Abstract
The aim of this work was to investigate the application of the nested PCR assay for the detection of Treponema pallidum (TP) DNA from the blood of patients with different stages of syphilis. In this study, a nested PCR method targeting the Tpp47 and polA genes (Tpp47-Tp-PCR and polA-Tp-PCR) was developed to detect TP-DNA in whole blood samples collected from 262 patients with different stages of syphilis (84 primary syphilis, 97 secondary syphilis, and 81 latent syphilis patients). The PCR assay detected T. pallidum DNA in 53.6% and 62.9% of the patients with primary and secondary syphilis, respectively, which was much higher than the detection levels in patients with latent syphilis (7.4%) (both p < 0.001). For primary syphilis, a low RPR (0–16) was correlated with a higher detection rate of TP-DNA, whereas for secondary syphilis, the higher detection rate of blood TP-DNA was correlated with higher blood RPR titers (at or beyond 32). For latent syphilis, TP-DNA was only detectable by PCR in the early phase of the latent infection. Thus, blood RPR titers were correlated with the blood T. pallidum burden, but the correlations varied with primary and secondary syphilis. The results indicate that nested PCR is a sensitive method for detecting blood TP-DNA and is especially useful for detecting early syphilis including primary syphilis and secondary syphilis. The findings also suggest that the PCR assay may be used to complement other methods to enhance the diagnosis of syphilis.
These authors contributed equally: Cuini Wang, Yuanyuan Cheng.
Introduction
The incidence of syphilis has experienced a sharp increase worldwide over the last several decadesCitation1. In China, cases of syphilis showed a markedly ascending trend from 2004 to 2013, with an annual percentage increase of 16.3% (95% confidence interval (CI) 13.8 to 18.8)Citation2. In 2016, 438,199 new syphilis cases were reportedCitation3.
Treponema pallidum is the etiological agent of syphilis and it is known that T. pallidum can disseminate to the central nervous system within days after exposure, leading to severe and irreversible symptomatic neurosyphilis if left untreatedCitation4. Previous studies have suggested that certain strain types of T. pallidum were associated with neurosyphilisCitation5,Citation6. However, few studies have assessed blood and cerebrospinal fluid (CSF) samples due to the difficulty in extracting and detecting low amounts of target DNA, and molecular typing of T. pallidum has mostly been done with specimens from moist skin lesionsCitation5. Therefore, data are still limited about T. pallidum in the blood and CSF, and there is still a lack of concrete evidence for the higher-risk strain types associated with neurosyphilis. If strain types can be determined directly from blood specimens, a large number of clinical samples may be analyzed, which will help identify the preferential strain types associated with neurosyphilis. In addition, the improved detection of T. pallidum will help in the early and rapid diagnosis of syphilis. Hence, an efficient technology to enhance the detection of T. pallidum DNA in the blood should be very useful for understanding the epidemiology and pathogenesis of syphilis.
At present, several PCR methods have been developed for the detection of T. pallidumCitation7–Citation13. However, in the case of single-step PCR using clinical specimens, the low quantity of DNA present in the specimens will greatly affect the sensitivity. To enhance the detection sensitivity, nested PCR was therefore used in this study for the detection of T. pallidum in whole blood samples from patients with syphilis. The correlation between the presence of T. pallidum DNA with blood rapid plasma reagin (RPR) titer and different stages of the disease was comprehensively evaluated.
Results
Nested PCR assays (Tpp47-Tp-PCR and polA-Tp-PCR) were used to detect T. pallidum DNA in blood samples from all patients
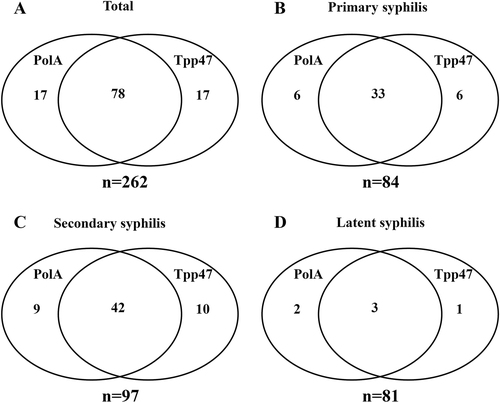
In total, 262 patients were included in this study. Among them, 84 (32.1%) patients were with primary syphilis, 97 (37.0%) with secondary syphilis, and 81 (30.9%) with latent syphilis (Table ). The PCR detection targeted polA and Tpp47. As shown in Fig. , 95 (36.3%) specimens were polA-Tp-PCR positive and 95 (36.3%) were Tpp47-Tp-PCR positive. In total, 112 (42.7%) patients were positive with Tpp47-Tp-PCR and/or polA-Tp-PCR. We obtained 78 concordant-positive samples and thus 150 concordant-negative Tp-PCR results; 17 specimens were negative for polA-Tp-PCR but positive for Tpp47-Tp-PCR, whereas 17 were negative for Tpp47-Tp-PCR but positive for polA-Tp-PCR. There were no significant differences between the two target genes (p = 1.000) and the κ-coefficient was 0.719.
Clinical characteristics of the 262 syphilis patients examined in this study
a The positive specimens of polA-Tp-PCR and/or Tpp47-Tp-PCR among all patients (n = 262). b The positive specimens of polA-Tp-PCR and/or Tpp47-Tp-PCR in the primary syphilis patients (n = 84). c The positive specimens of polA-Tp-PCR and/or Tpp47-Tp-PCR in the secondary syphilis patients (n = 97). d The positive specimens of polA-Tp-PCR and/or Tpp47-Tp-PCR in the latent syphilis patients (n = 81)
We also detected polA and Tpp47 using single-step PCR among 95 polA-positive samples and 95 Tpp47-positive samples by nested PCR. We found that there were only 23.2% (22/95) polA- and 26.3% (25/95) Tpp47-positive samples. The grayscale of DNA fragments of Tpp47 and polA were very weak compared with those of the nested PCRs (Supplementary Figure 1).
The detection rate of T. pallidum DNA in the blood from patients with different clinical stages of syphilis
As shown in Fig. , 45 (53.6%) specimens from 84 patients with primary syphilis were positive for Tpp47-Tp-PCR and/or polA-Tp-PCR, including 33 specimens positive for both Tpp47-Tp-PCR and polA-Tp-PCR, 6 specimens positive only for Tpp47-Tp-PCR, and 6 specimens positive only for polA-Tp-PCR.
For secondary syphilis, 42 (43.3%) specimens were positive for polA-Tp-PCR and Tpp47-Tp-PCR, 10 specimens were only positive for Tpp47-Tp-PCR, and 9 specimens were only positive for polA-Tp-PCR. In total, 61 (62.9%) specimens were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR (Fig. ).
Of the 81 patients with latent syphilis, 5 (6.2%) specimens were positive for polA-Tp-PCR and 4 (4.9%) were positive for Tpp47-Tp-PCR. Among the positive specimens, 2 were only positive for polA-Tp-PCR and 1 was only positive for Tpp47-Tp-PCR. There were 6 (7.4%) specimens positive for polA-Tp-PCR and/or Tpp47-Tp-PCR in latent syphilis (Fig. ).
In conclusion, the detection rate was the highest for secondary syphilis, but there was no significant difference in the detection rates between primary and secondary syphilis (p = 0.228). For latent syphilis, the detection rate was much lower compared with those of primary and secondary syphilis (both p < 0.001).
The relationship between blood RPR titer and the presence of T. pallidum DNA in different stages of syphilis
The association between the blood RPR titer and the presence of T. pallidum DNA in different stages of syphilis is shown in Table . The positive rate of T. pallidum DNA was higher when the blood RPR titer was lower for primary syphilis. There were 53 primary syphilis patients with blood RPR titers ranging from 0 to 16, of whom 32 (60.4%) were positive with polA-Tp-PCR and/or Tpp47-Tp-PCR. For the 22 patients with blood RPR titers ranging from 32 to 64, 10 (45.5%) were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR. There were only nine patients with blood RPR titers ≥ 128, of whom three (33.3%) were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR. From the results, it is clear that the detection rate of TP-DNA was the highest (60.4%) when the blood RPR titer ranged from 0 to 16, as opposed to compared with those when the RPR titer ranged from 32 to 64 (45.5%) or when it was at or beyond 128 (33.3%); however, these differences did not reach statistical significance (p = 0.176 and 0.126, respectively). Interestingly, 8 of the 84 patients with primary syphilis were negative for blood RPR and T. pallidum particle agglutination assay (TPPA), but 5 (62.5%) of the 8 patients were blood TP-DNA positive. There were eight patients who were non-reactive with blood RPR but positive for blood TPPA; of these, two (25%) were Tp-DNA positive.
The relationship between the blood RPR titer and the presence of T. pallidum DNA for all syphilis patients
In contrast to the situation with primary syphilis, the positive rate of T. pallidum DNA was higher for secondary syphilis when blood RPR titers were higher (Table ). All patients with secondary syphilis were reactive to blood RPR and TPPA. There were eight patients with blood RPR titers ≤ 16, of whom three (37.5%) were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR. When the blood RPR titer was in the range of 32 to 64, there were 49 patients of whom 33 (67.3%) were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR. When the blood RPR titer was at or beyond 128, there were 40 patients of whom 25 (62.5%) were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR. Thus, the detection rate of blood TP-DNA was higher when the blood RPR titer ranged from 32 to 64 (67.3%) or when it was at or beyond 128 (62.5%) than in patients with blood RPR titers ranging from 0 to 16 (37.5%); however, the differences were not statistically significant (p = 0.111 and 0.18, respectively).
There were only 6 positive specimens among 81 patients with latent syphilis. Fourteen patients had RPR titers at or beyond 128. Of these, four (28.6%) patients were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR. In addition, there were 27 patients with RPR titers ranging from 32 to 64, 2 (7.4%) of whom were positive for polA-Tp-PCR and/or Tpp47-Tp-PCR. Both had blood RPR titers of 64. None of the patients were TP-DNA positive when the blood RPR titer ranged from 0 to 16. Among the six patients, three were early latent patients and three patients were of unknown duration.
Discussion
The prompt diagnosis of early syphilis not only helps stop the transmission of the infection source but also prevents long-term consequences. However, in many circumstances, the diagnosis of early syphilis can be a challenge. For example T. pallidum could not be found at the site of bacterial infection; although it is very common clinically, its serum RPR/TPPA is negative. Therefore, a sensitive method for detecting T. pallidum is fundamental for the prompt diagnosis of early syphilis. In this study, the results showed that nested PCR might be a relatively sensitive method for detecting T. pallidum DNA in blood, especially in patients with primary and secondary syphilis. Our method was sensitive and the amount of target DNA fragments was low in the single-step PCR detection, indicating that the concentration of T. pallidum DNA was also low in the blood. When there were low amounts of templates in the samples, there was low sensitivity, instability, and poor repeatability using single-step PCR. Previous studies reported that, because Herpesvirus DNA was present in low copy numbers in abscess specimens, Herpesviral DNA could not be detected using primary PCR, and all abscess specimens required nested PCR for the detection of amplified DNACitation14. Our results confirmed that nested PCR provided a relatively sensitive method for detecting the pathogenic DNA in blood. To date, no specific genes have been recommended as a gold-standard target for TP-PCR. However, the Tpp47 and polA genes are the most commonly used targets for PCR in previously reported studiesCitation15. Tpp47 encodes a cytoplasmic membrane protein involved in cell wall synthesis and is partly specific for T. pallidum subsp. pallidumCitation16. polA encodes DNA polymerase I involved in DNA repair and replication in most bacteria and shows a number of unique features in T. pallidum subsp. pallidumCitation17. A recent study showed that Tp-PCR targeting the Tpp47 and polA genes showed very good congruence and only four discordant results were observed between the two methods among 272 specimens from patients with sexually transmitted ulcersCitation18. Our results also found good concordance between the two PCR targets (Fig. ) and were consistent with the findings reported in previous studies.
This study showed that T. pallidum DNA could be amplified from whole blood samples in different stages of syphilis, which will help in the early diagnosis of syphilis. For spirochetemia in the secondary stage, the detection rate was the highest in secondary syphilis, reaching 62.9%. The detection of T. pallidum DNA for primary syphilis was 53.6% (45/84). Primary syphilis appears as painless ulcers at the site of T. pallidum contact. The bacteria mainly proliferate locally and spread into the blood at the same time. Unfortunately, the local bacteria, which are expected to be found easily under dark-field microscopic examination from genital ulcer swabs in patients with primary syphilis, can be easily destroyed under many circumstances. For example, topical antibiotics can destroy them, making the diagnosis of primary syphilis challenging when combined with a negative RPR and TPPA result. Our detection rate was higher than that previously reported. Grange et al.Citation13 showed that the frequency of T. pallidum detection in whole blood using nested PCR was 13%. Quantitative PCR results from the study by Tipple et al.Citation11 reported a 21.4% detection rate. On the one hand, subtle or atypical dermatological or mucosal lesions of syphilis can lead to misclassification of disease stages, and therefore primary and secondary syphilis may coexist. On the other hand, we tried our best to obtain reliable patient histories and none of our patients were treated with antibiotics before blood was collected in this study.
An interesting finding of this study is that T. pallidum DNA was detected in five patients with primary syphilis, who were non-reactive to both blood RPR and TPPA, indicating that T. pallidum can enter the blood shortly after infecting skin or mucosal surfaces. This finding can help physicians make an early diagnosis for those patients with atypical syphilis manifestations and negative serological responses. A previous study also showed that T. pallidum DNA could be detected in 3 (27%) of 11 individuals who were exposed to patients with syphilis and had a non-reactive blood RPRCitation19. The entry of T. pallidum into the blood of experimentally infected animals occurs within 24 h of intratesticular inoculationCitation7, but it is unknown whether this also occurs in humans. It is known that humoral antibodies against cardiolipin and treponemal antigen usually do not appear until 1–4 weeks after the development of a chancreCitation20. Therefore, the interpretation of syphilis serology can be problematic for very early primary syphilis, because serological responses are delayed. Dark-field microscopy is very useful for new, moist primary lesions, but the detection sensitivity decreases as the lesions heal or are treated by local rinsing or antibiotic medications. This situation often requires patients to return for re-testing. Thus, T. pallidum DNA detection in blood provides not only an added value for the diagnosis of these cases of suspected primary syphilis but also gives evidence to facilitate proper and prompt treatment.
Another interesting finding of this study was that blood T. pallidum DNA was more commonly detected in patients with low serum RPR titers in primary syphilis, whereas in secondary syphilis T. pallidum DNA was more commonly detected in patients with higher serum RPR titers. Regrettably, the difference in the detection rates did not reach statistical significance. As serum RPR titers were relatively low in primary syphilis, only 9 patients with primary syphilis were recruited in this study with blood RPR titers at or beyond 128. On the contrary, serum RPR titers were higher in secondary syphilis and there were only 8 patients for whom serum RPR titers ranged from 0 to 16. The fact that only a few patients in these groups affected the statistics. This finding may therefore need more samples to be verified in further studies. RPR measures nontreponemal antibodies to lipoidal antigens, principally cardiolipin, released from damaged host cells and T. pallidumCitation21,Citation22. Therefore, RPR titers tend to correlate with disease activity and the T. pallidum burden. Our results suggest that when T. pallidum begins to disseminate into the blood from a local infection site, the small number of T. pallidum is not able to provoke enough humoral and cellular immune responses to clear pathogens at lower blood RPR titers for primary syphilis. On the contrary, secondary syphilis was observed at the stage of spirochetemia. T. pallidum may provoke an intense immune response to destroy the bacteria and, at the same time, the response may damage host cells, exposing the host to a greater concentration of lipoidal antigens and consequently increased titers of RPR. In the samples from latent syphilis, we detected T. pallidum DNA in only six patients with a serum RPR titer ≥ 64. Serum RPR titers were generally higher during early syphilis than during later stages of infectionCitation23. Thus, the six patients might be with early latent syphilis.
Our findings, together with those of previous studiesCitation13, Citation24,Citation25 showed that nested PCR can be used to detect T. pallidum DNA in the peripheral blood of patients with syphilis at various stages. We further analyzed the relationship between blood RPR titers and the detection of T. pallidum DNA, and found that blood RPR titers were correlated with the blood T. pallidum burden. Importantly, these correlations were different in primary and secondary syphilis. This method is useful for detecting T. pallidum DNA for suspected cases of primary syphilis, especially when clinical symptoms are atypical or in circumstances when T. pallidum cannot be found in the chancre, and blood RPR and TPPA are non-reactive. In these cases, the detection of blood T. pallidum DNA can avoid or delay misdiagnosis. Our results indicate that the blood PCR method can be used to complement other methods to enhance the diagnosis of syphilis.
Materials and methods
Ethics statement and subjects
The study was approved by the Ethics Committee of the Shanghai Skin Disease Hospital. Between September 2015 and April 2017, eligible patients who sought medical care in the Sexually Transmitted Disease Clinic of the Shanghai Skin Disease Hospital were asked to participate in this study. Participants were interviewed with a brief questionnaire to collect medical and social-demographic information. Patients with therapeutic interventions were excluded. The diagnosis of the clinical stage of syphilis was determined based on a combination of a compatible history, clinical manifestations and the results of nontreponemal and treponemal tests in the serum. All eligible patients who agreed to participate in the study provided their written informed consent.
Diagnostic criteria for primary, secondary, and latent syphilis
Primary syphilis was classified as having clinical manifestation of chancres or ulcers and one of the following laboratory confirmations: (i) positivity for spirochetes by dark-field microscopic examination; (ii) positive RPR confirmed by TPPA; or (iii) a negative result for spirochetes by dark-field microscopic examination and initially negative results of RPR and TPPA serologically becoming positive in the follow-up period (a maximum of 3 months).
Secondary syphilis was classified by (i) a positive RPR confirmed by TPPA and (ii) skin or mucocutaneous lesions.
Latent syphilis was defined by positive RPR confirmed by TPPA but without skin or mucocutaneous lesions, or any symptoms of syphilis; early latent syphilis was classified as an infection by T. pallidum within 2 years; and late latent syphilis was thereafter classified as having an infection after 2 years.
PCR detection of T. pallidum in the blood
DNA was extracted from 1 ml of whole blood with anti-coagulant EDTA using the QiaAmp DNA mini blood Kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer’s instructions. Nested PCR was performed initially with the outer primers of polA (sense: 5′-TGCGCGTGTGCGAATGGTGTGGTC-3′ and antisense: 5′-CACAGTGCTCAAAAACGCCTGCACG-3′). In a total volume of 50 μl, each reaction consisted of 10 μl of DNA, 25 μl of 2 × PCR master mix (Promega), and 0.6 μM primers. The PCR reaction was performed in an ABI 9700 thermocycler (Applied Biosystems, Foster City, CA) under the following cycling conditions: 94 °C for 4 min, 25 cycles at 94 °C for 1 min, 65 °C for 30 s, and 72 °C for 45 s, followed by 72 °C for 7 min. In the second round of PCR, 3 μl of the first reaction product was used in a total volume of 25 μl of reaction mixture with the sense primer (5′-GGATTGCATCCGCACGATAC-3′) and the antisense primer (5′-CAGCAGATGCAGATACCCCA-3′). This PCR reaction contained 12.5 μl of 2 × PCR master mix (Promega) and 0.6 μM primers. The cycling conditions consisted of 4 min at 94 °C then 30 cycles of 94 °C for 1 min, 60 °C for 30 s, and 70 °C for 30 s, followed by 72 °C for 7 min.
For the nested PCR amplifying the Tpp47 gene, the outer primers included 5′-GTCAGCCTGTAGTATCCCG-3′ (sense) and 5′-TTCTGCACGTAAGGTAAGC-3′ (antisense). The inner primer sequences were 5′-TGCCATAACTCGCCATCAGA-3′ (sense) and 5′-CAACACGGTCCGCTACGACTA-3′ (antisense). The reaction mix and the cycling conditions were similar to those of polA. Appropriate positive (Nichols strain of T. pallidum) and negative (distilled water) controls were used with each set of reactions.
Single-step PCR was performed to amplify the Tpp47 gene and the PCR reaction contained 12.5 μl of 2 × PCR master mix (Promega), 0.6 μM primers, and 10 μl of DNA. The inner primers of Tpp47 were used. The cycling conditions were similar to those of the second round of PCR of Tpp47. For single-step PCR amplifying the polA gene, the PCR reaction contained 12.5 μl of 2 × PCR master mix (Promega), 0.6 μM primers, and 10 μl of DNA. The inner primers of polA were used. The cycling conditions were similar to those of the second round of PCR of polA.
Samples were kept at 4 °C until analysis by gel electrophoresis. The PCR products were electrophoresed on a 2% agarose gel together with a 100 bp DNA ladder (Takara) at 100 V for 20 min. The gels were visualized after staining with ethidium bromide.
Statistical analysis
All statistical analyses were performed using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to calculate the median and interquartile range. In addition, the χ2-test and the Fisher’s exact test were performed to compare the proportion between the groups. Differences were considered to be statistically significant at two-sided P-values < 0.05.
Supplementary Figure 1
Download TIFF Image (890 KB)Supplementary Figure 1 legend
Download MS Word (13.1 KB)Acknowledgements
This work was supported by the National Natural Science Foundation of China [81371862 and 81572039], The Basic Research Project of Shanghai Science and Technology Commission [15JC1403000], and the Shanghai Natural Science Foundation [15ZR1437000, 16411961300].
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41426-018-0085-2.
References
- Rowley J. Toskin I., Ndowa F. World Health Organization, Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections–2008. Available at: http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf (accessed 3 December 2017).
- YangSEpidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend studyLancet Infect. Dis.201717 716 72510.1016/S1473-3099(17)30227-X
- Chinese Center for Disease Prevention and Control; Center for STI and AIDS Prevention and Control. Available at: http://www.ncstdc.org/ (accessed 26 November 2017).
- MarraCMNeurosyphilisCurr. Neurol. Neurosci. Rep.2004443544010.1007/s11910-004-0065-x
- DaiTMolecular typing of Treponema pallidum: a 5-year surveillance in Shanghai, ChinaJ. Clin. Microbiol.2012503674367710.1128/JCM.01195-123486273
- MarraCEnhanced molecular typing of Treponema pallidum: geographical distribution of strain types and association with neurosyphilisJ. Infect. Dis.20102021380138810.1086/6565333114648
- SalazarJCAssessment of the kinetics of Treponema pallidum dissemination into blood and tissues in experimental syphilis by real-time quantitative PCRInfect. Immun.2007752954295810.1128/IAI.00090-071932886
- HeymansRClinical value of Treponema pallidum real-time PCR for diagnosis of syphilisJ. Clin. Microbiol20104849750210.1128/JCM.00720-09
- CastroRDetection of Treponema pallidum sp pallidum DNA in latent syphilisInt J. STD AIDS20071884284510.1258/095646207782716901
- LeslieDEDevelopment of a real-time PCR assay to detect Treponema pallidum in clinical specimens and assessment of the assay’s performance by comparison with serological testingJ. Clin. Microbiol.200745939610.1128/JCM.01578-06
- TippleCGetting the measure of syphilis: qPCR to better understand early infectionSex. Transm. Infect.20118747948510.1136/sti.2011.0494943252622
- LiuHNew tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I geneJ. Clin. Microbiol.2001391941194610.1128/JCM.39.5.1941-1946.200188053
- GrangePAEvaluation of a PCR test for detection of Treponema pallidum in swabs and bloodJ. Clin. Microbiol.20125054655210.1128/JCM.00702-113295187
- ChenV1Herpesviruses in abscesses and cellulitis of endodontic originJ. Endod.20093518218810.1016/j.joen.2008.11.0182661131
- Gayet-AgeronAComparison of diagnostic accuracy of PCR targeting the 47-kilodalton protein membrane gene of Treponema pallidum and PCR targeting the DNA polymerase I gene: systematic review and meta-analysisJ. Clin. Microbiol.2015533522352910.1128/JCM.01619-154609724
- MiaoRMFieldsteelAHGenetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogensJ. Bacteriol.1980141427429293620
- RodesBLiuHJohnsonSGeorgeRSteinerBMolecular cloning of a gene (poIA) coding for an unusual DNA polymerase I from Treponema pallidumJ. Med Microbiol.20004965766710.1099/0022-1317-49-7-657
- Gayet-AgeronAPerformance of the 47-kilodalton membrane protein versus DNA polymerase I genes for detection of Treponema pallidum by PCR in ulcersJ. Clin. Microbiol.20155397698010.1128/JCM.03444-144390634
- MarfinAAAmplification of the DNA polymerase I gene of Treponema pallidum from whole blood of persons with syphilisDiagn. Microbiol Infect. Dis.20014016316610.1016/S0732-8893(01)00275-9
- LarsenSASteinerBMRudolphAHLaboratory diagnosis and interpretation of tests for syphilisClin. Microbiol.19958121
- BelisleJTFatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteinsJ. Bacteriol.19941762151215710.1128/jb.176.8.2151-2157.1994205333
- MatthewsHMYangTKJenkinHMUnique lipid composition of Treponema pallidum (Nichols virulent strain)Infect. Immun.197924713719414365
- SamoffEPre-treatment syphilis titers: distribution and evaluation of their use to distinguish early from late latent syphilis and to prioritize contact investigationsSex. Transm. Dis.20093678979310.1097/OLQ.0b013e3181b3566b
- GuanYSensitivity of nPCR for four types of membrane protein DNA and of two pairs of primers for Tpp47 DNA of Treponema pallidum in whole blood of congenital syphilis newbornsJ. Matern. Fetal Neonatal Med.2017221710.1080/14767058.2017.1378321
- MikalováLMolecular typing of syphilis-causing strains among human immunodeficiency virus-positive patients in Antwerp, BelgiumSex. Transm. Dis.20174437637910.1097/OLQ.0000000000000600
