Abstract
Understanding the processes driving parasite assemblages is particularly important in the context of zoonotic infectious diseases. Leptospirosis is a widespread zoonotic bacterial infection caused by pathogenic species of the genus Leptospira. Despite a wide range of animal hosts, information is still lacking on the factors shaping Leptospira diversity in wild animal communities, especially in regions, such as tropical insular ecosystems, with high host species richness and complex biogeographical patterns. Using a large dataset (34 mammal species) and a multilocus approach at a regional scale, we analyzed the role of both host species diversity and geography in Leptospira genetic diversity in terrestrial small mammals (rodents, tenrecs, and shrews) and bats from 10 different islands/countries in the western Indian Ocean (WIO) and neighboring Africa. At least four Leptospira spp. (L. interrogans, L. borgpetersenii, L. kirschneri, and L. mayottensis) and several yet-unidentified genetic clades contributed to a remarkable regional Leptospira diversity, which was generally related to the local occurrence of the host species rather than the geography. In addition, the genetic structure patterns varied between Leptospira spp., suggesting different evolutionary histories in the region, which might reflect both in situ diversification of native mammals (for L. borgpetersenii) and the more recent introduction of non-native host species (for L. interrogans). Our data also suggested that host shifts occurred between bats and rodents, but further investigations are needed to determine how host ecology may influence these events.
Introduction
Leptospirosis is the most widespread and prevalent zoonosis in the worldCitation1,Citation2. This emerging disease represents a major health concern on tropical islands, particularly in the Indian Ocean, where some of the highest human incidence rates have been reportedCitation2–Citation4. The causative organisms are spirochete bacteria of the genus Leptospira, which are diverse and divided into 10 currently recognized pathogenic speciesCitation5,Citation6. Leptospira infect a wide range of animals that may shed living bacteria in their urine and contribute to environmental contamination and indirectly to human infection. Among wild animals, terrestrial small mammals, especially rodents, are considered to be the main reservoirs for Leptospira infections in humansCitation5. In addition, evidence of Leptospira carriage in different bat speciesCitation7–Citation14 suggests the potential role of these animals in human leptospirosisCitation15. However, field-based studies of wild mammal populations often suffer from attempts to culture Leptospira, which often fails, hindering further genotyping. Thus, despite its importance for understanding leptospirosis epidemiology, there is currently only limited research on the genetic diversity and evolution of Leptospira in terrestrial small mammals and batsCitation10,Citation16,Citation17.
The western Indian Ocean (WIO) islands and neighboring countries on the African mainland are characterized by a large diversity of terrestrial small mammals and bats as well as several introduced speciesCitation18,Citation19. In these areas, leptospirosis is endemic and incidences in humans are reported to be among the highest worldwideCitation3,Citation4,Citation20. For instance, high incidence rates in humans are reported in the SeychellesCitation21 and MayotteCitation22. In contrast, leptospirosis is poorly documented in other regional countries and islands (i.e., Madagascar, Mauritius, Comoros, and South Africa), possibly due to under-diagnosisCitation20,Citation23,Citation24.
Epidemiological studies have revealed that depending on the geographic region, humans can be exposed to different Leptospira spp. For example, acute human infections on La Réunion are mainly caused by LeptospirainterrogansCitation25, while on Mayotte, the most prevalent bacterial species identified in acute cases are L. borgpetersenii, L. kirschneri, and L. mayottensisCitation26. Several reports have identified an important diversity of Leptospira lineages in wild mammals of the region. On Madagascar, introduced rats have been reported to be the only carriers of L. interrogansCitation27, while L. borgpetersenii and L. kirschneri infect endemic bats and terrestrial small mammalsCitation10. On Mayotte, an introduced tenrec species, Tenrec ecaudatus, is a main reservoir of L. mayottensisCitation28, which is also found in native tenrec populations in neighboring MadagascarCitation10; introduced rats are host to a broader range of pathogenic LeptospiraCitation8,Citation28. On La Réunion, introduced rats are only infected by L. interrogansCitation25, and the partial sequencing of a strain hosted by the endemic bat species Mormopterus francoismoutoui revealed a single lineage closely related to L. borgpeterseniiCitation29. However, information about Leptospira diversity is based on local surveys and often suffers from the use of a single gene for Leptospira genetic characterization. As a result of these limitations, the processes shaping the regional Leptospira diversity within wild animal communities inhabiting the WIO and neighboring African countries remain unclear.
The aim of the present study is to analyze Leptospira genetic diversity and structure within a large range of wild animal hosts at the WIO regional scale and in neighboring Africa. To this end, we used a large range of host species, including terrestrial small mammals and bats. Animals from the WIO region are mostly endemic and those from neighboring Africa are widely distributed species; in addition, we studied the introduced species from both of these regions. We employed an optimized multilocus genotyping approach to specifically examine the respective role of the host and the geography in shaping the regional diversity of this zoonotic bacterium.
Results
Samples and genotyping
In total, we used 127 Leptospira-positive samples from terrestrial small mammals and bats (Table and Table S1). Our dataset included 34 animal species: 57% were terrestrial small mammals and 43% were bats. Most of the animal species are indigenous to the region, with Nesomyinae and Tenrecidae representing endemic Malagasy adaptive radiationsCitation30,Citation31. Success in multilocus genotyping was highly variable depending on the samples. A complete multilocus scheme was obtained for only 101 samples (Table ).
Details of the Leptospira-positive samples from terrestrial small mammals and bats used in the analyses, the number of genetic sequences obtained, and the associated Leptospira spp
Leptospira spp. identification and geographic distribution
Among the 127 samples, we used a 473-bp fragment of the secY gene for 115 samples to identify Leptospira spp. (Figure S2). When secY sequences could not be obtained (n = 12 samples), we used a 452-bp fragment of the rrs2 gene instead (Figure S3). The secY phylogeny provided a much greater phylogenetic resolution compared to rrs2, and some discrepancies between the genes were observed especially for Leptospira in the Rousettus and Triaenops bats (see below). Overall, samples clustered in seven well-supported genetic clades (clades A–G, Fig. ). Four of these clades grouped with the referenced sequences of L. borgpetersenii (clade A), L. mayottensis (clade D), L. kirschneri (clade E), and L. interrogans (clade F). The three other clades as well as three single sequences (indicated with an asterisk in Fig. ) did not cluster with any reference sequences.
a Map of the sample locations. b Schematic representation of the Leptospira phylogenetic relationships based on the maximum-likelihood secY and rrs2 phylogenetic trees (details in Figures S1 and S2). Circle sizes are proportional to the number of samples included for each branch. Colors within the pie charts refer to the different countries as shown on the map. Samples denoted by an asterisk (*) refer to the sequences that could not be assigned to any clade
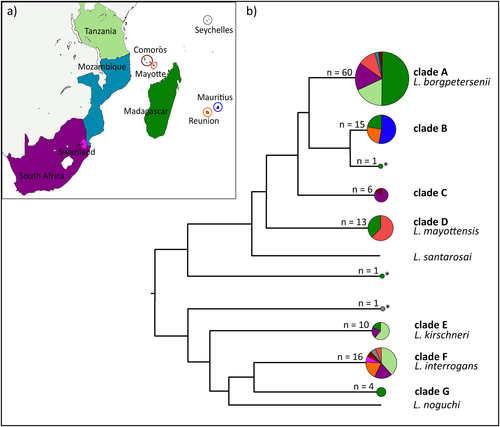
Most of the samples grouped in clade A (L. borgpetersenii), which was widely represented in the region and identified in six of the 10 sampled islands/countries (Fig. ) from a wide range of animals (i.e., 20 host species, Figure S2). Clade B was closely related to L. borgpetersenii but was not associated with any reference sequences. This clade was inclusive of Molossidae bats from Madagascar, La Réunion, and Mauritius, except for a Triaenops bat from Madagascar. Clade C incorporated Rousettus bats from both South Africa and the Comoros. Based on the secY phylogeny, it was closely related to L. borgpetersenii. However, two samples in this clade (samples 1574 and 1577) grouped with L. interrogans based on the rrs2 gene (Figure S3).
Clade D, corresponding to L. mayottensis, included Leptospira that infected terrestrial small mammals from Madagascar and Mayotte. Clade E, which was identified as L. kirschneri based on the reference material, was found in terrestrial small mammals from Madagascar, South Africa, and Tanzania and in Miniopterus bats from South Africa. Clade F, assigned to L. interrogans, was widespread in the region and was identified from seven of the 10 islands/countries. L. interrogans was found to infect rats (Rattus rattus) introduced at all the locations where this species was sampled (i.e., La Réunion, Seychelles, and Mayotte), as well as the insectivorous Nycteris bats in Swaziland, and frugivorous Rousettus bats in South Africa and the Comoros. Clade G was composed of Leptospira that exclusively infected Malagasy bats of the genera Triaenops and Pteropus; however, the phylogenetic position of Leptospira from Triaenops varied depending on the gene analyzed. These samples were embedded in the L. interrogans/L. noguchi group depicted in the secY phylogeny (Figure S2), although they were more external in the rrs2 phylogeny. As the Triaenops material occupied an intermediate position in both phylogenies, and we did not observe any double peaks in the Leptospira sequences for these samples; we considered that the incongruent phylogenies may be the result of the different evolution (e.g., recombination rate) of the studied genes, rather than co-infection, and we maintained these samples in the following multilocus analysis. Finally, a Pteropus bat sample from the Seychelles showed an intermediate position in the rrs2 tree (Figure S3) between L. borgpetersenii and the L. interrogans/L. kirschneri/L. noguchi group and did not cluster with any of the reference sequences.
Genetic diversity and population structure
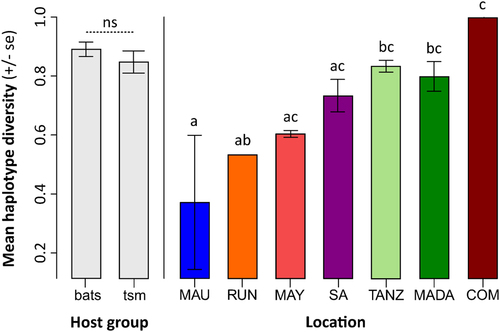
We analyzed a complete multilocus scheme of 2252 nucleotides for 101 samples. Haplotype diversity (HD) was calculated, except for the specimens from Mozambique, Swaziland, and the Seychelles, as these locations either contained no sample, or only one sample was available (see Table and Table S5). There was a significant difference in HD among the islands/countries (p < 0.001) but not between the terrestrial small mammals and bats (p = 0.512, Fig. ). The highest HD was recorded from the Comoros, but this might be an artifact of the two positive samples from this location being infected by different Leptospira spp. High HD was recorded for Madagascar, Tanzania, and South Africa, in contrast with lower HD on La Réunion and Mauritius. However, the Analysis of Molecular Variance (AMOVA) results (Table ) revealed no significant genetic structure among the islands/countries (ΦCT = 0.035, p = 0.248) and this was confirmed when the bats and terrestrial small mammals were analyzed separately (Table S4). When examining all the samples, there was not a clear separation between the terrestrial small mammal and bat hosts (ΦCT = 0.074, p = 0.049), and this was confirmed when analyzing only the Malagasy samples (Table S4). In the AMOVAs, most of the genetic variation was explained by differences among the host genera and species (Table and S4).
“tsm” refers to terrestrial small mammals. “MAU”=Mauritius, “RUN”=La Réunion, “MAY”=Mayotte, “SA” = South Africa, “TANZ” = Tanzania, “MADA” = Madagascar, “COM” = Union of the Comoros. Letters a–c above the bars refer to significantly different averages based upon a Tukey HSD test and “ns” = non-significant. Bars can have more than one letter to reflect the “overlap” between them
Results of the hierarchical AMOVA analysis
The coalescent analysis included all the previously identified clades, except clade C (i.e., Rousettus samples from South Africa and the Comoros), which was removed because of either failure to obtain the full genotype (samples 6, 1483, 1504, 1564) or suspected co-infections (samples 1577 and 1574, Figure S2 and S3). As shown in Fig. , L. borgpetersenii samples (clade A) were highly diversified and divided into at least seven genetic host-associated clusters (numbers 1–7), with each of these clusters infecting different groups of bats or terrestrial small mammals. Additionally, they were also different from the reference sequences. One exception was those infecting rats introduced on Mayotte and other terrestrial small mammals in Tanzania (see cluster 2), which grouped with strains from Ireland, Indonesia, and Portugal. L. borgpetersenii infecting rodents and other terrestrial small mammals on Madagascar were separated into two different clusters (numbers 5 and 3, respectively) according to the different host genera (Eliurus and Microgale). Moreover, L. borgpetersenii from insectivorous bats (Miniopterus, Scotophilus, and Nycteris) grouped into four different clusters (numbers 1, 4, 6, and 7), originating from Madagascar, Mozambique, South Africa, and the Comoros. Closely related to L. borgpetersenii, clade B (number 12) grouped all the Molossidae bat samples from Madagascar (Otomops madagascariensis, Mormopterus jugularis), La Réunion (M. francoismoutoui), and Mauritius (M. acetabulosus) as well as one sample from the Malagasy insectivorous bat Triaenops menamena of the family Rhinonycteridae. The two other samples from T. menamena clustered in a different group (clade G, number 10), which was related, but external, to the other L. borgpetersenii samples.
The genetic clades identified in Fig. are shown in the gray circle, and the major genetic groups within these clades (squares, numbers 1 to 12) are highlighted by dashed boxes. Animal silhouettes represent host groups. Sequences from the samples specific to this study are in black and coded with the sample ID, geographic location, and host species/genus. Colored circles at the tip of the branches correspond to the geographic locations as shown in Fig. . Reference samples are in gray and are coded as follow: Li = L. interrogans, Lk = L. kirschneri, and Lb = L. borgpetersenii (see Table S1 for details). Posterior probabilities higher than 80% are represented by white circles at the nodes. Clade C was not included (refer to Results section for more information)
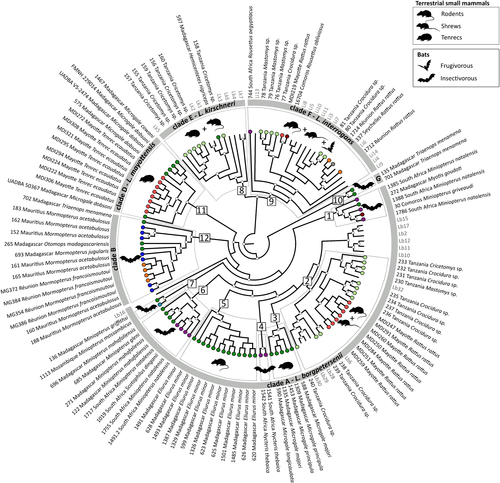
In contrast to L. borgpetersenii, there was less diversity within L. interrogans and L. kirschneri, and there was no clear diversification according to the host genus or species, although some sub-structuring was found. For L. interrogans, terrestrial small mammals and bats were infected with closely related strains. Indeed, this was particularly evident for L. interrogans infecting rats introduced on Mayotte and Rousettus fruit bats in the Comoros (number 9). Finally, L. kirschneri infecting rodents in Tanzania and tenrecs of the genus Hemicentetes on Madagascar were closely related to each other (number 8).
Table shows highly significant FST estimates among the different Leptospira clusters. In particular, Leptospira from Molossidae bats in clade B and Triaenops bats in clade G were highly differentiated from L. borgpetersenii samples in clade A.
Pairwise distance (FST) among the different genetic Leptospira clades in the western Indian Ocean islands and neighboring Africa
Discussion
We report here the most comprehensive multilocus sequence analysis study of Leptospira in wild animals of the WIO islands and adjacent continental Africa. We reveal a highly diversified assemblage of Leptospira in terrestrial small mammals and bats of the region, composed of at least four Leptospira species (L. interrogans, L. borgpetersenii, L. kirschneri, L. mayottensis) and several other Leptospira that are apparently not described. Our results highlight that Leptospira diversity in wild animals of the WIO is much higher than previously thought and that rodents, tenrecs, shrews, and bats, including introduced and endemic species, contribute to this regional diversity.
Yet-undescribed diversity
We reported for the first time the presence of L. kirschneri in Glauconycteris and Miniopterus bats in South Africa, as well as a closely related L. interrogans lineage in Pteropus rufus on Madagascar. This contrasts with a previous study that did not find antibodies to Leptospira in Pteropus rufusCitation32,Citation33. However, our observations should be taken with caution because they are based on a limited number of samples and only the rrs2 gene was analyzed. Indeed, Polymerase Chain Reaction (PCR) failures in achieving the complete multilocus (MLST) scheme occurred in 20% of the samples, which limits our capacity to identify these samples to the species level. PCR failures may be related to (i) the small amount of initial material, as we worked with DNA extracts from original samples and not isolates, (ii) potential mismatches in the primer regions, and (ii) the possible absence of the locus coding for surface-expressed proteins (e.g., lipL41), as the loss of genes is common in the evolution of pathogenic Leptospira strainsCitation34,Citation35. As the estimation of Leptospira diversity is strongly dependent on sample size and the analyzed locus, additional samples are needed for further genetic characterization to confirm that the suggested host species are actual reservoirs of these particular Leptospira spp.
Apart from the four already-described Leptospira taxa highlighted in our phylogenetic analyses, we also found yet-undescribed genetic clades. For instance, the Bayesian phylogeny and FST values showed that Leptospira from Molossidae bats (clade B) and Triaenops bats (clade G) were highly genetically divergent from L. borgpetersenii samples (clade A). Recent investigations on Mayotte led to the description of a new Leptospira, L. mayottensisCitation6, which probably originated from MadagascarCitation10. In this context, further genetic and serological characterization of Molossidae and Triaenops-associated Leptospira lineages may lead to the description of new leptospiral species that, so far, are only known from the WIO region.
Widespread distribution of L. borgpetersenii and L. interrogans
L. borgpetersenii and L. interrogans are largely distributed in our study region. L. interrogans was found in rodents at all sampled locations, and shrews in Tanzania. This observation is coherent with the fact that L. interrogans is commonly associated with rodents worldwideCitation17,Citation27,Citation36,Citation37. Interestingly, we also reported the presence of L. interrogans, or closely related lineages, in bats. In particular, L. interrogans from Rousettus in the Union of the ComorosCitation9 was almost genetically identical (based on our multilocus scheme) to the one infecting rats in the neighboring island of MayotteCitation28. These results are concordant with a previous study carried out in South America suggesting a rodent–bat transmission and that bats (particularly frugivorous bats) may transmit L. interrogans to humansCitation11. Further investigations are thus required to test whether bats are a natural reservoir of L. interrogans, and if transmission between terrestrial small mammals and bats may occur. Finally, in our study, L. borgpetersenii (clade A) was not found in four study regions, namely, Swaziland, Mauritius, La Réunion, and the Seychelles, which might be due to the absence of this strain locally, but more likely is because the typical host species were not sampled in our study. Indeed, on La Réunion, for example, L. borgpetersenii has been reported in cows and miceCitation25, which were hosts that were not analyzed in our study.
Distinct evolutionary histories?
Leptospira diversity in the WIO region was characterized by a lack of spatial structure but high host-associated structure, supporting that Leptospira diversity at a given location is strongly correlated to the local host assemblageCitation10. Therefore, we can expect that small oceanic islands, that have emerged in situ in recent geological time and had low numbers of native mammal species, would harbor reduced levels of Leptospira diversity. This hypothesis is supported by our HD results on La Réunion and Mauritius. As already reported for MadagascarCitation10,Citation14, Leptospira diversity was regionally shaped by strong host specificity. However, Leptospira diversity was not associated with host type (bats vs. terrestrial small mammals), although more samples from these two groups are needed for more definitive conclusions. In contrast, we found that the host genus and species were the main drivers of Leptospira diversity with patterns of host specificity varying depending on the Leptospira species being considered. Within the L. borgpetersenii (-like) lineages (clades A and B), there was clear host specificity at the genus and species level. This suggests that strong in situ diversification, favored by the regional island context and rich-endemic mammal fauna of the WIO, is a major driving process in the evolution of the highly diversified L. borgpeterseniiCitation10,Citation14. Based on genomic analysisCitation38 and environmental studiesCitation16,Citation39, L. borgpetersenii is thought to rely more on a direct host-to-host mode of transmission. This may facilitate host adaptation, especially in bat species roosting in close proximity. Co-diversification of L. borgpetersenii and its hosts is probably a long-term process, as shown by the presence of little or no haplotypic variation in lineages (clade B) on Madagascar, La Réunion, and Mauritius. Indeed, the islands of Mauritius and La Réunion were formed by volcanic activity approximately 5 and 1.8–1.1 million years ago, respectivelyCitation40, and each island has an endemic species of Mormopterus, which presumably has had no contact in recent geological time. We hypothesize that the diversification of Leptospira sheltered by the three Mormopterus likely follows colonization of Mauritius and La Réunion by an ancestral population from Madagascar that arrived with their leptospiral infections. In contrast to these probable endemic L. borgpetersenii lineages, we found that the only hosts carrying strains found elsewhere (Europe, Indonesia) were rats introduced on Mayotte and different native terrestrial small mammals in Tanzania, suggesting a distinct role of introduced Rattus in disseminating cosmopolitan L. borgpetersenii strains.
In contrast, our results suggest that L. interrogans (clade F) was less diversified and bat-borne genotypes were genetically related to those of terrestrial small mammals. This suggests a different evolutionary history compared to L. borgpetersenii. Our hypothesis is that the low diversity of L. interrogans in the WIO region may be associated with a recent human-associated introductionCitation41 of non-native infected rodents (Rattus spp. and possibly Mus spp.). Additionally, the transmission strategy of L. interrogans, relying on survival in humid environmentsCitation16,Citation38, may lead to reduced host adaptation and diversification. Additional sampling of bats and terrestrial small mammals in the WIO region and neighboring Africa, with a particular emphasis on the introduced species, will help test our hypothesis of the distinct evolutionary histories between L. borgpetersenii and L. interrogans.
Host ecology and patterns of mono vs. co-infection
We found different patterns of Leptospira infection among host species. Both mono-infection (host specificity) and co-infection (multiple clades/species) occurred (Table ). In bats for instance, the strong host specificity of Leptospira in Molossidae bats contrasted with the carriage of multiple Leptospira clades/species in the insectivorous Triaenops menamena, Miniopterus spp., Nycteris thebaica or in the frugivorous Rousettus aegyptiacus. In this latter species, co-infection with different Leptospira spp. was found in certain individuals (Figures S2 and S3). Patterns of co-infection have already been reported from South America in two bat species Uroderma bilobatum and Lonchophylla thomasiCitation11 and from the Comoros in R. obliviosusCitation9. However, the genetic data in other geographical regions are too scarce to ascertain if this is a general pattern in bats. Terrestrial small mammals were also found to be infected with different Leptospira species. This was the case for Rattus spp. on MayotteCitation8 and three species of terrestrial small mammals from TanzaniaCitation42. These observations are coherent with several field studies in the rodent populations worldwideCitation8,Citation16.
Co-infection may result from the exposure of hosts to diverse environments and should thus be highly dependent of host ecology. Indeed, as highlighted by a recent field study conducted in southeast Asia, different species of Leptospira have diverse habitat requirementsCitation16: L. borgpetersenii is much more abundant in dry habitats than L. interrogans, which is restricted to humid habitats, and rodents inhabiting both types of habitats are thus infected by both Leptospira speciesCitation38. Humid caves, used as roosting sites by N. thebaica and R. aegyptiacus in Swaziland and South Africa, may provide a source of L. interrogans and may explain why these bat species were found to be infected with both L. borgpetersenii and L. interrogans. Further studies should focus on understanding the ecological aspects of the host and the persistence of Leptospira species in different environments to disentangle patterns of infection and different epidemiological cycles that may occur in wild animals.
Human epidemiology
Human leptospirosis on WIO islands and in continental Africa has been generally associated with Leptospira spp. circulating in introduced animalsCitation8,Citation27,Citation43, such as rats or dogsCitation25, because these animals are in close proximity to humans and, thus, can act as a source of contaminating Leptospira. Our study reveals, however, a wide Leptospira diversity circulating in wild animals of the region, with specific lineages associated with the animal taxa present at a given locality. Further investigations should address whether humans may be directly or indirectly (e.g., by a bat-rat transmission cycle) exposed to the local leptospiral diversity sheltered by autochthonous mammals. Tenrec ecaudatus has been reported as a main reservoir of L. mayottensisCitation28, which is involved in 15% of human acute cases on MayotteCitation26. A recent study compared rrs2 Leptospira sequences from humans on La Réunion to those circulating in the endemic bat species M. francoismoutoui infected with the L. borgpetersenii clade B and showed that this bat species was probably not involved in the appearance of clinical cases. However, further studies focusing on public health should investigate the possibility that wild animal-associated strains may lead to symptomless or sub-clinical infections in humans. The positive relationship between host species richness and Leptospira diversity presented herein does not necessarily suggest a greater leptospirosis incidence in humans. Indeed, a previous study suggested a negative relationship between mammalian diversity and human incidence of leptospirosis in island ecosystems, which may be explained by both bioregulation and dilution effectsCitation44. To answer these questions, it will be necessary to improve current techniques for culturing Leptospira from field samples and to develop tests for Leptospira infection capable of detecting local host-associated Leptospira lineagesCitation45. Such investigations would allow for a greater understanding at the global level of the role of different host/reservoir species in human leptospirosis and the design of improved preventive measures.
Materials and methods
Sample collection and dataset
Our study was conducted with Leptospira samples from the four host groups (rodents, tenrecs, and shrews—referred to herein as terrestrial small mammals—and bats), from 10 different islands/countries in the WIO and continental Africa (Fig. and Table ). We used previously published dataCitation9,Citation10,Citation28,Citation42 (n = 75 samples) as well as new samples (n = 52, kidney tissue) from La Réunion, Mauritius, Seychelles, South Africa, Swaziland, Mozambique, and Madagascar. For some islands/countries, samples were obtained from a range of localities, and details are provided in Table S1. All samples included in this study were PCR-positive for Leptospira, following the protocol described in Dietrich et al.Citation10, except for the samples in Tanzania that were positive-cultured strainsCitation42.
Ethics statement
New samples were collected in accordance with the terms of the research permits issued by the national authorities; in Madagascar: Ministère des Forêts et de l’Environnement, Madagascar National Parks, Département de Biologie Animale (no. 253/11/MEF/SG/DGF/DCB.SAP/SCB, no. 294/10/MEF/SG/DGF/DCB.SAP/SCB, no. 350/10/MEF/SG/DGF/DCB.SAP/SCB, and no. 68/12/MEF/SG/DGF/DCB.SAP/SCB); in France (La Réunion): Direction de l’Environnement, de l’Aménagement et du Logement; in the Seychelles: Seychelles Bureau of Standards (no. AO157 and AO347) and Ministry of Environment; on Mauritius: National Parks and Conservation Service, in Mozambique: Museum de Historia Naturel and Universidade Eduardo Mondlane; in Swaziland: National Trust Commission for Mlawula Game Reserve; and in South Africa: Limpopo Department of Economic, Environment and Tourism (CPM006806), Gauteng Department of Agriculture, Conservation and Environment, South African National Parks (Kruger National Park–RB/2010/04), North West Department of Economic Development, Environment, Conservation and Tourism (000039 NW-07), Department of Agriculture, Forestry and Fisheries Section 20 permission (12/11/1/1/8). The ethical terms of the research protocol were approved by the CYROI Institutional Animal Care and Use Committee (Comité d’Ethique du CYROI no. 114, IACUC certified by the French Ministry of Higher Education and Research) under accreditation 03387 (LeptOI).
Leptospira genotyping
The multilocus sequence analysis was performed using a set of five genes according to previous studies investigating Leptospira diversityCitation26,Citation46. This includes the housekeeping genes adk, secY, and icdA, the 16S rRNA gene rrs2 and the lipL41 encoding a surface-expressed protein. Gene sequences were available for some samples from Tanzania, Comoros, Madagascar, and MayotteCitation9,Citation10,Citation28,Citation42. Additional gene sequences for previously published samples as well as genotyping of the new samples (see details in Table S1) were obtained using the amplification conditions described in Dietrich et al.Citation10 and the primer list detailed in Table S2. Nucleotide sequences generated in this study were deposited in GenBank under the Accession Numbers KP211557-KP211735, KP211741-KP211743, KP211745-KP211782, KP211785-KP211786, and KT599414-KT599433. Alignments for each gene used in the multilocus analysis are archived at Dryad doi:10.5061/dryad.hj029. Careful manual checking of chromatograms did show double peaks for some samples (see results) suggesting the presence of co-infection within the same individual. Such samples were removed from the multilocus analysis to avoid aberrant concatenation of distinct Leptospira lineages infecting the same host.
Identification of Leptospira spp.
To identify the major Leptospira clades and species from the maximum number of samples, we used the secY gene, which is highly discriminatory at this taxonomic levelCitation47 and for which we obtained a large dataset. When secY sequences could not be obtained, we used instead the rrs2 gene to infer Leptospira spp. because this gene was most easily amplified. Separate alignments were performed using the CLC Sequence Viewer 6 for the secY and rrs2 genes, including 47 reference sequences representing the major Leptospira spp. worldwide (see details in Table S3). JMODELTEST v.2.1.4 was used to search for the best-fit nucleotide substitution model using the Akaike Information Criterion (AIC)Citation48, and we constructed phylogenetic trees for the secY and rrs2 genes based on the maximum-likelihood (ML) method with 1000 bootstraps using PHYMLCitation49.
Genetic diversity and population structure
To infer the regional genetic structure and evolutionary relationships of Leptospira, we used a multilocus analysis, including the five genes described above. Separate alignments were conducted for each gene as mentioned above, and we then employed the coalescent Bayesian inference approach implemented in BEAST v.1.7.3Citation50 to infer Leptospira diversification. Thirty samples were added to our dataset as reference sequences (Table S3). We used partitioned data with a constant population size coalescent tree prior and unlinked substitution models (a HKY+I+G model of evolution was independently applied to each partition). Runs were initiated on a random starting tree with an unlinked strict clock for each gene (preliminary runs showed that the strict clock better suited the data as the uncorrelated lognormal and exponential relaxed clocks gave ESS values <200 for some parameters and lower posterior means, Figure S1). Analyses were run for 200 × 106 generations, sampling every 1000 generations, with the initial 10% discarded as burn-in. TRACER v.1.5.0Citation51 was then used to verify that the effective sample size of each parameter was higher than 200. The sampled posterior trees were summarized using TREEANNOTATOR v.1.6.2 to generate a maximum clade credibility tree (maximum posterior probabilities) and to calculate the mean age and 95% highest posterior density (HPD) interval for each node.
HD was calculated for each gene in DnaSP v5Citation52 and the average HD was compared among the locations and host groups (terrestrial small mammals vs. bats) through ANOVA and Tukey HSD (honestly significant difference) post hoc tests. We then used an AMOVA test to determine the significance of an a priori geographic and host-associated structure among samples based on the concatenated sequences using ARLEQUIN v.3.5.1.2Citation53. We defined “population” as samples from a single island or country obtained from the same host species (or genus in the case of Microgale). In a first analysis, populations were grouped by island/country. In cases when a single sample or a single host was available from a given island/country, these data were excluded (see details in Table ). The importance of geographic location on Leptospira structure was also tested separately for bats and terrestrial small mammals. In a second analysis, populations were grouped by host type (terrestrial small mammals vs. bats). We repeated this second analysis only with the samples from Madagascar, where bats and terrestrial small mammals have been extensively sampled. The ΦCT, ΦSC, and ΦST values were used to estimate the genetic differentiation among the different groups and the significance of the tests was assessed by 1023 permutations of populations between groups.
The level of differentiation among the previously identified Leptospira genetic clades/species was estimated by generating FST values in ARLEQUIN v.3.5.1.2 (distance method). The significance of the FST comparisons were assessed using permutation tests (110 permutations per comparison).
Supplemental Material(PDF 1432 kb)
Download PDF (1.4 MB)Acknowledgements
For their assistance with animal sampling, we are grateful to Voahangy Soarimalala (Madagascar); Julien Chotte, Coralie Foray, the Parc National de la Réunion, the Office National des Forêts, and the Réserve Naturelle Nationale de l’Etang St-Paul (La Réunion); the Conseil Général de Mayotte and Direction de l’Environnement, de l’Aménagement et du Logement de Mayotte, Betty Zumbo from Agence Régional de Santé Océan Indien, Marion Pannequin from Coopération des agriculteurs de Mayotte, Soufou Said, Catherine Dioniso and Fabrice Bosca from the Association Naturalistes Environnement et Patrimoine (Mayotte); Philippe Palmire, Jastin Bibi, Léon Biscornet, Jude Gédéon from the Ministry of Health and Pierre-André Adam from the Island Conservation Society (Seychelles); Teresa Kearney, Ernest Seamark, Wilderness Safaris (South Africa); Ara Monadjem (Swaziland); Sookhareea Rajendraprasad from the National Parks and Conservation Service (Mauritius); Carlos Bentos from Museum de Historia Naturel (Mozambique); and Ahmed Ouledi, Ramadhoini Ali Islam, and Mohamed Moulin (Union of the Comoros). We thank David Wilkinson and Julien Mélade for their help with the lab work. This work was supported by the CPER/Regional Council/European Regional Development Funds ERDF-POCT; Reunion, LeptOI (No. 32913) and FSOI (No. 31189) projects and the FEDER PO INTERREG V #RE6875. M.D.’s postdoctoral fellowship were financed by “RUN-Emerge: A Regpot European project funded by European Commission under FP7 program” and by the National Research Foundation, South Africa (NRF—92524). B.R. received postdoctoral grants from the abovementioned RunEmerge project, from “Fonds de Coopération Régionale” of the Préfecture de La Réunion and from the Dr. Ralph and Marian Falk Medical Research Trust to The Field Museum of Natural History, Chicago. Y.G. was supported by a fellowship from the French Ministry for National Education and Research at the University of La Réunion.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41426-018-0059-4).
References
- LevettPNLeptospirosisClin. Microbiol. Rev.200114 296 32610.1128/CMR.14.2.296-326.200188975
- CostaFGlobal morbidity and mortality of leptospirosis: a systematic reviewPLoS Negl. Trop. Dis.20159e000389810.1371/journal.pntd.00038984574773
- PappasGPapadimitriouPSiozopoulouVChristouLAkritidisNThe globalization of leptospirosis: worldwide incidence trendsInt. J. Infect. Dis.20081235135710.1016/j.ijid.2007.09.011
- YersinCHuman leptospirosis in the Seychelles (Indian Ocean): a population-based studyAm. J. Trop. Med. Hyg.19985993394010.4269/ajtmh.1998.59.933
- AdlerBde la Peña MoctezumaALeptospira and leptospirosisVet. Microbiol.201014028729610.1016/j.vetmic.2009.03.012
- BourhyPColletLBrisseSPicardeauMLeptospira mayottensis sp. nov., a pathogenic Leptospira species isolated from humansInt. J. Syst. Evol. Microbiol.2014644061406710.1099/ijs.0.066597-04811635
- BessaTAFThe contribution of bats to leptospirosis transmission in Sao Paulo City, BrazilAm. J. Trop. Med. Hyg.20108231531710.4269/ajtmh.2010.09-0227
- DesvarsASimilarities in Leptospira serogroup and species distribution in animals and humans in the Indian Ocean island of MayotteAm. J. Trop. Med. Hyg.20128713414010.4269/ajtmh.2012.12-01023391038
- LagadecEPathogenic Leptospira spp. in bats, Madagascar and Union of the ComorosEmerg. Infect. Dis.2012181696169810.3201/eid1810.1118983471613
- DietrichMDiversification of an emerging pathogen in a biodiversity hotspot: Leptospira in endemic small mammals of MadagascarMol. Ecol.2014232783279610.1111/mec.12777
- MatthiasMADiversity of bat-associated Leptospira in the Peruvian Amazon inferred by Bayesian phylogenetic analysis of 16S ribosomal DNA sequencesAm. J. Trop. Med. Hyg.2005739649742270400
- TulsianiSMThe role of fruit bats in the transmission of pathogenic leptospires in AustraliaAnn. Trop. Med. Parasitol.2011105718410.1179/136485911X128998384135014089791
- CoxTESmytheLDLeungLKPFlying foxes as carriers of pathogenic Leptospira speciesJ. Wildl. Dis.20054175375710.7589/0090-3558-41.4.753
- GomardYMalagasy bats shelter a considerable genetic diversity of pathogenic Leptospira suggesting notable host-specificity patternsFEMS Microbiol. Ecol.201692fiw03710.1093/femsec/fiw037
- DietrichMMühldorferKTortosaPMarkotterWLeptospira and bats: story of an emerging friendshipPLoS Pathog.201511e100517610.1371/journal.ppat.10051764643053
- CossonJFEpidemiology of Leptospira transmitted by rodents in Southeast Asia.PLoS Negl. Trop. Dis.20148e290210.1371/journal.pntd.00029024046967
- LiSSource tracking of human leptospirosis: serotyping and genotyping of Leptospira isolated from rodents in the epidemic area of Guizhou province, ChinaBMC Infect. Dis.20131310.1186/1471-2334-13-75
- O’BrienJBats of the western Indian Ocean islandsAnimals2011125929010.3390/ani10302594513465
- RussellJCColeNCZuëlNRocamoraGIntroduced mammals on Western Indian Ocean islandsGlob. Ecol. Conserv.2016613214410.1016/j.gecco.2016.02.005
- DesvarsAMichaultABourhyPLeptospirosis in the western Indian Ocean islands: what is known so far?Vet. Res.2013448010.1186/1297-9716-44-803852700
- BovetPHealth situation and issues in the Seychelles in 2012Med. Sante Trop.20130111
- BourhyPIsolation and characterization of new Leptospira genotypes from patients in Mayotte (Indian Ocean)PLoS Negl. Trop. Dis.20104e72410.1371/journal.pntd.00007242889827
- RatsitorahinaMRahelinirinaSMichaultAHas Madagascar lost its exceptional leptospirosis free-like status?PLoS ONE201510e012268310.1371/journal.pone.01226834396993
- GomardYSerologic evidence of leptospirosis in humans, Union of the Comoros, 2011Emerg. Infect. Dis.20142072072210.3201/eid2004.1312073966392
- GuernierVHuman leptospirosis on Reunion Island, Indian Ocean: are rodents the (only) ones to blame?PLoS Negl. Trop. Dis.201610473310.1371/journal.pntd.0004733
- BourhyPHuman Leptospira isolates circulating in Mayotte (Indian Ocean) have unique serological and molecular featuresJ. Clin. Microbiol.20125030731110.1128/JCM.05931-113264139
- RahelinirinaSFirst isolation and direct evidence for the existence of large small-mammal reservoirs of Leptospira sp. in MadagascarPLoS ONE20105e1411110.1371/journal.pone.00141112991340
- LagadecEIdentification of Tenrec ecaudatus, a wild mammal introduced to Mayotte Island, as a reservoir of the newly identified human pathogenic Leptospira mayottensisPLoS Negl. Trop. Dis.201610e000493310.1371/journal.pntd.00049335004980
- DietrichMLeptospira and Paramyxovirus infection dynamics in a bat maternity enlightens pathogen maintenance in wildlifeEnviron. Microbiol.2015174280428910.1111/1462-2920.12766
- Soarimalala, V. & Goodman, S. M. Les petits mammifères de Madagascar. (Association Vahatra, Antananarivo, 2011).
- EversonKMSoarimalalaVGoodmanSMOlsonLEMultiple loci and complete taxonomic sampling resolve the phylogeny and biogeographic history of Tenrecs (Mammalia: Tenrecidae) and reveal higher speciation rates in Madagascar’s humid forestsSyst. Biol.20166589090910.1093/sysbio/syw034
- LhuillerMLeptospiroses in Madagascar (bacteriological and serological study)Arch. Inst. Pasteur Madagascar197864429439
- Kolochine-ErberBBrygooERResearch on leptospirosis in MadagascarBull. Société Pathol. Exot.195649686698
- XuYWhole genome sequencing revealed host adaptation-focused genomic plasticity of pathogenic LeptospiraSci. Rep.2016610.1038/srep200204735792
- FoutsDEWhat makes a bacterial species pathogenic?: Comparative genomic analysis of the genus LeptospiraPLoS Negl. Trop. Dis.20161015710.1371/journal.pntd.0004403
- HimsworthCGEcology of Leptospira interrogans in Norway rats (Rattus norvegicus) in an inner-city neighborhood of Vancouver, CanadaPLoS Negl. Trop. Dis.20137e227010.1371/journal.pntd.00022703688548
- VillanuevaSYAMHigh virulence in hamsters of four dominant Leptospira serovars isolated from rats in the PhilippinesMicrobiology201416041842810.1099/mic.0.072439-0
- BulachDMGenome reduction in Leptospira borgpetersenii reflects limited transmission potentialProc. Natl Acad. Sci. USA2006103145601456510.1073/pnas.06039791031599999
- MasonMREncinaCSreevatsanSMunoz-ZanziCDistribution and diversity of pathogenic Leptospira species in peri-domestic surface waters from south central ChilePLoS Comput. Biol.201610e0004895
- GillotPYLefèvreJCNativelPEModel for the structural evolution of the volcanoes of Réunion IslandEarth Planet. Sci. Lett.199412229130210.1016/0012-821X(94)90003-5
- TollenaereCPhylogeography of the introduced species Rattus rattus in the western Indian Ocean, with special emphasis on the colonization history of MadagascarJ. Biogeogr.20103739841010.1111/j.1365-2699.2009.02228.x
- NalamKGenetic affinities within a large global collection of pathogenic Leptospira: implications for strain identification and molecular epidemiologyPLoS ONE20105e1263710.1371/journal.pone.00126372929200
- DesvarsANazeFBenneveauACardinaleEMichaultAEndemicity of leptospirosis in domestic and wild animal species from Reunion Island (Indian Ocean)Epidemiol. Infect.20131411154116510.1017/S0950268812002075
- DerneBTFearnleyEJLauCLPaynterSWeinsteinPBiodiversity and leptospirosis risk: a case of pathogen regulation?Med. Hypotheses20117733934410.1016/j.mehy.2011.05.009
- TanXTAmranFChee CheongKAhmadNIn-house ELISA screening using a locally-isolated Leptospira in Malaysia: determination of its cut-off pointsBMC Infect. Dis.20141410.1186/s12879-014-0563-74212092
- AhmedNMultilocus sequence typing method for identification and genotypic classification of pathogenic Leptospira speciesAnn. Clin. Microbiol. Antimicrob.200652810.1186/1476-0711-5-281664579
- VictoriaBConservation of the S10-spc-alpha locus within otherwise highly plastic genomes provides phylogenetic insight into the genus LeptospiraPLoS ONE20083e275210.1371/journal.pone.00027522481283
- DarribaDTaboadaGLDoalloRPosadaDjModelTest 2: more models, new heuristics and parallel computingNat. Methods2012977210.1038/nmeth.21094594756
- GuindonSGascuelOA simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihoodSyst. Biol.20035269670410.1080/10635150390235520
- DrummondAJRambautABeast: Bayesian evolutionary analysis by sampling treesBMC Evol. Biol.2007721410.1186/1471-2148-7-2142247476
- Rambaut, A. & Drummond, A. J. TRACER: MCMC trace analysis package. http://beast.bio.ed.ac.uk/Tracer (2007).
- LibradoPRozasJDnaspv5: a software for comprehensive analysis of DNA polymorphism dataBioinformatics2008251451145210.1093/bioinformatics/btp187
- ExcoffierLLavalGSchneiderSARLEQUIN (version 3.0): an integrated software package for population genetics data analysisEvol. Bioinform. Online20051475010.1177/117693430500100003
