients with MDR-TB. Approximately 89% of the enrolled patients had suffered TB previously (Table ). In this research, the results obtained using molecular diagnosis were for the most part consistent with the results obtained by conventional culture+DST. Only four patients who were diagnosed using molecular methods displayed resistance to two types of drugs. The culture method verified resistance to one type of drug. Despite the differences between individual samples, these differences did not affect the use of second-line drugs. Only two patients whose diagnoses were made using the chip hybridization method harbored sensitive isolates. The culture method verified RFP resistance in these patients. These two patients were treated with first-line drug regimens for 65 and 73 days, respectively, until the DST showed resistance.
Table 1 Demographic and clinical characteristics of patients tested using conventional culture methods or molecular technology
Treatment outcomes
All patients (n=92) received a first-line drug regimen for some time. Among patients receiving first-line anti-TB drugs, the duration of therapy was 22 days in Group B and 154 days in Group A (P<0.05). Relative to Group B, the start of second-line pharmacotherapy in sufferers in Group A was delayed (5 vs 73 days, P<0.05) (Table ).
Table 2 Therapeutic outcome of MDR-TB patients diagnosed using conventional culture and molecular diagnosis technology (n=92)
Specialized measures used to control contagion
All patients diagnosed using molecular technology were eventually hospitalized for MDR-TB therapy and placed in drug resistance sickrooms. In contrast, patients diagnosed by standard culture and drug susceptibility test methods were placed in sickrooms designated for patients with drug-susceptible infections.
Of the 92 inpatients with MDR-TB, the patients who were enrolled in Group A spent longer in sickrooms designated for patients with drug-susceptible infections (33 vs 4 days, P<0.05) and were likely to spend extended periods in hospital wards (33 vs 21 days, P<0.05) compared with the patients in Group B. Thirty-eight MDR-TB patients started extramural hospital treatment using first-line drugs before their drug susceptibility test results were known. Of these 38 patients, the mean time until hospitalization and starting second-line drug pharmacotherapy was 73 days in Group B compared with 126 days in Group A (P<0.05) (Table ).
Table 3 Specialized control measures for contagion
Laboratory index
At the third follow-up, the ESR was decreased; a greater reduction occurred in Group B than in Group A (67% vs 48%, P<0.05). At the sixth follow-up, there was no obvious difference in ESR between Groups A and B (P>0.05).
Complications
All patients received anti-TB treatment, and some patients (33%) had complications. Gastrointestinal symptoms were the most common (70%), followed by sinus problems (30%), hepatotoxicity (20%) and delayed healing of incisions (17%). No patients experienced neurological symptoms or hearing impairment. The complication rate was significantly lower in Group B than in Group A (10% vs 61%, P<0.05) (Table ). In Group A, seven patients had sinus problems, and four patients experienced delayed wound healing. In Group B, two patients had sinus problems and one patient experienced delayed wound healing. These complications were resolved by symptomatic treatment.
Table 4 Complications (n=30)
Discussion
Patients with spinal TB are often seen by physicians in general hospitals, in which there is a lack of protective measures and limited experience in diagnosis, making the treatment and control of TB difficult. The ineffective anti-TB regimens that are often prescribed under these conditions may cause acquired drug resistance and local recurrence. The application of molecular diagnosis technology resulted in an apparent improvement in the clinical therapeutic effect among MDR-TB patients with spondylitis in this study.
Some researchers have paid close attention to nosocomial transmission and to the cross-infection of patients with drug-susceptible TB by patients with MDR-TB.Citation15, Citation16 Using molecular probe technology to detect TB, we are able to rapidly diagnose MDR-TB and begin the use of second-line anti-TB drugs as soon as possible. At the same time, we can control TB nidus, achieve earlier surgical treatment for spinal TB, shorten patients’ hospitalization times and improve the effects of drug treatments.
Although many articles pay close attention to the diagnostic efficiency of rapid molecular tests,Citation17, Citation18 very few studies have determined the clinical efficacy of these tests. Only three published papers have reported the clinical effects of using probe assays to detect MDR-TB.Citation8, Citation19, Citation20 Two of these studies were conducted in South Africa and assessed the starting time of MDR-TB therapy. One study reported that the time to use second-line anti-TB drugs was reduced from 78 to 62 days after use of the MTBDRplus method, and the other reported a similar decrease from 80 to 55 days. Both studies emphasized that clinical and experimental operating problems delayed the delivery and consequently the interpretation of research results. A very recent paper evaluating the clinical effects of using the Xpert method reported the clinical effects of using the method to detect MDR-TB in much more detailCitation8 and noted a marked reduction in the time to initiation of second-line drugs. In our research, the experimenter rapidly communicated the data obtained through molecular diagnosis technology to physicians, and this obviously shortened the time before the initiation of treatment with second-line drugs.
Drug therapy is the basis of spinal TB treatment. Furthermore, the use of individualized, appropriate anti-TB drug therapy can significantly improve the effects of surgery, especially for patients with MDR-TB of the spine. Optimal management of MDR-TB relies on the early detection of the disease in such patients.Citation15 Our previous studies have confirmed that the gene chip is a feasible and accurate tool for the species identification of M. tuberculosis and for the diagnosis of MDR-TB.Citation13 In this research, we observed improved therapeutic effects in patients in whom the gene chip was used for diagnosis. For example, local symptoms can be eased significantly or even disappear with the use of medication, thus avoiding surgery. Almost all of these cases occurred in Group B, and Group B was superior to Group A in clinical outcome. In addition, more patients in Group B accessed surgical treatment at an earlier time.
Preventing the spread of TB infection in hospitals is a problem that has been too long ignored in strategies for infection control for MDR-TB, especially in low- and middle-income developing countries.Citation19, Citation20 The main reason for this is the lack of effective detection and treatment of MDR-TB. Our research shows that the use of accurate and rapid molecular diagnostic technology to detect MDR-TB can improve clinical therapeutic effects and markedly reduce the length of hospital stay of patients with MDR-TB in drug-susceptible wards. At the same time, it is essential to reduce the chances of nosocomial infection by MDR-TB and to improve the effects of surgery for patients with spinal TB. We also observed that accurate and rapid detection of MDR-TB can dramatically shorten the time that patients use ill-suited first-line drug treatments outside the hospital setting and that it can effectively prevent the transmission of MDR-TB.
Since China formulated its national plan for the prevention and cure of TB in 1991, there has been a great deal of progress in TB control. At present, the standard TB treatment in China mainly uses four kinds of first-line drugs and other anti-TB chemotherapeutic drugs. However, all drugs can cause adverse reactions of varying degrees and frequencies. When such reactions occur, the patient’s adherence to treatment is often reduced, and this directly affects the prevention and control of TB. The incidence of adverse reactions to anti-TB drugs has increased significantly over time. In this research, we analyzed the adverse drug reactions that occurred in two groups of patients before using second-line drug treatment. Overall, gastrointestinal symptoms and hepatotoxicity occurred most frequently, and no neurological symptoms or hearing impairment occurred. The rate of adverse drug reactions was markedly lower in the molecular diagnosis group than in the conventionally diagnosed group. We consider that this may have been due to the earlier confirmed drug resistance status of these patients and the resulting decrease in the duration of their treatment with first-line drugs. A shortcoming of this study was that it did not analyze the participants’ adverse reactions to second-line drugs. In the future, we will conduct an in-depth study of the adverse reactions to all prescribed drugs; thereby, we expect to achieve more comprehensive results. In the current study, the occurrence of sinus problems and delayed wound healing in 3 and 2 patients, respectively, in group A may be related to the delay in drug resistance detection and/or to the delay in adjusting the chemotherapy regimen in these patients.
Our study has some limitations. First, it is a retrospective study with a small sample size. Second, it is uncertain whether our laboratory findings can be extrapolated to patients in other urban or even rural areas. However, the results of this research have important value as a guideline for other studies.
Conclusion
In summary, molecular diagnostic technology offers a rapid and accurate diagnostic tool for M. tuberculosis identification and resistance testing. Using this tool, we can rapidly analyze the drug resistance of spinal TB specimens, increase clinical efficacy and decrease delays in diagnosis, thereby reducing the need to build large numbers of advanced biosafety facilities. However, because infrastructure and trained professionals are required for the use of this technology, it is currently available only in a limited number of reference laboratories, which reduces its clinical utility in poverty-stricken zones. Thus, more integral and fully automatic gene detection systems should be developed. In addition, additional genetic mutations related to first-line and second-line drug resistance should be considered for incorporation into future gene detection systems because of the dissemination of M. tuberculosis strains with resistance to second-line drugs. Furthermore, a large sample prospective cohort study with long-term follow-up should be designed and conducted to further assess the efficiency of methods for the molecular diagnosis of spinal TB.
Acknowledgments
We acknowledge the data collectors and the study participants of the Laboratory Department of Southwest Hospital and the Infectious Disease Medical Center of Chongqing for their altruistic assistance during drug resistance testing.
References
- World Health OrganizationGlobal Tuberculosis Control 2014.Geneva: WHO.2014.
- Kipiani M, Mirtskhulava V, Tukvadze Net al.Significant clinical impact of a rapid molecular diagnostic test (genotype MTBDRplus assay) to detect multidrug-resistant tuberculosis. Clin Infect Dis 2014;59: 1559–1566.
- Rüsch S, Gaby E, Casal M.Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol 2006;44: 688–692.
- Gu Y, Wang G, Dong Wet al.Xpert MTB/RIF and GenoType MTBDRplus assays for the rapid diagnosis of bone and joint tuberculosis. Int J Infect Dis 2015;36: 27–30.
- Li R, Ruan Y, Sun Qet al.Effect of a comprehensive programme to provide universal access to care for sputum-smear-positive multidrug-resistant tuberculosis in China: a before-and-after study. Lancet Glob Health 2015;3: e217–e228.
- Nathavitharana RR, Hillemann D, Schumacher SGet al.Multicenter noninferiority evaluation of Hain GenoType MTBDRplus version 2 and Nipro NTM+MDRTB line probe assays for detection of rifampin and isoniazid resistance. J Clin Microbiol 2016;54: 1624–1630.
- Javed N, Aslam M, Mushtaq MAet al.Role of gene Xpert in diagnosis of tuberculous pleural effusion: comparison with pleural biopsy. Eur Respir J 2014;44: 2655.
- Boehme CC, Nicol MP, Nabeta P.Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicenter implementation study. Lancet 2011;377: 1495–1505.
- Cabibbe AM, Miotto P, Lazzeri Eet al.New DNA microarray platform platform for detection of MDR Mycobacterium tuberculosis and of drug-resistant malaria. Clin Microbiol Infect 2011;17 (Suppl 3): 591–592.
- Denkinger CM, Pai M.Using cerebrospinal fluid for the diagnosis of tuberculous meningitis with GeneXpert. Eur Respir J 2014;44: 1095–1096.
- Steingart KR, Schiller I, Horne DJet al.Xpert (R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;21: 1–116.
- Baig S, Qayyum S, Saifullah Net al.Role of GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampacin(R) resistance in patients in high risk DRTB. Eur Respir J 2014;44: 2672.
- Zhang Z, Li L, Luo Fet al.Rapid and accurate detection of RMP- and INH- resistant Mycobacterium tuberculosis in spinal tuberculosis specimens by CapitalBio™ DNA gene chip: a prospective validation study. BMC Infect Dis 2012;12: 1–7.
- Harris AD, Bradham DD, Baumgarten Met al.The use and interpretation of quasi-experimental studies in infectious diseases. Clin Infect Dis 2004;38: 1586–1591.
- Geis S, Bettge-Weller G, Goetsch Uet al.How can we achieve better prevention of progression to tuberculosis among contacts? Eur Respir J 2013;42: 1743–1746.
- Van Cutsem G, Isaakidis P, Farley Jet al.Infection control for drug-resistant tuberculosis: early diagnosis and treatment is the key. Clin Infect Dis 2016;15 (Suppl 3): 238–243.
- Tortoli E, Russo C, Piersimoni Cet al.Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J 2012;40: 442–447.
- Lim TK, Chew MY, Ng Jet al.Xpert MTB/RIF testing of pooled induced sputum. Eur Respir J 2014;44: 2602.
- Abubakar I, Zignol M, Falzon Det al.Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis 2013;13: 529–539.
- Nardell E.Turning off the spigot: reducing drug-resistant tuberculosis transmission in resource-limited settings. Int J Tuberc Lung Dis 2011;14: 1233–1243.
