Abstract
Ebolavirus vaccines based on several adenoviral vectors have been investigated in preclinical studies and clinical trials. The use of adenovirus serotype 2 as a vector for ebolavirus vaccine has not been reported. Herein, we generated rAd2-ZGP, a recombinant replication-incompetent adenovirus serotype 2 expressing codon-optimized Zaire ebolavirus glycoprotein, and evaluated its immunogenicity in mice and rhesus macaques. rAd2-ZGP induced significant antibody and cell-mediated immune responses at 2 weeks after a single immunization. The glycoprotein (GP)-specific immune responses could be further enhanced with a booster immunization. Compared to protein antigens, Zaire ebolavirus GP and Zaire ebolavirus-like particles, rAd2-ZGP could induce stronger cross-reactive antibody and cell-mediated immune responses to heterologous Sudan ebolavirus in mice and rhesus macaques. In rAd2-ZGP-immunized macaques, GP-specific CD8+ T cells could secret IFN-γ and IL-2, indicating a Th1-biased response. In adenovirus serotype 5 seropositive macaques, rAd2-ZGP could induce robust antibody and cell-mediated immune responses, suggesting that the efficacy of rAd2-ZGP is not affected by pre-existing immunity to adenovirus serotype 5. These results demonstrated that rAd2-ZGP can be considered an alternative ebolavirus vaccine for use in adenovirus serotype 5 seropositive subjects or as a sequential booster vaccine after the subjects have been immunized with a recombinant adenovirus serotype 5-based vaccine.
Introduction
Ebolavirus (EBOV) belongs to a genus of the Filoviridae family and consists of five species, including Zaire, Sudan, Bundibugyo, Reston, and Taï Forest EBOVsCitation1. Among these species, Zaire EBOV (ZEBOV) and Sudan EBOV (SEBOV) have the highest pathogenicity in humansCitation1. EBOV infection usually leads to severe hemorrhagic fever diseases with high fatalityCitation2. The 2013–2016 epidemic caused by ZEBOV in West Africa has become the largest outbreak recorded, with more than 28,000 people affected and a death toll of at least 12,000Citation3. The economic and health burdens posed by EBOV highlighted the need to develop safe and effective prophylactic vaccines.
Recombinant adenoviruses (rAd) have been extensively explored as vaccine vectors for many pathogens, including HIV, mycobacterium tuberculosis, malaria, influenza virus, and EBOVCitation4–Citation7. rAd vectors expressing EBOV glycoprotein (GP), the major surface protein mediating the attachment and entry of EBOVCitation8, effectively protected mice and non-human primates (NHPs) against lethal EBOV infectionCitation6, Citation9. At least three rAd-vectored vaccines, including recombinant human adenovirus serotype 5 (rAd5), human adenovirus serotype 26 (rAd26), human adenovirus serotype 35 (rAd35), and chimpanzee adenovirus type 3 (ChAd3), have been investigatedCitation10–Citation13. Recently, the rAd5-vectored vaccine expressing GP of the ZEBOV Makona strain (GenBank accession number KJ660346) underwent a phase II clinical trial in Sierra Leone and showed good safety and immunogenicityCitation14. The rAd5-GP-induced antibody response to EBOV peaked at day 28 after immunization but declined by 85% during the following 6 monthsCitation14, Citation15, suggesting that a booster immunization later might be necessary. Of particular note, rAd5-GP vaccine-induced GP-specific antibody and T cell responses were weakened by the presence of pre-existing anti-Ad5 antibody in a phase I trial conducted in China and a phase II trial conducted in Sierra Leone, which suggested that pre-existing anti-Ad5 immunity might dampen the immunogenicity of rAd5-vectored EBOV vaccinesCitation14–Citation16. Therefore, there is a need to evaluate more EBOV vaccines based on other rAd serotypes for immunization in Ad5-seropositive people and for immunization in people who previously received rAd5-vectored vaccines.
Both neutralizing antibody and cell-mediated immune (CMI) responses play important roles in the protection against EBOV infection by rAd-vectored vaccines in rodent and NHP modelsCitation17–Citation19. A strong association has been observed between the titers of GP-specific neutralizing antibodies and the protective efficacy of rAd5-GP in rodent and NHP challenge modelsCitation18–Citation20. It was also demonstrated that rAd5-GP-induced CD8+ T cell responses are critical for preventing the establishment of infection through clearing of EBOV-infected host cells in NHPsCitation13. Although rAd5 appeared to be a safe and effective vector for an EBOV vaccine, there is a high prevalence of pre-existing anti-Ad5 neutralizing antibodies in human populations, especially in Asia and AfricaCitation16, Citation21, Citation22. We therefore investigated whether recombinant human adenovirus serotype 2 (rAd2) could be used as another adenovirus vector for EBOV vaccines. Ad2 and Ad5 both belong to human adenovirus subgroup C but are distinct in sero-reactivityCitation23. rAd2 has been shown to be a safe and efficient vector in humans in many gene therapy clinical trialsCitation24. Given that pre-existing anti-Ad5 neutralizing antibodies have minimal cross-neutralizing activity to Ad2 and people who are Ad5 seropositive might not be Ad2 seropositiveCitation25, we proposed that Ad2 could be exploited as another vector for an EBOV vaccine.
In this study, we generated an rAd2 vector carrying a codon-optimized GP gene encoding the contemporary ZEBOV Makona strain (rAd2-ZGP). We investigated the immunogenicity of rAd2-ZGP by assessing GP-specific antibody and CMI responses in mice and NHPs. We also evaluated the immunogenicity of purified GP of ZEBOV (ZGP) and virus-like particles of the ZEBOV Makona strain (ZVLPs).
Results
Characterization of rAd2-ZGP, ZGP, and ZVLPs
We generated rAd2-ZGP, a recombinant replication-incompetent adenovirus serotype 2 carrying the GP of the contemporary ZEBOV Makona strain (GenBank accession number KJ660346) (Supplementary Figure S1A). The antigen components in three EBOV vaccine candidates were confirmed by SDS-PAGE followed by western blot analysis as described in the Methods (Supplementary Figure S1B). We estimated that the accumulated level of GP expressed by 5 × 108 vp rAd2-ZGP on cultured Vero cells was ~1 μg (between 0.64 and 1.28 μg) by 72 h post infection (Supplementary Figure S1C). The GP of ZEBOV 2014 (ZGP) with a truncated mucin-like domain and a transmembrane domain (~75 kDa) was produced in Sf9 cells using the baculovirus expression system. The ZVLPs were generated by expressing full-length GP and VP40 of the ZEBOV Makona strain. Under electron microscopy, these filamentous ZVLPs were ~70 nm in diameter and 500–1000 nm in length, resembling the filovirus particles in size and morphology (Supplementary Figure S1D).
rAd2-ZGP elicited specific antibody responses to homologous ZEBOV and heterologous SEBOV in mice
To evaluate whether rAd2-ZGP can elicit GP-specific antibody responses, female Balb/c mice were intramuscularly immunized with either a lower dosage of 5 × 108 vp rAd2-ZGP or a higher dosage of 5 × 109 vp rAd2-ZGP. Mice injected with either 2 µg ZGP, 20 µg ZGP, 2 µg ZVLPs, or 20 µg ZVLPs were evaluated in parallel. Mice injected with either 5 × 108 vp rAd2-Empty, 5 × 109 vp rAd2-Empty, or PBS were used as mock controls (Fig. ). The antibody response was evaluated at 3 weeks after the single immunization. Although 5 × 108 vp rAd2-ZGP is estimated to produce ~1 µg GP in cultured Vero cells, it generated a much higher level of GP-binding IgG antibodies to autologous ZEBOV GP than both the 2 and 20 µg ZGP and ZVLPs. We also assessed if the GP-specific antibody response has any cross-reactivity to heterologous SEBOV GP. Although ZEBOV GP shares 67% amino-acid identity with SEBOV GP (Supplementary Figure S1E), rAd2-ZGP could elicit significant cross-reactive antibodies to heterologous SEBOV GP (Fig. ). To further assess the quality of GP-specific antibodies, we measured the neutralizing antibodies using a neutralization assay based on reporter lentiviruses pseudo-typed by ZEBOV GP or SEBOV GP. A single immunization with rAd2-ZGP elicited a significantly higher level of neutralizing antibodies to ZEBOV than did the ZGP and ZVLP vaccines (Fig. , Supplementary Figure S2). A single immunization with rAd2-ZGP, but not ZGP or ZVLPs, induced apparent cross-reactive neutralizing antibodies to heterologous SEBOV (Fig. , Supplementary Figure S2). Both GP-specific binding antibodies and neutralizing antibodies increased with a higher dosage of immunization (Fig. , Supplementary Figure S2).
a Seven-week-old Balb/c female mice were intramuscularly immunized with 5 × 108 vp rAd2-ZGP, 5 × 109 vp rAd2-ZGP, 2 µg ZGP, 20 µg ZGP, 2 µg ZVLPs, or 20 µg ZVLPs. Mice injected with PBS, 5 × 108 vp or 5 × 109 vp rAd2-Empty were used as control groups. b Three weeks after immunization, serum samples were collected and subjected to ELISA analysis of IgG antibodies that bind to ZEBOV GP and SEBOV GP. The titers were calculated as reciprocal endpoints. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. c Three weeks after immunization, the serum samples were measured for the neutralizing activities to ZEBOV GP pseudo-typed lentivirus or SEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative control sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 5). d Mice immunized with higher dosage groups were sacrificed 3 weeks after immunization. Splenocytes were isolated and stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay and imaged with an ELISpot reader. Data are shown as the number of SFCs in one million splenocytes. e CD8+ T cells secreting IFN-γ were determined with an ICS assay after stimulation with a peptide pool derived from ZEBOV GP. Data are shown as the mean ± SD (n = 5). f Splenocytes were stimulated with either ZEBOV GP or SEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million splenocytes. Comparisons between groups were performed by one-way ANOVA, comparisons of the neutralizing antibody titers between low- and high-dose immunizations in the rAd2-ZGP group were performed by Student’s t test, and a P-value < 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001
We also tested a prime-boost immunization regime using either 5 × 108 vp rAd2-ZGP, 2 µg ZGP, or 2 µg ZVLPs with a booster immunization of the same vaccine 6 weeks after the first immunization. Mice injected with PBS and 5 × 108 vp rAd2-Empty were used as mock controls (Fig. ). Although all vaccines generated binding antibodies to ZEBOV GP after the two immunizations, rAd2-ZGP appeared to elicit the strongest IgG antibodies that bind to homologous ZEBOV GP (Fig. ). rAd2-ZGP stimulated the strongest neutralizing antibodies to homologous ZEBOV, especially after the booster immunization (Fig. , Supplementary Figure S2C). A booster immunization of rAd2-ZGP induced cross-reactive IgG binding antibodies and neutralizing antibodies to heterologous SEBOV (Fig. , Supplementary Figure S2F).
a Seven-week-old Balb/c female mice were intramuscularly injected with 5 × 108 vp rAd2-ZGP, 2 µg ZGP, or 2 µg ZVLPs. Six weeks later, mice received a booster immunization of the same vaccine. Mice injected with PBS or 5 × 108 vp rAd2-Empty were used as control groups. b Three weeks after each immunization, serum samples were collected and subjected to ELISA analysis of IgG antibodies that bind to ZEBOV GP and SEBOV GP. The titers were calculated as reciprocal endpoints. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. c Three weeks after each immunization, serum samples were measured for neutralizing activities to the ZEBOV GP pseudo-typed lentivirus or SEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 5). d Mice were sacrificed 3 weeks after the booster immunization. Splenocytes were isolated and stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay and imaged with an ELISpot reader. Data are shown as the number of SFCs in one million splenocytes. e CD8+ T cells secreting IFN-γ were determined using an ICS assay after stimulation with a peptide pool derived from ZEBOV GP. Data are shown as the mean ± SD (n = 5). f Splenocytes were stimulated with either ZEBOV GP or SEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million splenocytes. Comparisons between groups were performed by one-way ANOVA; comparisons of the neutralizing antibody titers between prime and booster immunizations in the rAd2-ZGP group were performed by Student’s t test. A P-value < 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001
rAd2-ZGP elicited cell-mediated immune responses to homologous ZEBOV and heterologous SEBOV in mice
The rAd5-vectored EBOV vaccine has been shown to generate CMI responses, which also contribute to the protection against EBOV infectionCitation13, Citation19. We therefore examined whether rAd2-ZGP induces CMI responses. Mice immunized with higher dosages (5 × 109 vp rAd2-ZGP, 20 µg ZGP, or 20 µg ZVLPs) were sacrificed at 3 weeks after a single immunization. Mice immunized with lower dosages (5 × 108 vp rAd2-ZGP, 2 µg ZGP, or 2 µg ZVLPs) were sacrificed at 3 weeks after the second immunization. Splenocytes were stimulated with a ZEBOV GP peptide pool. rAd2-ZGP induced significant ZEBOV GP-specific IFN-γ+ spot forming cells (SFCs). In contrast, ZVLPs only induced weak ZEBOV GP-specific IFN-γ+ SFCs, and ZGP induced little ZEBOV GP-specific IFN-γ+ SFCs (Figs. and ). We also measured IFN-γ+ CD8+ T cells in mice that received either one immunization of the higher dosage or two immunizations of the lower dosages. rAd2-ZGP induced the most robust GP-specific IFN-γ+ CD8+ T cell responses compared with ZGP and ZVLPs (Figs. and , and Supplementary Figure S3).
To assess whether the CMI response is cross-reactive to heterologous SEBOV, mouse splenocytes were stimulated with purified ZEBOV GP or SEBOV GP. Although the use of GP protein is not as efficient as that of GP peptides for measuring GP-specific IFN-γ+ SFCs, rAd2-ZGP appeared to induce IFN-γ+ SFCs responsive to ZEBOV GP, and rAd2-ZGP also elicited, to a less extent, IFN-γ+ SFCs responsive to SEBOV GP (Figs. and ). In contrast, ZGP and ZVLPs induced no significant IFN-γ+ SFCs responsive to SEBOV GP. These results suggested that rAd2-ZGP could elicit strong CMI responses, including IFN-γ+CD8+ T cell responses to autologous ZEBOV. rAd2-ZGP can also elicit cross-reactive CMI responses to heterologous SEBOV in immunized mice.
rAd2-ZGP induced robust antibody responses to homologous ZEBOV and heterologous SEBOV in rhesus macaques
To evaluate the antibody response elicited by rAd2-ZGP in NHPs, Chinese rhesus macaques were injected intramuscularly with 1 × 1011 vp rAd2-ZGP, 400 µg ZGP, 400 µg ZVLPs, or PBS. The same macaque received a booster immunization of the same vaccine 4 weeks after the first immunization (Fig. ). rAd2-ZGP rapidly induced a high level of IgG antibodies that bind to ZEBOV GP 2 weeks after one immunization, whereas ZGP and ZVLPs required two immunizations to elicit comparable antibody responses (Fig. , Table ). rAd2-ZGP generated a high titer of neutralizing antibodies against autologous ZEBOV 2 weeks after one immunization (Fig. , Supplementary Figure S4A, Table ). Although no significant enhancement in ZEBOV GP-binding antibodies was observed, a booster immunization with rAd2-ZGP further increased the titer of neutralizing antibodies against ZEBOV (Fig. , Supplementary Figure S4B, Table ). In contrast, ZGP and ZVLPs generated weaker GP-binding antibodies and neutralizing antibodies than did rAd2-ZGP after one immunization. Even after a booster immunization, the neutralizing antibodies elicited by ZGP and ZVLPs were still lower than those by rAd2-ZGP (Fig. , Supplementary Figure S4A-B, Table ). Consistent with the results in mice, rAd2-ZGP also induced a cross-reactive antibody response to SEBOV in rhesus macaques. Binding antibodies to SEBOV GP and neutralizing antibodies to SEBOV were detected in macaques 2 weeks after one immunization with rAd2-ZGP and increased further after a booster immunization, but at a much lower magnitude than to ZEBOV (Fig. , Supplementary Figure S4C-D, Table ). In contrast, ZGP and ZVLPs generated little neutralizing antibodies to SEBOV even after two immunizations (Fig. , Supplementary Figure S4C-D, Table ). These results demonstrated that rAd2-ZGP can effectively and rapidly generate antibody responses to autologous ZEBOV. rAd2-ZGP can elicit some cross-reactive GP-binding antibodies and neutralizing antibodies to heterologous SEBOV.
a Chinese rhesus macaques were divided into four groups and immunized intramuscularly with either 1 × 1011 vp rAd2-ZGP, 400 µg ZGP, 400 µg ZVLPs, or PBS. After 4 weeks, macaques received a booster immunization of the same vaccine. b At 0, 2, 4, and 6 weeks after the first immunization, serum samples were collected and subjected to ELISA analysis of IgG antibodies to ZEBOV GP and SEBOV GP. The titers were calculated as reciprocal endpoints. Control serum samples were run each time the assay was performed. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. c Serum samples were measured for the neutralizing activities to ZEBOV GP pseudo-typed lentivirus or SEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 4). d PBMCs were collected 2 weeks after the first and second immunizations and were stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million PBMCs. e PBMCs were collected at 2 weeks after each immunization. PBMCs were stimulated with either ZEBOV GP or SEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million PBMCs. f Qualitative profiles of ZEBOV GP-specific CD8+ T cell responses in PBMCs at 2 weeks after a booster immunization. GP-specific CD8+ T cells secreting IFN-γ+, IL-2, and TNF-α were determined with ICS analysis. Data are presented as the mean ± SD (n = 4). Comparisons between groups were performed by one-way ANOVA; comparisons of the neutralizing antibody titers between prime and booster immunizations in the rAd2-ZGP group were performed by Student’s t test. A P-value < 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001
Reciprocal ELISA titers and neutralizing antibody titers 2 weeks after each immunization in rhesus macaques
rAd2-ZGP elicited cell-mediated immune responses to autologous ZEBOV and heterologous SEBOV in rhesus macaques
To evaluate CMI responses elicited by rAd2-ZGP in NHPs, GP-specific enzyme-linked immunospot (ELISpot) assay and intracellular cytokine staining (ICS) assays were performed using freshly isolated peripheral blood mononuclear cells (PBMCs) from these rhesus macaques (Fig. ). After the first immunization, macaques immunized with rAd2-ZGP developed the strongest IFN-γ+ ELISpot response to ZEBOV GP peptides, which were further enhanced after a booster immunization, whereas macaques immunized with ZGP and ZVLPs only generated a weak IFN-γ+ ELISpot response after a booster immunization (Fig. ). To assess whether the CMI response was cross-reactive to heterologous SEBOV, macaque PBMCs were stimulated with either autologous ZEBOV GP or heterologous SEBOV GP protein. rAd2-ZGP generated IFN-γ+ SFCs responsive to ZEBOV GP, especially after a booster immunization (Fig. ). rAd2-ZGP also induced, but to a less extent, IFN-γ+ SFCs responsive to SEBOV GP. In contrast, ZGP and ZVLPs induced no significant cross-reactive CMI response to SEBOV GP. Given that antigen-specific CD8+ T cells secreting cytokines play important roles in the clearance of EBOV-infected cellsCitation13, Citation18, Citation26, we further analyzed ZEBOV GP-specific CD8+ T cells for the production of Th1 cytokines, including IFN-γ, IL-2, and TNF-α, by an ICS assay using a flow cytometer. Macaques immunized with rAd2-ZGP produced ZEBOV GP-specific CD8+ T cells producing IFN-γ, IL-2, and TNF-α (Fig. , Supplementary Figure S5). In contrast, macaques immunized with ZGP and ZVLPs generated IFN-γ-, IL-2-, and TNF-α-secreting CD8+ T cells at much lower levels (Fig. , Supplementary Figure S5). We also measured IFN-γ+ ELISpot responses to ZEBOV GP peptides, ZEBOV GP, and SEBOV GP in macaques immunized with rAd2 vector carrying an unrelated Zika virus antigen, rAd2-zika, and we found no significant GP-specific CMI response in macaques immunized with rAd2-zika (Supplementary Figure S6).
Taken together, these results demonstrate that rAd2-ZGP could elicit significant CMI responses against autologous ZEBOV and generate cross-reactive CMI responses to heterologous SEBOV.
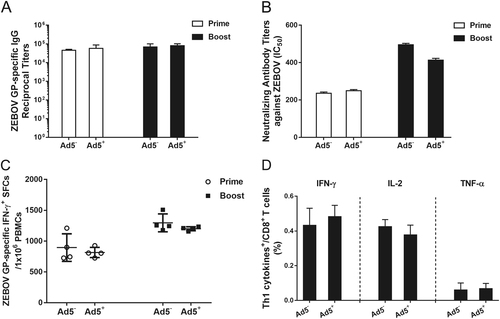
rAd2-ZGP could induce antibody and cell-mediated immune responses in Ad5-seropositive rhesus macaques
To investigate whether rAd2-ZGP is effective in inducing GP-specific immune responses in subjects who have pre-existing anti-Ad5 neutralizing antibodies, rhesus macaques that had been previously immunized with rAd5 were immunized with 1 × 1011 vp rAd2-ZGP followed by a booster immunization 4 weeks later. These Ad5-seropositive macaques were injected with Ad5 over 1 year ago and had Ad5 neutralizing antibody (mean titer 1:2630) but little cross-neutralization to Ad2 (mean titer 1:173) before immunization with rAd2-ZGP (Supplementary Table S1). rAd2-ZGP-induced GP-specific antibody and CMI responses were compared with Ad5-seronegative macaques immunized with the same immunization regimen. rAd2-ZGP induced similar levels of GP-specific IgG antibodies and neutralizing antibodies to ZEBOV in Ad5-seropositive macaques as in Ad5-seronegative macaques (Fig. ). Comparable IFN-γ+ SFCs were detected in macaques with or without pre-existing anti-Ad5 neutralizing antibodies (Fig. ), suggesting that pre-existing anti-Ad5 immunity did not affect the efficacy of rAd2-ZGP in inducing ZEBOV GP-specific CMI responses. rAd2-ZGP provoked similar CD8+ T cell responses with a Th1 cytokine profile of IFN-γ+, IL-2, and TNF-α. Pre-existing anti-Ad5 neutralizing antibodies did not dampen the immunogenicity of rAd2-ZGP. Therefore, rAd2-ZGP is similarly effective in inducing GP-specific antibody and CMI responses in both Ad5-seronegative and Ad5-seropositive subjects.
Chinese rhesus macaques seropositive for Ad5 neutralizing antibodies were immunized intramuscularly with 1 × 1011 vp rAd2-ZGP. After 4 weeks, these macaques received a booster immunization of the same vaccine. a Serum samples were collected and subjected to ELISA analysis of IgG antibodies that bind to ZEBOV GP. The titers were calculated as reciprocal endpoints. Control serum samples were run every time the assay was performed. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. b Serum samples were measured for the neutralizing activities to ZEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 4). c PBMCs were collected 2 weeks after each immunization. PBMCs were stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million PBMCs. Data are presented as the mean ± SD (n = 4). d Qualitative profiles of ZEBOV GP-specific CD8+ T cell responses in PBMCs 2 weeks after a booster immunization. GP-specific CD8+ T cells secreting IFN-γ, IL-2, and TNF-α were determined with ICS analysis. Data are presented as the mean ± SD (n = 4)
Discussion
Preventing EBOV epidemic outbreaks requires prophylactic vaccines that rapidly induce protective host immune responses, including neutralizing antibody and cytotoxic lymphocyte responsesCitation17–Citation19. The trimeric surface GP of EBOV, which mediates viral entry and contains the major epitopes for neutralizing antibodies, has been adopted as the principal antigen for EBOV vaccines, including those based on purified GPCitation27, VLPsCitation28, DNA vectorCitation29 and recombinant viral vectors such as recombinant adenoviral vectors and the vesicular stomatitis viral vectorCitation9–Citation11, Citation14, Citation30, Citation31. These vaccine candidates, tested individually in different laboratories, exhibited varying degrees of immunogenicity and protective efficacy in mice, guinea pigs, and NHPsCitation32.
In this study, we constructed rAd2-ZGP, a recombinant replication-incompetent rAd2-vectored EBOV vaccine. We investigated in two animal species the antibody and CMI responses induced by rAd2-ZGP and, in parallel, by two protein-based EBOV vaccines, ZGP and ZVLPs. We found the following: (i) rAd2-ZGP generated robust GP-binding IgG antibody and neutralizing antibody responses to autologous ZEBOV (Figs. –); (ii) rAd2-ZGP elicited robust GP-specific CMI responses with CD8+ T cells secreting Th1 cytokines in rhesus macaques, whereas ZGP and ZVLPs induced weak CMI responses to EBOV (Fig. ); (iii) rAd2-ZGP, but not ZGP or ZVLPs, induced some cross-reactive antibody and CMI responses to heterologous SEBOV (Figs. –); and (iv) pre-existing anti-Ad5 immunity showed no attenuation of the immunogenicity of rAd2-ZGP (Fig. ).
Replication-incompetent adenovirus vectors showed good performance in inducing antibody and T cell responses, which both play important roles in the protection of rAd-based vaccines against EBOV infectionCitation17–Citation19. Recently, an rAd5-vectored EBOV vaccine was proved to be safe and immunogenic in clinical trials and was approved by the Chinese FDA (NCT02326194)Citation14, Citation15. However, the declining tendency of the immune response at 6 months after rAd5-GP immunization in vaccinees suggested that repeated immunization might be needed to boost and ensure protection in humansCitation14. In this study, we demonstrated that an EBOV vaccine based on rAd2, which has also been used extensively in human gene therapy trials and has good safety records in humans, could induce robust antibody and CMI responses against autologous EBOV in mice and rhesus macaques (Figs. –). These antibody and CMI responses also showed significant cross-reactivity to a heterologous SEBOV (Figs. –). These results suggested that rAd2-ZGP, if proved to be safe and protective in further preclinical and clinical studies, could be a potential candidate vaccine to prevent EBOV infection.
Previous studies suggested that rAd-vectored EBOV vaccines based on different serotypes might exhibit different levels of immunogenicity and therefore might differ in protective efficacyCitation26, Citation31. An rAd5-based EBOV vaccine conferred effective protection against EBOV infection in rhesus macaquesCitation13, whereas rAd26- or rAd35-based vaccines did not exhibit the same level of protectionCitation31. One possible explanation is that these adenovirus serotypes have different cell tropisms and might use different cell surface receptors for viral entry, which could affect the target cell preference and therefore the antigen expression and presentationCitation33. We selected Ad2 because it shares the same cellular receptor with Ad5 and might thus have similar cell tropism as well as immunogenicity to rAd5-based vaccinesCitation34. Ad2 and Ad5 belong to adenovirus subgroup C, use the coxsackievirus and adenovirus receptors for virus attachment and use the αvβ3 or αvβ5 integrins for virus internalizationCitation35. rAd2-ZGP induced high levels of GP-binding and neutralizing antibodies in mice and rhesus macaques, which is comparable to the results in other studies using rAd5-GPCitation6, Citation13. Because this level of immune response showed complete protection against EBOV challenge in the context of an rAd5-based vaccine, rAd2-ZGP might have similar protective effects against EBOV infectionCitation6, Citation9, Citation13, Citation18. The antibody and CMI responses induced by rAd2-ZGP also showed some cross-reactivity to heterologous SEBOV, which has not been reported for rAd5-vectored EBOV vaccines. These features can be valuable for vaccines to be used in an EBOV epidemic region with multiple circulating strainsCitation36. Importantly, the rapid generation of protective immune responses rendered rAd2-ZGP suitable for pre- and post-exposure immunization in the case of an EBOV outbreak.
There is a report that purified GP provided only partial protection against EBOV infection when given alone or as a booster vaccine with a DNA vaccineCitation37, but GP could protect rodents from lethal EBOV challenge when fused with an Fc fragment or formulated as Ebola immune complexesCitation27, Citation38. Our study also showed that the GP protein-based vaccine primarily induced an antibody response that was limited to autologous ZEBOV without significant CMI responses (Figs. –). Further modifications of the protein antigen or its combination with potent adjuvants might be considered to enhance the immunogenicity and protective efficacy of GP protein-based vaccines. ZVLPs, consisting of GP and matrix protein VP40, have been shown to induce EBOV-specific antibody and CMI responses in mice and NHPsCitation28, Citation39. Immunization with a mammalian-derived VLP conferred protection against autologous EBOV in both small animals and NHPs, but much larger dosages, multiple immunizations and the presence of adjuvants were requiredCitation39, Citation40. In our study, ZVLPs could elicit CMI responses, but the responses were much weaker than those for rAd2-ZGP (Figs. –), possibly due to the low dosage, the limited immunization, and the absence of adjuvants. Therefore, ZVLPs alone cannot elicit efficient protective immune responses after a single immunization and might not be effective in inducing cross-reactive neutralizing antibodies and CMI responses.
Although the exact mechanisms of protection of an effective EBOV vaccine remain to be clarified, it is likely that neutralizing antibody and CMI responses play important roles in controlling viral infection. In this study, we found that a significant proportion of ZEBOV GP-specific CD8+ T cells secreting IFN-γ, IL-2, and TNF-α, which have been shown to be associated with protection, were generated in macaques immunized with rAd2-ZGP (Fig. ). This result highlighted the property of rAd2-ZGP in eliciting Th1-biased T cell responses, which might contribute to the clearance of EBOV-infected host cells.
Pre-existing anti-Ad5 immunity is a concern for the application of rAd5-vectored vaccines because it could attenuate the rAd5-mediated antigen expression and therefore decrease the immunogenicityCitation41. In some African countries, ~90% of residents are seropositive for anti-Ad5 neutralizing antibodiesCitation16. This high seroprevalence might weaken the capacity of rAd5-vectored vaccines in inducing EBOV-specific immunity, as observed in a clinical trial conducted in this population in comparison with other populations with relatively lower anti-Ad5 neutralizing antibody seroprevalenceCitation14, Citation15. The rAd2-ZGP described in this study, however, could potentially circumvent the pre-existing anti-Ad5 neutralizing antibodies. rAd2-ZGP induced robust GP-specific neutralizing antibody and CMI responses in macaques with pre-existing anti-Ad5 immunity (Fig. ). The antibody and CMI responses were comparable to those in Ad5-seronegative macaques. Although Ad2 and Ad5 might co-circulate in populations of developing countries, their neutralizing antibodies do not cross-neutralize each other. People who are seropositive for anti-Ad5 neutralizing antibodies are not always seropositive for anti-Ad2 neutralizing antibodiesCitation25. Therefore, rAd2-ZGP might be considered an alternative EBOV vaccine candidate for people with pre-existing anti-Ad5 neutralizing antibodies or could be used as a booster vaccine for people who have been previously immunized with an rAd5-based vaccine.
We also noted that a booster immunization with rAd2-ZGP did not significantly increase ZEBOV GP-specific binding antibodies, which was similar to the findings of a previous study by another laboratoryCitation6. However, a booster immunization with rAd2-ZGP enhanced the titer of neutralizing antibodies against ZEBOV (Fig. ), suggesting that a booster immunization can increase the quality of GP-specific antibodies. The booster immunization also significantly increased the cross-reactive binding antibodies to SEBOV GP (Fig. ). Moreover, the CMI responses to ZEBOV and SEBOV were increased after a booster immunization (Fig. ). These results suggested that although the pre-existing anti-Ad2 antibodies might exert some dampening effects on repeated immunization with rAd2-ZGP, the quality of antibody responses and the quantity of CMI responses can still be enhanced.
We generated an rAd2-vectored EBOV vaccine expressing GP and evaluated its immunogenicity in mice and rhesus macaques. We showed that rAd2-ZGP induced robust GP-specific antibody and CMI responses to autologous EBOV, even in the presence of anti-Ad5 immunity. Importantly, immune responses induced by rAd2-ZGP showed cross-reactivity to heterologous SEBOV. The rapid generation of EBOV-specific immune responses renders rAd2-ZGP suitable for pre- and post-exposure immunization in the case of an EBOV outbreak. rAd2-ZGP is worth further investigating in EBOV-challenged NHP models.
Materials and methods
Ethics statement
All animal experiments were performed at the Animal Experimental Center of the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences. The experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC# 2015006). The Chinese rhesus macaques that participated in this study were free of simian immunodeficiency virus, simian T lymphotropic virus type 1, and simian retrovirus.
Vaccine preparation
rAd2-ZGP, a replication-incompetent adenovirus type 2 carrying the EBOV glycoprotein-encoding gene, was constructed as previously describedCitation42. ZGP, the coding sequence for GP from the contemporary ZEBOV Makona strain (GenBank accession number KJ660346), was codon-optimized for mammalian expression and synthesized (Genscript Inc., Nanjing, China). The ZGP gene was placed into the adenovirus shuttle vector pGA1 to obtain pGA1-ZGP. The pGA1-ZGP was linearized and subjected to homologous recombination with the linearized Ad2 backbone with deletion of the E1 and E3 genes in competent E. coli BJ5183 cells (Invitrogen, CA, USA). The resultant adenoviral plasmid pAd2-ZGP was linearized and transfected into HEK293 cells to rescue rAd2-ZGP. To generate purified ZGP, the coding sequence corresponding to residues 33–311 and 464–632 of GP from the ZEBOV Makona strain (GenBank accession number KJ660346) was integrated into a baculovirus vector, with a gp67 signal sequence fused to the N-terminus. ZGP protein was expressed in Sf9 cells and purified as previously describedCitation8. To generate ZVLPs, a recombinant baculovirus expressing full-length GP and VP40 from the ZEBOV Makona strain (GenBank accession number KJ660346) was generated and produced in Sf9 cellsCitation43. ZVLPs were purified through a discontinuous sucrose gradient (10, 30, and 50%) at 28,000 × g for 90 min at 4 °C. For morphology analysis, the ZVLP sample was set on a carbon-coated copper grid, stained with 1% phosphotungstic acid and examined on a Tecnai G2 Spirit (FEI, OR, USA) at an accelerating voltage of 120 kV.
Western blot analysis of GP
To analyze the rAd2-ZGP-mediated expression of GP, 1 × 106 Vero cells were infected by rAd2-ZGP at 5 × 109 viral particles (vp). At 12, 24, 36, 48, 60, and 72 h post infection, the cells were harvested. One-tenth of the rAd2-ZGP-infected cell lysates and GP standard samples (0.08, 0.16, 0.32, 0.64, 1.28, and 2.56 µg) were subjected to SDS-PAGE followed by western blot analysis. To verify the GP component in the candidate vaccines, the rAd2-ZGP-infected cell lysates at 72 h post infection, purified ZGP and ZVLPs were subjected to SDS-PAGE followed by western blot analysis with anti-GP polyclonal antibodies (Sino Biological, China) and anti-VP40 antibodies (Abcam, MA, USA).
Evaluation of immunogenicity of vaccine candidates in mice
Seven-week-old female Balb/c mice were injected intramuscularly with two immunization strategies as follows: (1) single immunization with either a lower dosage or a higher dosage of each vaccine candidate, including rAd2-ZGP (5 × 108 vp or 5 × 109 vp per mouse in phosphate-buffered saline, PBS), ZGP (2 or 20 µg per mouse with alum), ZVLPs (2 or 20 µg per mouse in PBS), and rAd2 empty vector (rAd2-Empty, 5 × 108 vp or 5 × 109 vp per mouse in PBS), and (2) prime-boost immunization with a lower dosage of each vaccine candidate, including rAd2-ZGP (5 × 108 vp per mouse in PBS), ZGP (2 µg per mouse with alum), ZVLPs (2 µg per mouse in PBS), and rAd2-Empty (5 × 108 vp per mouse in PBS). At 3 weeks after the first immunization, blood samples were collected. Mice receiving a single immunization with the higher dosages were euthanatized for isolation of splenocytes for further analysis. At 6 weeks after the first immunization, mice receiving a lower dosage of vaccine candidates were boosted similarly as with the first immunization. At 3 weeks after the booster immunization, the mice were euthanatized. Serum samples were collected, and splenocytes were isolated for further analysis.
Evaluation of immunogenicity of vaccine candidates in rhesus macaques
Twenty 4–6-year-old Chinese rhesus macaques were randomly divided into five groups with both male and female macaques in each group. Each group received two intramuscular vaccinations at 4-week intervals as follows: (1) four macaques received 1 ml PBS as a mock treatment control; (2) four macaques who had not been exposed to adenovirus received two doses of rAd2-ZGP (1 × 1011 vp in PBS); (3) four Ad5-seropositive macaques, who were immunized with rAd5 over 1 year ago, received two doses of rAd2-ZGP (1 × 1011 vp in PBS); (4) four macaques received two doses of purified ZGP (400 µg in PBS with alum); and (5) four macaques received two doses of ZVLPs (400 µg in PBS). Blood samples were collected, and the serum samples and peripheral blood mononuclear cells were isolated for further analysis as previously describedCitation44. Macaques receiving rAd2-ZGP were tested for serum neutralizing antibodies to Ad2 and Ad5 before and after immunization of rAd2-ZGP.
Enzyme-linked immunosorbent assay
EBOV GP-specific binding antibodies were analyzed by enzyme-linked immunosorbent assay (ELISA) as described previouslyCitation45. In brief, 96-well flat-bottom plates were coated with 100 µl purified EBOV GP at 1 µg/ml in PBS at 4 °C overnight. After washing and blocking, serum samples were serially diluted in twofold increments and added in duplicate. After incubation at room temperature for 2 h, the plates were washed, and HRP-labeled secondary antibody was added (Proteintech, IL, USA). After incubation for another 1 h at room temperature, the plates were washed and developed with TMB/E substrate (Merck Millipore, MA, USA). Finally, the reaction was stopped, and the OD450 values were read. The ELISA titers were calculated as a reciprocal endpoint. A negative serum control was run each time the assay was performed. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the negative sera plus 3 standard deviations (SDs).
Micro-neutralization assay
The micro-neutralization (MN) assays were performed using lentivirus pseudo-typed with EBOV GP as previously describedCitation45. In brief, EBOV GP pseudo-typed lentiviruses were prepared by co-transfection of the HIV-1 proviral vector pNL4–3.Luc.R-E− and expression vectors encoding GP from the ZEBOV Makona strain (GenBank accession number KJ660346) or GP from SEBOV (GenBank accession number KR063670) into 293T cells. Subsequently, the pseudo-typed viruses were harvested, titered, and then mixed with serially diluted serum samples at 100 TCID50 per well. After incubation at 37 °C for 30 min, 90–100% confluent Huh-7 cells were infected with the mixture for 4 h. Then, the infection mixture was replaced with complete DMEM medium. After 48 h of incubation, the luciferase activity was detected with a Luciferase Assay System (Promega, WI, USA). The neutralizing activity was measured as the decrease of luciferase expression relative to negative control sera. The half maximal inhibitory concentration (IC50) values were then calculated as the reciprocal of the serum dilution at which 50% neutralization was achieved using GraphPad Prism 7.00Citation13.
The titers of neutralizing antibodies against Ad5 and Ad2 were detected with an MN assay as previously describedCitation46. Briefly, HEK293 cells were seeded into 96-well plates at 3 × 104 cells per well. After 24 h, serial dilutions of heat-inactivated serum samples were incubated with Ad2-SEAP or Ad5-SEAP at 4 × 106 vp per well for 1 h at 37 °C. The mixtures were added to the 96-well plates and incubated for 24 h at 37°C. Subsequently, supernatants were harvested, and the SEAP activity was detected using a Phospha-Light System according to the manufacturer’s instructions (Thermo Fisher Scientific, IL, USA). The relative light units (RLUs) were recorded, and the neutralizing titers were calculated as the reciprocal dilutions that inhibited 50% of the RLU values.
ELISpot assay
IFN-γ ELISpot assays were performed using freshly isolated splenocytes or PBMCs. In brief, sterile 96-well microtiter plates (Merck Millipore, MA, USA) were coated with IFN-γ coating antibody (R&D Systems, MN, USA) at 4 °C overnight. The plates were then washed and blocked at 37 °C for 2 h. Mouse splenocytes or monkey PBMCs were isolated using a density gradient medium (LymphoprepTM, Vancouver, Canada), seeded at 5 × 105 cells per well and stimulated with a peptide pool of GP from the ZEBOV Makona strain (GenBank accession number KJ660346) (Chinese Peptide Company, China) at 2 µg/ml per peptide or with ZEBOV GP or SEBOV GP (GenBank accession number AKB09538) (Sino Biological, China) at 20 µg/ml. After incubation for 24 h, the plates were incubated with biotinylated detection antibodies and developed with alkaline phosphatase-conjugated streptavidin (BD PharMingen, CA, USA) and NBT/BCIP reagent (Pierce, IL, USA). Finally, the spots were counted with an ELISpot reader (Bioreader 4000, BIOSYS, Germany).
ICS assay
ICS assays were performed as previously describedCitation5. In brief, freshly isolated mouse splenocytes or monkey PBMCs were seeded into 96-well plates (2 × 106 cells per well) and incubated with an EBOV GP peptide pool (2 µg/ml). One hour later, brefeldin A (BD Phamingen, CA, USA) was added and incubated for another 10 h. The cells were then harvested, stained with surface antibodies (CD3-Pacific blue, CD8-APC-Cy7, CD4-FITC; BD Biosciences, CA, USA) for 1 h, then permeabilized and stained with intracellular antibodies (IFN-γ-PE, IL-2-APC, TNF-α-PE-Cy7; BD Biosciences, CA, USA). Finally, the cells were detected with an LSR Fortessa SORP instrument (BD Biosciences, CA, USA).
Statistical analysis
Flow cytometric data were analyzed using FlowJo version 7.6 (Tree Star, Inc., Ashland, USA). Statistical analyses and graphical presentations were conducted with GraphPad Prism version 6.0 (GraphPad Software, Inc., CA, USA). Differences among groups were tested with one-way ANOVA. Differences in the same vaccine groups were determined by Student’s t test. Throughout the text, figures, and legends, the following terminology is used to show statistical significance: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Data availability
The authors declare that all relevant data are available from the corresponding author upon request.
Supplementary Table S1
Download PDF (84.2 KB)Supplementary Figure S1
Download PDF (244.3 KB)Supplementary Figure S2
Download PDF (157.7 KB)Supplementary Figure S3
Download PDF (206.2 KB)Supplementary Figure S4
Download PDF (161.2 KB)Supplementary Figure S5
Download PDF (419.1 KB)Supplementary Figure S6
Download PDF (135.2 KB)Acknowledgements
We thank the staff at the Animal Center of GIBH for their excellent technical assistance. This study was supported by the National Natural Science Foundation of China (Nos. 31470892 and 91442102), the National Key Research and Development Project (2016YFC1200900), the National Science and Technology Major Project of the Ministry of Science and Technology of China (2015ZX09102025002), the Guangzhou Health Care and Cooperative Innovation Major Project (Nos. 201508020252 and 201803040004), and a grant of the CAS Youth Innovation Promotion Association to Liqiang Feng (No. 2014328).
Author contributions
L.C., L.F., and Y. F. conceived and designed this study. Y.F., C.L., P.H., Q.W., X.Z., Y.Z., Y.S., S.Y., C.Y., Y.F., C.W., and L.Q. performed the experiments and collected and analyzed the data. L.C., L.F., and Y. F. wrote the manuscript. W.X., Y.L., C.S., F.G.G. and X.X. read and revised the manuscript. All authors edited and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41426-018-0102-5).
References
- FeldmannHGeisbertTWEbola haemorrhagic feverLancet2011377 849 86210.1016/S0140-6736(10)60667-83406178
- FeldmannHJonesSKlenkHDSchnittlerHJEbola virus: from discovery to vaccineNat. Rev. Immunol.2003367768510.1038/nri1154
- BaizeSEmergence of Zaire Ebola virus disease in GuineaN. Engl. J. Med.20143711418142510.1056/NEJMoa1404505
- HoelscherMADevelopment of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in miceLancet200636747548110.1016/S0140-6736(06)68076-82762105
- ZhangYEffects of the fusion design and immunization route on the immunogenicity of Ag85A-Mtb32 in adenoviral vectored tuberculosis vaccineHum. Vaccin. Immunother.2015111803181310.1080/21645515.2015.10421934514253
- SullivanNJAccelerated vaccination for Ebola virus haemorrhagic fever in non-human primatesNature200342468168410.1038/nature01876
- CreechCBRandomized, placebo-controlled trial to assess the safety and immunogenicity of an adenovirus type 35-based circumsporozoite malaria vaccine in healthy adultsHum. Vaccin. Immunother.201392548255710.4161/hv.260384162066
- WangHEbola viral glycoprotein bound to its endosomal receptor Niemann-pick C1Cell201616425826810.1016/j.cell.2015.12.044
- WuSAn adenovirus vaccine expressing Ebola virus variant makona glycoprotein is efficacious in guinea pigs and nonhuman primatesJ. Infect. Dis.2016214S326S33210.1093/infdis/jiw2505050474
- MilliganIDSafety and immunogenicity of novel adenovirus type 26– and modified vaccinia ankara–vectored ebola vaccines: a randomized clinical trialJAMA20163151610162310.1001/jama.2016.4218
- TapiaMDUse of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trialLancet Infect. Dis.201616314210.1016/S1473-3099(15)00362-X4700389
- LedgerwoodJEA replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adultsVaccine20102930431310.1016/j.vaccine.2010.10.037
- SullivanNJCD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primatesNat. Med.2011171128113110.1038/nm.2447
- ZhuFCSafety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trialLancet201638962162810.1016/S0140-6736(16)32617-4
- ZhuFCSafety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trialLancet20153852272227910.1016/S0140-6736(15)60553-0
- XiangZChimpanzee adenovirus antibodies in humans, sub-Saharan AfricaEmerg. Infect. Dis.2006121596159910.3201/eid1210.0600783290939
- WongGImmune parameters correlate with protection against ebola virus infection in rodents and nonhuman primatesSci. Transl. Med.2012414615810.1186/1479-5876-10-146
- SullivanNJMartinJEGrahamBSNabelGJCorrelates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal ruleNat. Rev. Microbiol.2009739340010.1038/nrmicro2129
- ChoiJHA single dose respiratory recombinant adenovirus-based vaccine provides long-term protection for non-human primates from lethal Ebola infectionMol. Pharm.2015122712273110.1021/mp500646d
- RichardsonJSEnhanced protection against Ebola virus mediated by an improved adenovirus-based vaccinePLoS ONE20094e530810.1371/journal.pone.00053082669164
- SunCEpidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern ChinaVaccine2011293837384110.1016/j.vaccine.2011.03.042
- SumidaSMNeutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon proteinJ. Immunol.20051747179718510.4049/jimmunol.174.11.7179
- ChroboczekJBieberFJacrotBThe sequence of the genome of adenovirus type 5 and its comparison with the genome of adenovirus type 2Virology199218628028510.1016/0042-6822(92)90082-Z
- Fernandes, P., Silva, A. C., Coroadinha, A. S. & Alves, P. M. in David T. Curiel Adenoviral Vectors for Gene Therapy (Second Edition) Ch. 6 (Academic Press, San Diego, 2016).
- LiQNeutralizing antibodies against adenovirus type 2 in normal and HIV-1-infected subjects: implications for use of Ad2 vectors in vaccinesHum. Vaccin. Immunother.2017131810.1080/21645515.2017.1278976
- StanleyDAChimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challengeNat. Med.2014201126112910.1038/nm.3702
- KonduruKEbola virus glycoprotein Fc fusion protein confers protection against lethal challenge in vaccinated miceVaccine2011292968297710.1016/j.vaccine.2011.01.1133070761
- WarfieldKLEbola virus-like particles protect from lethal Ebola virus infectionProc. Natl Acad. Sci. USA2003100158891589410.1073/pnas.2237038100307663
- LiWCharacterization of immune responses induced by Ebola virus glycoprotein (GP) and truncated GP isoform DNA vaccines and protection against lethal Ebola virus challenge in miceJ. Infect. Dis.2015212S39840310.1093/infdis/jiv1864564543
- MarziAVSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strainScience201534973974210.1126/science.aab3920
- GeisbertTWRecombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against Ebola virus challengeJ. Virol.2011854222423310.1128/JVI.02407-103126236
- KeshwaraRJohnsonRFSchnellMJToward an effective Ebola virus vaccineAnnu. Rev. Med.20176837138610.1146/annurev-med-051215-030919
- BarouchDHImmunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunityJ. Immunol.20041726290629710.4049/jimmunol.172.10.6290
- ParksREveleghCGrahamFUse of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administrationGene Ther.199961565157310.1038/sj.gt.3300995
- ZhangYBergelsonJMAdenovirus receptorsJ. Virol.200579121251213110.1128/JVI.79.19.12125-12131.20051211528
- ParkDJEbola virus epidemiology, transmission, and evolution during seven months in Sierra LeoneCell20151611516152610.1016/j.cell.2015.06.0074503805
- Mellquist-RiemenschneiderJLComparison of the protective efficacy of DNA and baculovirus-derived protein vaccines for EBOLA virus in guinea pigsVirus Res.20039218719310.1016/S0168-1702(02)00338-6
- PhoolcharoenWA nonreplicating subunit vaccine protects mice against lethal Ebola virus challengeProc. Natl Acad. Sci. USA2011108206952070010.1073/pnas.11177151083251076
- WarfieldKLEbola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challengeJ. Infect. Dis.2007196S43043710.1086/520583
- WarfieldKLInduction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infectionJ. Immunol.20051751184119110.4049/jimmunol.175.2.1184
- GilbertSCAdenovirus-vectored Ebola vaccinesExpert. Rev. Vaccin.2015141347135710.1586/14760584.2015.1077122
- XiaoLEnhancement of SIV-specific cell mediated immune responses by co-administration of soluble PD-1 and Tim-3 as molecular adjuvants in miceHum. Vaccin. Immunother.20131072473310.4161/hv.273404130272
- ZhengXTreatment with hyperimmune equine immunoglobulin or immunoglobulin fragments completely protects rodents from Ebola virus infectionSci. Rep.2016610.1038/srep241794828711
- SunCJMucosal priming with a replicating-vaccinia virus-based vaccine elicits protective immunity to simian immunodeficiency virus challenge in rhesus monkeysJ. Virol.2013875669567710.1128/JVI.03247-123648167
- ZhangQPotent neutralizing monoclonal antibodies against Ebola virus infectionSci. Rep.2016610.1038/srep258564867612
- ZhengXSeroprevalence of neutralizing antibodies against adenovirus type 14 and 55 in healthy adults in Southern ChinaEmerg. Microbes Infect.2017610.1038/emi.2017.295520307
