Abstract
Acinetobacter baumannii is one of the most challenging nosocomial pathogens due to the emergence and widespread of antibiotic resistance. We aimed to provide the first analysis of global prevalence of antibiotic resistance in A. baumannii infections, by synthesizing data and knowledge through a systematic review. We searched studies reporting antibiotic resistance in A. baumannii infections using the Medline, Embase, Web of Science, and Cochrane databases from January 2000 to December 2016. Studies were eligible if they investigated and reported antibiotic resistance in A. baumannii infections with inpatients or outpatients in hospital. Our investigation showed a high prevalence of resistance to the common prescribed antibiotics in A. baumannii infections in both OECD (Organization for Economic Co-operation and Development) and non-OECD countries. Strikingly, though OECD countries have substantially lower pooled prevalence of resistance compared to non-OECD countries based on the data during 2006–2016, a further investigation in a time scale disclosed a faster increase in OECD countries during the past 11 years, and currently both of them have a comparable prevalence of resistance (2011–2016). Tigecycline and colistin are still active but their resistances are expected to become common if the preventative measures are not taken. Antibiotic resistance in A. baumannii infection developed fast and is a crisis for both OECD and non-OECD countries. A “post-antibiotic era” for A. baumannii infection is expected in the next 10–20 years without immediate actions from pharmaceutical companies and governments.
Introduction
Antimicrobial resistance (AMR) has become a global crisis due to escalating evolution of resistance coupled with a diminished antibiotic pipeline. A recent high profile report estimates that, by 2050, 10 million people will die from AMR per year if the current situation continues uncontrolledCitation1. However, this prediction was challenged due to the lack of detailed data on AMR burden, its morbidity and mortality, the modeling of future scenarios, etcCitation2. Therefore, accessing the global prevalence of antibiotic resistance of the pathogens and investigating its developmental trend will aid our understanding of the current situation as well as make proper estimation of future scenario. Thus, this will be highly important for policy making.
The microorganisms that are mainly involved in antibiotic resistance are the so called ESKAPE pathogens, standing for Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae, capable of “escaping” from common antibacterial treatmentsCitation3. A. baumannii is one of the most challenging pathogens among them due to its particular antibiotic resistance characteristics. World Health Organization has recently published its first ever list of antibiotic resistant “priority pathogens” to secure and guide research and development related to new antibiotics, among which A. baumannii was being selected as priority 1 (critical), highlighting its serious threats to public healthCitation4. A. baumannii is an opportunistic Gram-negative bacterium and responsible for a broad range of nosocomial infections, the most important of which are ventilator-associated pneumonia and bloodstream infections, and the mortality rates can reach 35%Citation5. It is particularly problematic due to the frequency of multi-drug resistance (MDR) and the high epidemic potentialCitation6. Currently, A. baumannii has developed resistance to almost all known antibiotics, and the MDR has been widely documentedCitation7. On the other hand, the emergence and widespread of antibiotic resistance have diminished the options of effective therapeutic drug for A. baumannii infection, and a clinician has to choose the previously abandoned antibiotic colistin, which is generally associated with more serious adverse effectCitation8. Most importantly, it was reported that clinical isolates resistant to colistin have emerged in certain geographical areasCitation9, making the last resort of antibiotics in human medicine ineffective.
Given the epidemic potential of antibiotic resistant A. baumannii, it is important to know the current prevalence of antibiotic resistance and its developmental trend, the latter of which is critical for the estimation of future scenario. However, the previous studies on the prevalence of antibiotic resistance were largely limited to either certain country, certain timeframe, selected antibiotics, or lack of detailed information and systematic analysis. Hence, we sought to evaluate the global prevalence of pooled resistance in A. baumannii infections to the most commonly prescribed antibiotics in hospital by summarizing data identified in the published literatures based on OECD (Organization for Economic Co-operation and Development) status. Antibiotics are obtained mostly only by prescription in the more developed OECD countries, whereas many antibiotics can be purchased over counters without the need for a prescription in developing non-OECD countriesCitation10,Citation11.
Methods and materials
Search strategy and selection criteria
The Medline, Embase, Web of Science, and Cochrane databases were systematically searched for references published from January 2000 to December 2016 without language restrictions. The Medical Subject Heading terms in the literature retrieval were “drug resistance”, “antimicrobial resistance”, and “bacterial resistance”, and searched with different combinations of other key text words. We also searched the relevant patents, websites, conference proceedings, government and national report, and open access material through Web of Science and Google Scholar. We manually checked the reference lists of the original key studies (studies cited by multiple articles) to identify other potential studies. Our search was restricted to A. baumannii studies only and has excluded the data of A. baumannii complex. The detailed search strategy is provided in Supplementary Table S1.
Two reviewers (RX, JZ) independently screened all the titles and abstracts. We obtained the full-text papers that fulfill the inclusion criteria for evaluation. The studies were strictly filtered and screened with the following eligible standards: the tested A. baumannii isolates in the included studies were collected from infected patients in hospitals. Only bacterial isolates clearly being identified as A. baumannii were included. The antimicrobial susceptibility testing was following eligible guidelines. The antibiotic resistance was explored, tested, and confirmed in hospital or related research participating laboratories or institutions.
The commonly prescribed antibiotics included in this study were: carbapenems (imipenem or meropenem), amikacin, ampicillin-sulbactam, tobramycin, ceftazidime, piperacillin-tazobactam, cefepime, colistin, and tigecyclineCitation12. Fluoroquinolones (ciprofloxacin) were not included in this analysis due to either the nearly saturated antibiotic resistance or the lack of enough data (Supplementary Figure S1 and Supplementary Table S2). The MDR A. baumannii was defined as resistance to three or more classes of antimicrobial agents including aminoglycosides, β-lactams (excluding the intrinsic resistant agents: penicillin and cephalosporins), carbapenems, fluoroquinolones, and tetracyclines.
Data extraction and quality evaluation
Two reviewers independently retrieved and evaluated data from the selected studies. The following information were extracted: journal, author, publication year, article design, article country, economic situation, recruited patients, recruitment location and time, antimicrobial susceptibility testing, species identification, antimicrobial agents, and antibiotic sensitivities. The extracted results were stratified by OECD status of the studies following previous meta-analysisCitation13. OECD countries often have strong economic vitality and are usually regarded as “developed” countries, whereas non-member countries are regarded as “developing” countries.
The Cochrane collaboration risk of bias tool was carried out to assess the quality of studies by two independent reviewers. We applied the Critical Appraisal Skills Program checklist (http://www.casp-uk.net/) to assess the selection bias for the cohort and case–control papers. The quality assessment charts were generated for “good,” “adequate,” and “poor” reporting based on the Cochrane recommended traffic light systemCitation14.
Data synthesis and analysis
The statistical analyses were carried out with the package Metafor in R software and the whole process for this systematic review and meta-analysis followed the PRISMA guidelineCitation15,Citation16. This package integrates a collection of functions that allow the user to fit fixed or random effects models to implement meta-regression analysis. The 95% confidence interval (CI) for the estimates of resistant A. baumannii was calculated. We used forest plots to show the pooled prevalence of resistance for each antibiotic. The pooled prevalence of antibiotic resistance data, measured based on the OECD status of the studies, were characterized with forest plots for each antibiotic, along with 95% CI. The pooled prevalence of drug resistance for each antibiotic was explored and investigated in all the study countries. Furthermore, the pooled prevalence of antibiotic resistance at different time interval was investigated and compared. The heterogeneity was assessed by the I2 statistics, where I2 values suggested the level of heterogeneity. The pooled prevalence of antibiotic resistance for each antibiotic was analyzed by three periods (years of 2000–2005, 2006–2010, and 2011–2016), stratified by OECD status. The random-effect meta-analysis was implemented to analyze these studies, and the pooled odds ratios were calculated for each period. Finally, we constructed a meta-regression model to quantify the variation of resistant estimates for each antibiotic between different periods using the odds ratios.
Results
Study characteristics and quality of the included studies
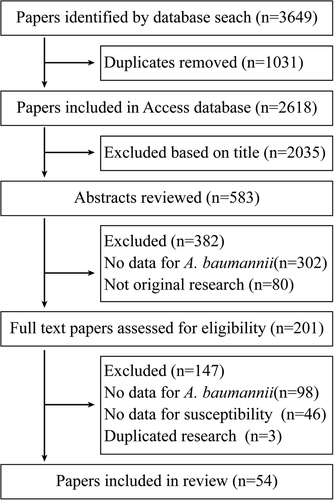
We identified 54 studies in our analysis. The detailed reference retrieval process and study assessment were shown in Fig. . The characteristics of these studies were summarized in Table and Fig. . Thirty five studies from OECD countries reported resistance of 57,188 isolates and 19 studies from non-OECD countries reported 7395 isolates. Antimicrobial sensitivity testing and breakpoints for all antibiotics was detailed in Supplementary Table S2, and for tigecycline and colistin were provided in Supplementary Table S3 and Supplementary Table S4. The breakpoints for tigecycline were mainly according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and US Food and Drug Administration (FDA) criteria (Supplementary Table S3). The A. baumannii species identification was detailed in Supplementary Table S2.
The characteristics of included papers on antibiotic resistance of A. baumannii infections by OECD status of each country
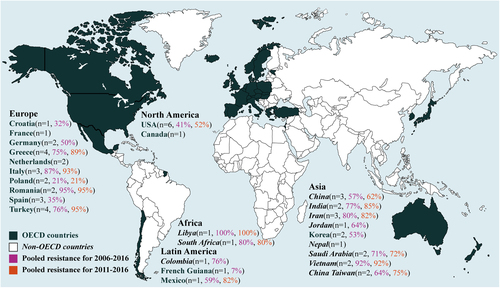
Fig. 2 Geographical distribution of the prevalence of antibiotics resistance in A. baumannii infections to imipenem (%) by two periods. n is the number of included studies per country
The assessment of the study quality was carried out based on the Cochrane collaboration risk of bias tool. The overall quality of the reported studies was generally good for the key risk of bias (Supplementary Figure S2).
The pooled prevalence of antibiotic resistance
During our initial analysis of the resistant status, we found limited data in non-OECD countries before 2005, suggesting a lack of awareness of antibiotic resistance in A. baumannii during this period. Therefore, we calculated the pooled prevalence based on the data during 2006–2016. The pooled prevalence of resistance for each antibiotic was calculated after fitting the random-effect regression, displayed in forest plots (Supplementary Figure S3–12) and summarized in Table . The pooled prevalence of antibiotic resistance during 2006–2016 in non-OECD countries is much higher than that of OECD countries for nearly all the studied antibiotics except colistin and tigecycline, the resistance to which are comparable (Table ). In the case of imipenem, the pooled prevalence of resistance in OECD countries is 53.8% (95% CI 36.8–70.8), in contrast to 76.8% (67.4–86.2) in non-OECD countries. A similar observation was noted on other antibiotics, among which meropenem and amikacin in non-OECD countries have the largest difference in the pooled prevalence of resistance than those in OECD countries. The pooled prevalence of resistance to cefepime is higher but less dramatic in non-OECD countries compared to that in OECD countries. On the other hand, the antibiotic resistance in different countries within OECD varies significantly. As shown in Fig. , French Guiana has the lowest pooled prevalence of imipenem resistance at 7%, whereas Italy and Romania at 87 and 95%, respectively. Similar results were also observed on other antibiotics (Supplementary Figure S4–10).
The pooled prevalence of antibiotic resistance to the commonly prescribed antibiotics in OECD and non-OECD countries during 2006–2016
Tigecycline is one of the “last-resort” antimicrobial agents for antibiotic resistance in A. baumannii infection. Though it has been used for only about 10 years, significant percentage of resistance has been observed in both OECD and non-OECD countries without significant difference between them (Table ). Colistin, another last resort of treatment for drug-resistant A. baumannii infection, becomes especially important when tigecycline is found to be insusceptibleCitation17,Citation18. Our analysis showed that the pooled prevalence of colistin resistance were lower at 1.4% (0.2–2.6) and 1.3% (0.1–2.7) in OECD and non-OECD countries during 2006–2016, respectively, suggesting that colistin is still the most active agent against A. baumannii infections and potentially effective in clinical practice, although its clinical effectiveness needs more investigations.
The developmental trend of antibiotic resistance
To get further insight of antibiotic resistance, we analyzed the resistance data by a time scale (Table ). We included data for the duration of 2000–2005 in OECD countries so to get more information about the resistance development. In contrast to the aforementioned observation that non-OECD countries have significant higher prevalence of antibiotic resistance (Table ), the pooled prevalence of resistance to each antibiotic during 2011–2016 was comparable between OECD and non-OECD countries, though it was slightly higher in non-OECD countries (Table ; Fig. ). The gap of resistance between OECD and non-OECD countries was getting smaller during past 11 years except for tobramycin and ceftazidime (Supplementary Figure S13). For example, the overall pooled prevalence of resistance to imipenem (2006–2016) in OECD is 23% lower than that in non-OECD countries (Table ). However, the difference between them becomes negligible during 2011–2016, with 77.8% (66.7–88.4) in non-OECD and 73.9% (54.8–95.6) in OECD countries (Fig. ; Table ). The increase in the prevalence of antibiotic resistance in OECD countries was shown to be overall faster than non-OECD countries in the past 11 years (Fig. ): For example, the pooled resistance for imipenem increased 22.3% (from 51.6% in 2006–2010 to 73.9% in 2011–2016) in OECD countries, in contrast to 5.7% (from 72.1 to 77.8%) in non-OECD countries (Table ; Fig. ).
The pooled prevalence of antibiotic resistance to the commonly prescribed antibiotics at three different periods in OECD and non-OECD countries
Fig. 3 a The pooled prevalence of antibiotic resistance to the common antibiotics except for tigecycline and colistin during the three periods (years of 2000–2005, 2006–2010, and 2011–2016) in OECD and two periods (years of 2006–2010 and 2011–2016) in non-OECD countries. b The proportion of antibiotic resistance growth between different time period in OECD and non-OECD countries
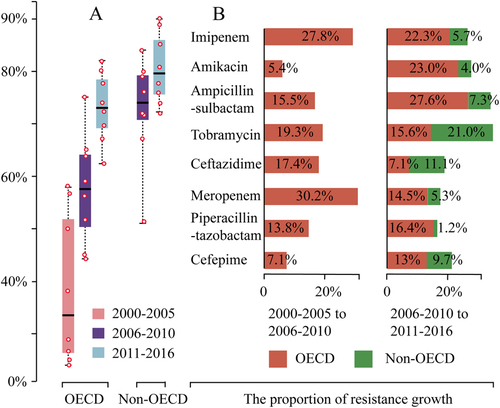
In addition, our analysis demonstrated that A. baumannii has a high propensity to develop resistance rapidly (Fig. ). In the case of imipenem, the pooled resistance is only 23.8% in OECD countries during 2000–2005, it rapidly increased 50.1 up to 73.9% (54.8–95.6) during 2011–2016 after about 11 years’ time usage only. Similar results were also observed for other antibiotics (Table ).
The pooled prevalence of MDR
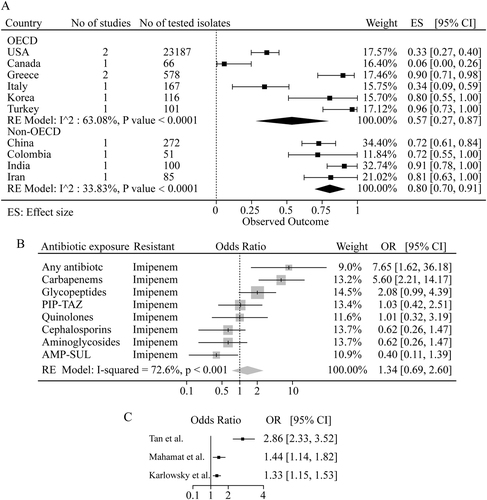
We further analyzed the MDR isolates during 2006–2016. Our analysis disclosed a high MDR prevalence in both OECD and non-OECD countries, with 56.9% (27.3–86.5) and 80.4% (69.9–90.8) respectively (Fig. ). The prevalence of MDR in Greece and Turkey were the highest at 90% (81–98) and 96% (83–100) respectively, in contrast to 6% (0–26) in Canada, indicating a significantly different status of MDR within OECD countries. This difference was less significant within non-OECD countries (Fig. ).
Fig. 4 a The pooled prevalence of MDR in A. baumannii isolates from 2006–2016 in OECD and non-OECD countries. b The pooled crude odds ratios between antibiotic resistance in A. baumannii infections and prior exposure to any antibiotic. c The pooled crude odds ratios between antibiotic resistance in A. baumannii infections and ICU or non-ICU hospitalization. PIP-TAZ piperacillin-tazobactam, AMP-SUL ampicillin-sulbactam
MDR A. baumannii infections are often associated with high mortalityCitation19. Our analysis showed that the pooled mortality of MDR can reach up to 42.7% (36.7–48.7) worldwide (Supplementary Figure S14) and varies significantly in different countries. For example, America has the lowest pooled mortality of MDR at 29%, whereas Turkey and Korea have nearly twice of that at 64 and 52%, respectively.
The risk factors for antibiotic resistance
We also investigated the relationship between previous antibiotics exposure and imipenem resistance. As shown in Fig. , an odds ratio of imipenem resistance at 7.65 (1.62–36.18) was observed with patients previously exposed to any antibiotic. Especially, previous exposure to carbapenems (meropenem and imipenem) and glycopeptides resulted in a significant resistance to imipenem, with odds ratios at 5.60 (2.21–14.17) and 2.08 (0.99–4.39), respectively, suggesting that the previous exposure to carbapenems and glycopeptides is the risk factor for imipenem resistance.
A meta-regression analysis on the data from three early studiesCitation20–Citation22 showed higher odds ratios of antibiotic resistance in patients from intensive care unit (ICU) compared to non-ICU (Fig. ). Especially, antibiotic resistance reported by Tan and colleagues demonstrated the highest odds ratio of 2.86 (2.33–3.52) in ICU compared to non-ICUCitation21.
Discussion
To our knowledge, we provide the first systematic review and meta-analysis on the global prevalence of antibiotic resistance in A. baumannii infection on the commonly prescribed antibiotics. Our initial analysis found that OECD countries have substantially lower pooled prevalence of antibiotic resistance (2006–2016), which is consistent with our initial expectation as OECD countries have a better antibiotics management system. However, a different conclusion was drawn when we analyzed the prevalence following a time scale. We found that the prevalence of resistance was increasing rapidly in the last 16 years (OECD countries) or 11 years (non-OECD countries). Strikingly, the prevalence increase in OECD countries was even faster and outstanding, which resulted in a similar level of antibiotic resistance to non-OECD countries during 2011–2016 (Fig. ). The pooled prevalence during 2011–2016 should maximally reflect the current status of antibiotic resistance. Thus, we concluded that the current prevalence of antibiotic resistance in A. baumannii infection is similar between OECD and non-OECD countries, both of which are facing the same degree of severity of antibiotic resistance. In addition, our analysis demonstrated how rapid the antibiotic resistance to A. baumannii infections expanded, providing a theoretical basis for the proper estimation of the future scenario, which has been challenged for the accuracy in the government report due to the lack of scientific evidenceCitation1,Citation2.
The rapid expansion of antibiotic resistance discovered in OECD countries could be mainly due to the spread of already established clones or because of acquisition of resistance determinants by susceptible strains. Despite it is not clear which is the main cause, several researches supported the latterCitation23–Citation27. Indeed, the pan-European epidemic clonal I, II and III have been universally identified throughout many countries in EuropeCitation28. However, not every country in Europe has high prevalence of antibiotic resistance. For example, Turkey and Greece have particularly high antibiotic resistance rates. In contrast, Croatia and Poland have an overall much lower resistant rate (Supplementary Figure S3–10). Thus, it is unlike that the rapid increase of antibiotic resistance was only caused by the spread of certain clonal type. The observation could be the combination of several factors: the influence of cross-border exchange, such as transfer of patients, traveler, medical tourism and refugees from the regions, where antibiotic resistance is higher, including Iran and Iraq;Citation29 Overuse and/or unrestricted availability of antibiotic agents such as carbapenems;Citation30 The difference in antibiotics management and infection control measures, and the environmental pollutionCitation31.
The high global prevalence of antibiotic resistance found in our studies indicates that most of the antibiotics are likely ineffective to A. baumannii infection except tigecyline and colistin, the resistance is much lower (Table ). Thus, the use of the antibiotics should be cautious, as they might not be effective for the patients. A proper bacterial susceptibility test should be given to understand the resistance profile if antibiotic resistance was suspected. Especially when the patients have previous exposed to antibiotics or hospitalized in ICU. Based on our analysis, susceptibility test to imipenem might be particularly necessary if the patient has been treated with carbapenem and glycopetides previously. On the other hand, continuous surveillance of A. baumannii resistance should be implemented to understand the local antibiotic resistance situation and guide the therapeutic practice. Strict infection control policy and antibiotic management should also be enforced to reduce the emergence and spread of pan-antibiotic resistance.
MDR has always been the most serious concern as it can lead to the failure of antimicrobial therapy and increased mortalityCitation19. We showed that both OECD and non-OECD countries have high MDR rates. On the other hand, the pipeline of new antibiotics for treatment is running dry. The only option we have now is tigecycline which was licensed in 2005, and the previous abandoned antibiotic colistinCitation32. Our analyses showed that tigecycline and colistin have the least drug resistance currently (Table ). Both of them could be potentially used for therapeutic purpose on MDR A. baumanii infection. However, the effectiveness of tigecycline was challenged in a recent studyCitation18. Colistin has a good susceptibility compared to other tested antibiotics. This is likely due to the fact that colistin has been limited from being used during the last several decades due to nephrotoxicity. Now, colistin has been revaluated for treatment of MDR A. baumannii for its low resistanceCitation33. However, resistance to colistin is also emerging. A recent study from America reported 50% resistance to colistinCitation34. Most importantly, our analysis found that the development of drug resistance in A. baumannii is fast (Fig. ). It took only 11 years for the percentage of resistance to imipenem increased from 23.8 to 73.9% in OECD countries (Table ). Thus, we would expect that resistance to tigecycline and colistin could be common in another ten to 20 years’ time if no preventative measures were taken, which may result in an absolute “post-antibiotic era” for pan-antibiotic resistant A. baumannii infections. The public awareness of such severity of pan-antibiotic resistance in A. baumannnii infections should be increased and the investment by pharmaceutical companies and governments on the new antibiotic research and development should be encouraged.
We noticed that different methods were used for A. baumannii identification and antibiotics susceptibility testing in the reports used for our systematic review and meta-analysis, which could be the limitation of our study.
In conclusion, the antibiotic resistance in A. baumannii infection has become a global crisis. OECD countries have demonstrated a faster increase in antibiotics resistance in the past 16 years and reached a comparable antibiotic resistance prevalence with non-OECD countries currently. The fast development of antibiotic resistance in A. baumannii infection warn of an absolute “post-antibiotic era” for antibiotic resistant A. baumannii infections if resistance to tigecycline and colistin become common in the next 10–20 years. Thus, immediate actions should be taken to prevent and control further development of antibiotics resistance in A. baumannii infection. Research and development on new antibiotics for A. baumannnii infection should be highly encouraged.
Supplementary materials
Download MS Word (3.4 MB)Acknowledgements
This work was supported by the Macau Science and Technology Development Fund (Grant No.: FDCT/066/2015/A2 and FDCT/131/2016/A3), Research Committee of University of Macau (Grant No.: MYRG2016-00073-FHS and MYRG2016-00199-FHS). We thank Dr. Youyan Nie from National Institute of Education, Nanyang Technological University, for her critical review as well as revision on the manuscript.
Authors contributions
R.X. and J.Z. designed the systematic review and meta-analysis protocol. R.X. and J.Z. did the data collection, and R.X., X.D.Z., and J.Z. worked on the data analysis. R.X. and J.Z. performed data interpretation. R.X., B.P., Q.Z., X.D.Z., and J.Z. drafted the manuscript. All authors provide important intellectual revision of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41426-018-0038-9).
References
- O’Neill, J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist.20, 1–16 (2014).
- de KrakerMEStewardsonAJHarbarthSWill 10 million people die a year due to antimicrobial resistance by 2050?PLoS Med.201613 e100218410.1371/journal.pmed.10021845127510
- BoucherHWBad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of AmericaClin. Infect. Dis.2009481 1210.1086/595011
- WHO Publishes List of Bacteria for which New Antibiotics are Urgently Needed2017Geneva,WHO
- AntunesLCViscaPTownerKJAcinetobacter baumannii: evolution of a global pathogenPathog. Dis.20147129230110.1111/2049-632X.12125
- D’ArezzoSEpidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy)Clin. Microbiol. Infect.20091534735710.1111/j.1469-0691.2009.02668.x
- PotronAPoirelLNordmannPEmerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiologyInt. J. Antimicrob. Agents20154556858510.1016/j.ijantimicag.2015.03.001
- NationRLFramework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensusLancet Infect. Dis.20151522523410.1016/S1473-3099(14)70850-3
- QureshiZAColistin-resistant Acinetobacter baumannii: beyond carbapenem resistanceClin. Infect. Dis.2015601295130310.1093/cid/civ0484462660
- MorganDJNon-prescription antimicrobial use worldwide: a systematic reviewLancet Infect. Dis.20111169270110.1016/S1473-3099(11)70054-83543997
- CizmanMAntibiotic policies in Central Eastern EuropeInt. J. Antimicrob. Agents20042419920410.1016/j.ijantimicag.2004.03.016
- FishbainJPelegAYTreatment of acinetobacter infectionsClin. Infect. Dis.201051798410.1086/653120
- BryceAGlobal prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysisBMJ2016352i93910.1136/bmj.i9394793155
- Higgins, J. P. & Green, S. Cochrane handbook for systematic reviews of interventions version 5.1. 0. The cochrane collaboration5, (2011). www.handbook.cochrane.org.
- MoherDPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253510.1136/bmj.b25352714657
- ViechtbauerWConducting meta-analyses in R with the metafor packageJ. Stat. Softw.20103614810.18637/jss.v036.i03
- TasinaEEfficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysisLancet Infect. Dis.20111183484410.1016/S1473-3099(11)70177-3
- NiWTigecycline treatment experience against multidrug-resistant Acinetobacter baumannii infections: a systematic review and meta-analysisInt. J. Antimicrob. Agents20164710711610.1016/j.ijantimicag.2015.11.011
- FitzpatrickMAInfluence of ACB complex genospecies on clinical outcomes in a U.S. hospital with high rates of multidrug resistanceJ. Infect.20157014415210.1016/j.jinf.2014.09.004
- KarlowskyJASurveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001Antimicrob. Agents Chemother.2003471681168810.1128/AAC.47.5.1681-1688.2003153334
- TanREpidemiology and antimicrobial resistance among commonly encountered bacteria associated with infections and colonization in intensive care units in a university-affiliated hospital in ShanghaiJ. Microbiol. Immunol. Infect.201447879410.1016/j.jmii.2012.11.006
- MahamatAClinical epidemiology and resistance mechanisms of carbapenem-resistant Acinetobacter baumannii, French Guiana, 2008–2014Int. J. Antimicrob. Agents201648515510.1016/j.ijantimicag.2016.03.006
- GogouVEvolution of multidrug-resistant Acinetobacter baumannii clonal lineages: a 10 year study in Greece (2000-09)J. Antimicrob. Chemother.2011662767277210.1093/jac/dkr390
- VillalonPClonal diversity of nosocomial epidemic Acinetobacter baumannii strains isolated in SpainJ. Clin. Microbiol.20114987588210.1128/JCM.01026-103067678
- Da SilvaGJSequence types of Portuguese carbapenem-resistant Acinetobacter baumannii isolates collected over 10 yearsJ. Antimicrob. Chemother.2010652254225610.1093/jac/dkq274
- Di PopoloAMolecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing schemeClin. Microbiol. Infect.20111719720110.1111/j.1469-0691.2010.03254.x
- DiancourtLThe population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic poolPLoS One20105e1003410.1371/journal.pone.00100342850921
- van DesselHIdentification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitalsRes. Microbiol.200415510511210.1016/j.resmic.2003.10.003
- PourhajibagherMAntimicrobial resistance of Acinetobacter baumannii to Imipenem in Iran: a systematic review and meta-analysisOpen Microbiol. J.201610324210.2174/18742858016100100324814728
- CantonRRapid evolution and spread of carbapenemases among Enterobacteriaceae in EuropeClin. Microbiol. Infect.20121841343110.1111/j.1469-0691.2012.03821.x
- YilmazGCharacterization and toxicity of hospital wastewaters in TurkeyEnviron. Monit. Assess.201718910.1007/s10661-016-5732-2
- MarakiSEpidemiology and antimicrobial sensitivities of 536 multi-drug-resistant gram-negative bacilli isolated from patients treated on surgical wardsSurg. Infect.20121332633110.1089/sur.2011.115
- LazureanuVInfection with Acinetobacter baumannii in an intensive care unit in the Western part of RomaniaBMC Infect. Dis.20161610.1186/s12879-016-1399-04928153
- LeshoEEmergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infectionsJ. Infect. Dis.20132081142115110.1093/infdis/jit293