Abstract
Enteroviruses infect millions of humans annually worldwide, primarily infants and children. With a high mutation rate and frequent recombination, enteroviruses are noted to evolve and change over time. Given the evidence that human enteroviruses are commonly found in other mammalian species and that some human and animal enteroviruses are genetically similar, it is possible that enzootic enteroviruses may also be infecting human populations. We conducted a systematic review of the English and Chinese literature published between 2007 and 2017 to examine evidence that enteroviruses may be zoonotic. Of the 2704 articles screened for inclusion, 16 articles were included in the final review. The review of these articles yielded considerable molecular evidence of zooanthroponosis transmission, particularly among non-human primates. While there were more limited instances of anthropozoonosis transmission, the available data support the biological plausibility of cross-species transmission and the need to conduct periodic surveillance at the human–animal interface.
These authors contributed equally: Jane K. Fieldhouse, Xinye Wang
These authors contributed equally: Kerry A. Mallinson, Rick W. Tsao
Introduction
Enteroviruses (EVs) are positive-sense, single-stranded RNA viruses in the family Picornaviridae that infect millions of people worldwide on an annual basis, especially infants and children under the age of oneCitation1. More than 300 serotypes of EV have been identified and demonstrated to cause a variety of diseases and morbidityCitation2. Among the most notable EVs known to infect humans are coxsackie-, echo-, polio-, and rhinoviruses. While a majority of non-polio EV infections are asymptomatic or cause mild respiratory disease, more severe disease outcomes such as aseptic meningitis, acute flaccid paralysis (AFP), and acute hemorrhagic conjunctivitis are associated with certain EV types. Enterovirus 71 (EV-71), for example, is a type most commonly associated with severe hand, foot, and mouth (HFMD) disease. Enterovirus D68 (EV-D68) is a type that has caused sporadic but severe respiratory disease outbreaks across the United States, Asia, Africa, and Europe in recent yearsCitation3,Citation4.
In 2012, a comparative analysis of phylogenic and genetic classification demonstrated a close relationship between animal and human picornavirusesCitation5. In February 2013, the International Committee on Taxonomy of Viruses (ICTV) approved changes to EV and rhinovirus species names to remove all reference to the host species names. Former species names had been selected based upon the host species from which the virus was originally isolated; however, after many of the human EV species were identified and isolated in non-human hosts, the proposal was made to drop the designation of the viruses as human, bovine, porcine, and simian. Today, EVs are classified into 12 species, including enterovirus A–L (EV-A, B, C, D, E, F, G, H, J, K, and L) and rhinovirus A–C (RV-A, B, and C) (Table ). EVs E–G chiefly cause disease in livestock, such as cattleCitation6 and pigsCitation7, while several simian EVs (e.g., EV-H and EV-J) have been isolated from both captive and wild non-human primates (NHPs)Citation2,Citation8. Enterovirus I has historically been excluded for fear of confusion with enterovirus 1; however, a dromedary camel enterovirus was proposed as a new species of EV I to the ICTV in June 2016Citation9.
Species within the Enterovirus genus
Given the evidence that human EVs may be commonly found in other mammalian species, it is possible EVs that naturally circulate in animal populations may also be infecting human populations. With multiple genotypes, a high mutation rate, and frequent recombinationCitation10, EVs have considerable potential for cross-species infection. Recognizing that the term “zoonoses” can be confusingCitation11, for the purposes of this review, we defined the generic term “zoonoses” as a “two-way street” where a pathogen causing disease might move from either animals to humans or from humans to animalsCitation12,Citation13. Where we sought to be directionally more specific, we employed the term “anthropozoonosis”, which we embraced as “a disease causing pathogen that is transmitted from animals to humans”, and “zooanthroponosis”, sometimes referred to as “reverse zoonoses”, as “a disease causing pathogen that is transmitted from humans to animals.” Therefore, in this report, we sought to review the English and Chinese scientific literature for evidence that EVs may be zoonotic.
Methods
In August 2017, we searched the English literature published between 2007 and 2017 on ProQuest, PubMed, Scopus, and Web of Science databases. The systematic review of Chinese literature published between 2007 and 2017 was conducted in September 2017 on the Chinese National Knowledge Infrastructure (CNKI), Wanfang Data and Weipu Data databases. Both reviews followed the standard systematic review procedures established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). In each of these databases, we empirically chose to search for citations during the last 10 years as molecular methods for virus detections have markedly increased during the last decade as compared to older diagnostic methods such as cell culture and immunoassays. The search was additionally limited to include the following publication categories: review, article, dissertation, thesis, or journal. Articles were included in the search if they mentioned “enterovirus”, “rhinovirus”, a virus that belongs to the EV genus (“coxsackie”, “echovirus”, “poliovirus”, “pleurodynia”, “simian virus 6”, “SV6”, and “unclassified simian virus”), or a disease caused by an EV (“Hand, foot, and mouth disease”, “Bornholm disease”, and “Swine vesicular disease”) as well as a term of interest (“human infection”, “transmiss*”, “transfer”, “cross-species”, “interspecies”, “zoono*”, “anthropono*”, or “zooanthropono*”) in either the title, abstract, or keywords. The search methods were designed to capture any documentation of zoonotic transmission, regardless of the terms used to describe the type of transmission.
The following search string was used for the English databases: (enterovirus* OR rhinovirus* OR coxsackie* OR echovirus* OR poliovirus* OR “Hand, foot, and mouth disease” OR “Bornholm disease” OR pleurodynia OR “Simian virus 6” OR SV6 OR “unclassified simian virus*” OR “Swine vesicular disease”) AND (“human infection” OR transmiss* OR transfer OR cross-species OR interspecies OR zoono* OR anthropono* OR zooanthropono*). Articles published in languages other than English were not considered during the English literature review.
Concurrently with the English literature review, we conducted a systematic review of the Chinese literature due to the high annual disease burden of EVs in China. Between 2008 and 2013 alone, China experienced epidemic outbreaks and saw over 9 million reported cases of HFMD caused predominantly by EV serotypes A71 (EV-A71) and Coxsackievirus A16 (CV-A16)Citation14,Citation15. From a One Health perspective, China is also seeing increasingly frequent and intense contact between humans and domestic livestock largely due to the rapid increase in demand for meat, poultry, and dairy products. Furthermore, habitat loss and illegal wildlife trade have recently increased human exposure to wild animalsCitation16. Conceivably, Chinese populations may be more likely to experience animal EV infections than humans in other societies.
The search terms used for the Chinese literature review were based on the English search terms: (SU = ‘enterovirus (Changbingdu)’ OR SU = ‘coxsackie (Kesaqibingdu)’ OR SU = ‘echovirus (Aikebingdu)’ OR SU = ‘rhinovirus (Bibingdu)’ OR SU = ‘poliovirus (Xiaoermabizheng)’ OR SU = ‘hand, foot, and mouth disease (Shouzukoubing)’ OR SU = ‘bornholm disease (Liuxingxingxiongjitong)’ OR SU = ‘pleurodynia (Xiongmotong)’ OR SU = ‘simian virus (Yuanhoubingdu)’ OR SU = ‘Swine vesicular disease (Zhushuipaobing)’) AND (SU = ‘zoonoses (Renchugonghuan)’ OR SU = ‘human infection (Renganran)’). All articles were in Chinese. The search terms “cross-species”, “anthropono*”, and zooanthropono*” were not included when searching in the Chinese databases as these terms greatly limited the number of articles generated. EndNote X8 was utilized to compile articles and remove duplicates for both the English and the Chinese literature reviews.
Results
Search results
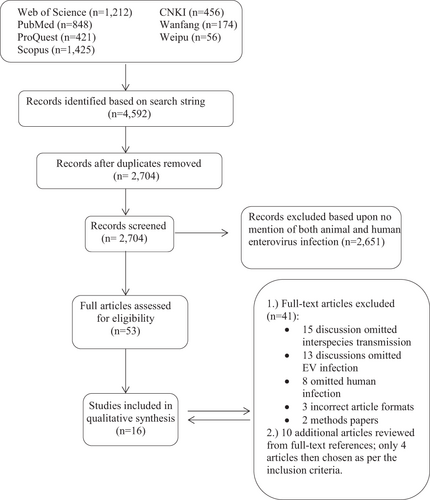
A total of 4592 articles were identified by our search strategy. After duplicates were removed, a total of 2704 articles were screened for inclusion (Fig. ). The title, abstract, and keywords of all English articles captured by the search were independently reviewed by two authors (J.K.F. and R.W.T. reviewed articles by authors A-L; X.W. and K.A.M. reviewed articles by authors M-Z). The abstracts of 686 Chinese articles were reviewed by X.W. Articles that made no mention of both animal and human infection of an EV were not considered for full-text review. Following the screening of both English and Chinese articles, 53 English articles were selected for full-text review, which was conducted independently by each author for inclusion. After the full review, 41 articles were removed due to omission of interspecies transmission, omission of EV infection, omission of human infection, incorrect article format or the article being a methods paper (Fig. ). From the 12 articles selected for final inclusion in the qualitative synthesis, an additional ten articles were identified from the references as potentially significant and were, therefore, reviewed in full. Four of the ten additional articles met the inclusion criteria; thus, a total of 16 English publications were finally chosen in this review (Table ).
Fig. 1 Flow chart of the literature search process. Based on the search strategy, 4592 articles were identified in total, which included 3906 English articles and 686 Chinese articles. Duplicates were removed
Publications found to be important in considering the zoonotic potential of enteroviruses
Five of the 16 manuscripts included in the review were published prior to 2013 when the ICTV approved changes to EV species names. The 16 selected publications described studies conducted across 11 distinct countries with 43% of the studies (n = 7) clustered in Central African countries, 25% (n = 4) in Europe, 12% (n = 2) in the United States, and 19% (n = 3) in Asia. Eleven of the 16 studies (69%) discussed the zooanthroponoses of EVs (primarily among NHPs) and two of the articles (13%) discussed possible evidence of anthropozoonoses; however, through phylogenetic or molecular evidence, all 16 articles included in the final analysis documented instances of animal EV infection in humans or human EV infection in animals.
Evidence of anthropozoonosis
Two of the studies included in the review documented possible evidence of anthropozoonotic infection of humans with animal EVsCitation17. The 2008 Gür et al.Citation17 study collected a total of 3020 serum samples from eight species in different regions of Turkey. A microneutralization test provided serological evidence of bovine enterovirus type 1 (BEV-1) in 74 out of 244 healthy adults (30.3%) in Konya, Turkey. After cattle (64.8%), humans had the highest ratio of seropositivity for BEV-1 compared to four other species that tested positive, including sheep (32.8%), goats (27.6%), horses (12.8%), and dogs (3.2%). As a serological study, this article could not provide evidence of active infection or transmission route; however, based on the previous evidenceCitation18, the authors suggest the possible cause of human BEV-1 infection could be via contact with infected animals and/or contaminated cattle feces. All samples came from reportedly healthy adults living in urban areas, where contact with infected cattle feces was presumably lower than in rural areas. During this systematic review we found no other publication that documented seropositivity for BEV-1 in humans subsequent to this 2008 study.
Grützmacher et al. reported several respiratory disease outbreaks occurring simultaneously among gorilla populations and humans residing in nearby research camps in the Dzanga Sangha Protected Areas of the Central African RepublicCitation19. The study noted that respiratory symptoms manifested in the human population prior to the first respiratory signs being noticed among the gorilla population. In the first outbreak investigated, one of the 16 human specimens (fecal samples and throat swaps) collected tested positive for EV by PCR and viral sequencing. When analyzed using the National Center for Biotechnology Information’s (NCBI) Basic Local Alignment Search Tool (BLAST), the human specimen most similarly matched swine vesicular virus (isolate ITL 2/92 5′ UTR; Accession: AY875991.1) and human EV-71 (isolate 17001, gene for polyprotein, partial cds; Accession: AB575924.1), with 89 and 90% similar identity, respectively. Additionally, one of the seven fecal samples tested positive for an EV with 90% identity to Simian agent 5 (strain B165, complete genome; Accession: AF326751.2) based on the NCBI database. In the third outbreak, 12 out of 25 fecal samples collected from gorillas were positive for EV. Interestingly, sequences of these positive samples were identical with the human EV sequence detected in the first outbreak, which also matched with a high percentage both swine vesicular disease virus (SVDV) and EV-A 71. SVDV is believed to be evolved from coxsackie B virus serotype 5 (CBV-5) and is generally regarded as an animal EVCitation20,Citation21. Although this study does not provide evidence of how humans were infected with animal EVs, these results still demonstrate the possible zoonotic potential of EVs.
Evidence of zooanthroponosis
As compared to the evidence of anthropozoonotic EV transfer from animals to humans, we found that the majority of publications included in the final review (n = 11) demonstrated evidence of zooanthroponosis EV transfer from humans to animals. Ten of these publications documented evidence of human EV infections in NHPs, specifically chimpanzees, gorillas, mandrills, and Old-World monkeys (OWMs) such as macaques and baboons. One publication provided evidence that human EVs might also infect rodentsCitation22.
Interestingly, six of these 11 publications were from studies clustered in Central Africa: three cited studies of NHP EV-infections only in CameroonCitation23–Citation25, one in both Cameroon and the Democratic Republic of the Congo (DRC)Citation26, one in the DRCCitation27, and one in GabonCitation28.
Harvala et al. screened chimpanzee and gorilla stool samples in the jungles of Cameroon where there was reportedly very minimal human contactCitation23. The study found chimpanzee EVs that closely related to human EV-A and -D species in the complete VP1 region, including EV-A76 strains and a new species D type, assigned as EV-D111. Although it is unclear if the EVs detected in these fecal samples were indigenous to the chimpanzee populations or were directly or indirectly transmitted from humans, scholars still provide evidence about the possible cross-species transmission of EVs between humans and NHPs in Cameroon. Subsequently, in their 2014 publication, Harvala et al.Citation26 similarly detected human EVs in stool samples from chimpanzees, gorillas, and bonobos in other forested areas of Cameroon and the DRC, including EV-A76. In addition, the VP4 sequences of viruses isolated from chimpanzees had close identity to EV-A89 and a newly identified species A type (EV-A119) was detected from chimpanzees and gorilla in this study (both VP4 and VP1 sequences). These two types of EVs that have been demonstrated to infect humansCitation24,Citation25,Citation29. Citing the Harvala et al. studiesCitation23,Citation26, Sadeuh-Mba et al. found similar results when characterizing stool specimens collected from both captive and wild NHPs, with 18 out of 21 EVs (including EV-A76, EV-A71, Echovirus-15, Echovirus-29, EV-B82, Coxsackievirus-A13, and Coxsackievirus-A24) detected in chimpanzees and gorillas and one of six monkey-derived EVs reportedly circulating in human populationsCitation25. In their previous study of human EVs circulating in both healthy children and patients with AFP, Sadeuh-Mba et al. isolated several viruses that had been detected in wild chimpanzees, including EV-D111 and EV-A76Citation24. Using viral isolation, RT-PCR and sequencing, the 2014 Sadeuh-Mba et al. publication also provides strong evidence to support the hypothesis that the diversity of EVs in NHPs might be much broader than previously understood in the Harvala et al. studiesCitation23,Citation26. For example, two new EVs, sharing only 55.6–57.9% nucleotide and 52.2–53.2% amino acid VP1 sequence identity with the closest EV (SV6), were identified during this study and proposed as new EV types EV-122 and EV-123.
Mombo et al. was the first report to document that a human EV-C99 was associated with AFP symptoms in a captive chimpanzee in CongoCitation27. Two years later, Mombo et al. screened fecal samples from wild-living primates (include OWMs and apes) and through nucleic acid detection detected a novel human EV-B type (EV-B11) in mandrills, as well as human EV-A type (closely related to EV90) and EV-B type (EV107 and EV-B112) in chimpanzeesCitation28. These findings are all consistent with previously published reports in this review.
Studies across the USCitation30, EuropeCitation31, and AsiaCitation32,Citation33 supported the findings of human EV infection in NHPs, predominantly among captive primates. Interestingly, Oberste et al. found that nearly all EVs detected in synanthropic NHPs in Dhaka, Bangladesh, were human EVs based on VP1 sequencing, whereas only 12.5% of the detected EVs among NHPs in a zoo in Dhaka were human EVsCitation32,Citation33. A 2016 study in China provided some of the only evidence of EV infection among species other than NHPs. The EVs detected in rodents shared 72% identity with human coxsackievirus A11 based on the nucleotide sequence from 295-632 of the 5′UTR (Tibet2015) region and 74% identity with EV-D68 based on 179-628 of 5′UTR (NX2015) regionCitation22.
Supporting phylogenic evidence
It is well documented that SVDV originated in humans as human coxsackie virus B5, which transferred to pigs between 1945 and 1965Citation34,Citation35. Through recombination and phylogenetic analysis, Bruhn et al. recently suggested that SVDV may have originated from a single recombinant eventCitation36. More recently, a virus isolate (T75) from a 1975 SVDV outbreak in Central Russia was propagated, sequenced, and compared to an isolate from the first reported outbreak of SVDV in the Soviet Union (strain O72). Lomakina et al. found the T75 strain shared 80.0–90.4% identity with human coxsackievirus B4 in the VP1 region using Sanger sequencing. However, unlike the CVB5-related SVDV, which has caused several epizootic outbreaks since the 1960s, T75 has not become an established swine pathogenCitation37. Our understanding that SVDV originated as a human EV supports the finding that cross-species transfer of EVs occurs in animal species beyond NHPs.
Discussion
The majority of the studies included in this review clearly documented that animal infection with several HEV types (including EV-A, B, C, and D) have at least occasionally occurred. Interestingly, among these studies, the reverse zoonoses of EVs between NHP populations and humans are discussed most frequently. It also became evident through the review that there have been a number of diverse HEVs identified in NHPs. For instance, the Harvala et al. studiesCitation23,Citation26 and Sadeuh-Mba et al. studiesCitation24,Citation25 detected diverse EV species, such as species A (A76, A89, A90, A119), species B (B110), and species D (D111, D120) through screening of fecal samples collected from wild chimpanzees and gorilla in Central African countries (primarily in Cameroon). These EV types have been also detected in humans or were found to be closely related to previously detected HEV types. Similarly, the Oberste et al. studies conducted in Bangladesh also reported that several types of HEVs (including species A, B, and C) detected from both captive and wild NHPs (primarily Rhesus macaques)Citation32,Citation33. It is worth noting that EV-A76 was detected from both human samples and urban rhesus macaques in Bangladesh as well, although EV-A76 was not the most prevalent picornavirus detected among NHPs in the study. In similar studies conducted in Denmark and the USA, although researchers did not find the same types of HEVs as previously described in the studies conducted in Bangladesh and Central Africa, authors did find several new types of EVs that also closely related to HEVs circulating in rhesus macaques, including Coxsackie B3 (CB3, now assigned into species B)Citation31, simian enterovirus 46 (SV46), and EV92Citation30. These results may indicate that NHPs are susceptible to more diverse types of HEVs than previously expected.
In contrast to human infections with HEVs, which have been widely studied in the past decades, NHP infections with HEVs have not historically received as much attention. Based on the evidence from a growing number reported instances of NHP infection with diverse HEVs, as well as several instances of human infection with non-human EVs, there are compelling data to show the possibility for cross-species EV transmissionCitation23–Citation26,Citation32,Citation33,Citation38. It is, therefore, important to understand the relationship between humans and NHPs and consider the susceptibility of NHPs to HEV infection.
Numerous previous reports mentioned that NHPs shared more than 90% of human DNA. Among these NHPs, chimpanzees (>99% with human DNA) were reported to be humans’ closest living relativesCitation38,Citation39. Our findings in this review support the hypothesis that NHPs (especially chimpanzees) may be at an increased risk for infection with human diseases because of their genetic similarity and due to less of a species barrier for pathogen transfer. However, the condition that this phenomenon is hinged on is close contact between NHPs and humans. It is well documented that there has been an increase in contact between NHPs and humans in recent decades, especially in Central African countries and South Asian countriesCitation40. For instance, Wolfe et al. and Betsem et al. describe how the growing demand for bushmeat and intensified deforestation of tropical forests in Central Africa have escalated humans’ hunting activitiesCitation41,Citation42. These hunting practices increase wild NHPs’ proximity to humans and heighten the risk of human pathogen infections in NHPs. In addition, due to rapid urbanization, an increase in population density and change in land use (i.e. forest encroachment) in recent decades, many South Asian countries (i.e. Singapore, and Bangladesh) have reported the NHPs (e.g. Rhesus macaques and long-tailed macaques) thrive in new human habitats, also thereby increasing the interactions between humans and NHPsCitation33,Citation43.
In 2007, Wolfe et al. reported, “primates constitute only 0.5% of all vertebrate species but have contributed about 20% of our major human diseases”Citation44. Indeed, there are many human infectious diseases with NHP origins that are well-known and documented, including HIV/AIDSCitation45, dengueCitation46, hepatitis BCitation47, and malariaCitation48. Although no studies included in this review demonstrated human infection with an NHP EV, many of them indicate that NHP infection with human EVs might amplify human viruses or even facilitate recombination with primate EVs, yielding a novel source of future emerging diseases in humans. Thus, future studies exploring cross-species transmission of EVs between NHPs and humans are still needed.
The zoonotic transfer of EVs is not, however, limited to NHPs and humans; other animal EVs have also been found to infect humans and diverse animal species, such as BEV and SVDV. Subsequent to the Gür et al.Citation17 study of BEV-1 antibodies in humans, horses, dogs, goats, and sheep, McClenahan et al. (2013) found that alpacas had been infected with BEVs in the United StatesCitation49. The review also demonstrated the close relationship between SVDV and an HEV-B serotype, coxsackievirus B5 (CVB5), suggesting that SVDV was produced by the recombination between CVB5 and one other EV-B serotypeCitation17,Citation37. These findings highlight the potential for cross-species transmission of SVDV. Cross-species transmission of animal EVs may also increase the risk for new emerging infectious diseases, as animal EVs may more easily mutate or recombine with other viruses in an animal host.
This systematic review had a number of limitations. First, as we limited the online review to studies published during the past ten years (focusing upon the more frequent use of molecular methods), the review may have missed some early reports of possible EV zoonoses. For example, we learned of the 1973 report documenting probable SVDV infections among workers at the Animal Virus Research Institute (United Kingdom) through serological assessmentCitation21 through an external reviewer of the manuscript. Similar, non-molecular evidence of zoonoses may have been missed. Second, through our search strategy we focused heavily upon zoonoses, and may have missed some important reports of cross-species infections among nonhuman animalsCitation50. We cannot exclude the possibility that partial genomes of viruses detected in animal feces were pathogens passing through the digestive system without active infection. Additionally, this review only found limited articles demonstrating evidence of anthropozoonosis EV infection. Although the findings of these studies demonstrate the potential for animal EVs to infect humans, these results are not generalizable. For example, as a serological study, the Gür et al. studyCitation17 cannot provide evidence of active BEV-1 infection. In the other study demonstrating possible evidence of anthropozoonosis , Grutzmacher et al. found only one human fecal sample testing positive for an animal EV. While our findings demonstrate that EVs may be transmitted from animals to humans, the current scientific data are still somewhat sparse and provide suggestive, but not conclusive, evidence.
However, all of these findings convey an important message: the diversity of EVs and potential for cross-species transmission of EVs between humans and animals should not be underestimated. Further surveillance for cross-species EV infections among both humans and animals seem warranted. By leveraging a One Health approach, veterinarians, epidemiologists, and public health stakeholders will most effectively be able to provide targeted surveillance for EVs circulating in humans and animals. We recognize the barriers to active surveillance for EVs, including access to timely diagnostic tests, cross-reactivity in serology, and the limitations of primer-based molecular assays in detecting human as opposed to new animal EVs. As molecular diagnostic techniques continue to evolve, however, researchers may turn to more sensitive surveillance techniques, such as unbiased next-generation DNA sequencing as a solution to some of these hurdles.
Conclusions
This review found considerable molecular evidence supporting the occurrence of zooanthroponoses of EV infection, particularly among NHPs. While it is more limited, serological evidence of BEV-1 in humans and phylogenic evidence of an SVDV-like infection in a human fecal sample, demonstrate the possibility of anthropozoonotic transfer. From a One Health perspective, we must consider that anthropogenic factors such as deforestation combined with an overall global increase in industrialized animal production has led to intensified and more frequent interactions between humans and animals. In particular, should contact between humans and NHPs increase the risk of zoonotic transfer, active surveillance for EV infections will be increasingly important due to deforestation and the resulting trend of escalated interactions between wild primate populations and humans. Given our understanding of the possibility for zoonotic transfer, and the knowledge that zooanthroponotic transfer of EVs may commonly occur, surveillance for cross-species transmission of enteroviruses is recommended, regardless of the directionality of the transfer.
Acknowledgements
We wish to thank Dr. Dean Erdman, formerly of the US Centers for Disease Control and Prevention, for reviewing an earlier version of this manuscript. This systematic review was supported by Professor Gregory Gray’s Duke Global Health Institute discretionary funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- PalaciosGObersteMSEnteroviruses as agents of emerging infectious diseasesJ. Neurovirol.200511 424 43310.1080/13550280591002531
- Van NguyenDHigh rates of infection with novel enterovirus variants in wild populations of mandrills and other old world monkey speciesJ. Virol.2014885967597610.1128/JVI.00088-14
- AlexanderJJPBadenLPallanschMAAndersonLJEnterovirus 71 infections and neurologic disease—United States, 1977–1991J. Infect. Dis.199416990590810.1093/infdis/169.4.905
- MidgleyCMSevere respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014MMWR Morb. Mortal. Wkly. Rep.20146379894584696
- GorbalenyaCLAlexanderEToward genetics-based virus taxonomy: comparative analysis of a genetics-based classification and the taxonomy of picornavirusesJ. Virol.2012863905391510.1128/JVI.07174-11
- ZhuLIdentification of a novel enterovirus E isolates HY12 from cattle with severe respiratory and enteric diseasesPLoS ONE20149e9773010.1371/journal.pone.0097730
- AnbalaganSHesseRAHauseBMFirst identification and characterization of porcine enterovirus G in the United StatesPLoS ONE20149e9751710.1371/journal.pone.0097517
- NixW. A.JiangB.MaherK.StrobertE.ObersteM. S.Identification of Enteroviruses in Naturally Infected Captive PrimatesJournal of Clinical Microbiology20084692874287810.1128/JCM.00074-08
- WooPCYA novel dromedary camel enterovirus in the family Picornaviridae from dromedaries in the Middle EastJ. Gen. Virol.2015961723173110.1099/vir.0.000131
- LukashevANVakulenkoYAMolecular evolution of types in non-polio enterovirusesJ. Gen. Virol.2017982968298110.1099/jgv.0.000966
- MessengerAMBarnesANGrayGCReverse zoonotic disease transmission (zooanthroponosis): a systematic review of seldom-documented human biological threats to animalsPLoS ONE20149e8905510.1371/journal.pone.0089055
- RabozziGEmerging zoonoses: the “One Health Approach”Saf. Health Work20123778310.5491/SHAW.2012.3.1.77
- EvansBLeightonFA history of One HealthRev. Sci. Tech.20143341342010.20506/rst.33.2.2298
- LiuWCo-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot, and mouth disease in Central ChinaPLoS ONE20149e9605110.1371/journal.pone.0096051
- TakahashiSHand, foot, and mouth disease in china: modeling epidemic dynamics of enterovirus serotypes and implications for vaccinationPLoS Med.201613210.1371/journal.pmed.1001958
- WuJianyongLiuLanlanWangGuolingLuJiahaiOne Health in ChinaInfection Ecology & Epidemiology2016613384310.3402/iee.v6.33843
- GürSYapkiçOYilmazASerological survey of bovine enterovirus type 1 in different mammalian species in TurkeyZoonoses Public Health20085510611110.1111/j.1863-2378.2007.01095.x
- TaylorMWBovine enterovirus-1: characterization, replication and cytopathogenic effectsJ. Gen. Virol.19742317317810.1099/0022-1317-23-2-173
- GrutzmacherKSCodetection of respiratory syncytial virus in habituated wild western lowland gorillas and humans during a respiratory disease outbreakEcohealth20161349951010.1007/s10393-016-1144-6
- GravesJHSerological relationship of swine vesicular disease virus and Coxsackie B5 virusNature197324531431510.1038/245314a0
- BrownFGoodridgeDBurrowsRInfection of man by swine vesicular disease virusJ. Comp. Pathol.19768640941410.1016/0021-9975(76)90009-8
- DuJDistribution and characteristics of rodent picornaviruses in ChinaSci. Rep.2016610.1038/srep34381
- HarvalaHDetection and genetic characterization of enteroviruses circulating among wild populations of chimpanzees in Cameroon: relationship with human and simian enterovirusesJ. Virol.2011854480448610.1128/JVI.02285-10
- Sadeuh-MbaSAHigh frequency and diversity of species C enteroviruses in Cameroon and neighboring countriesJ. Clin. Microbiol.20135175977010.1128/JCM.02119-12
- Sadeuh-MbaSACharacterization of enteroviruses from non-human primates in Cameroon revealed virus types widespread in humans along with candidate new types and speciesPLoS Negl. Trop. Dis.20148e305210.1371/journal.pntd.0003052
- HarvalaHCo-circulation of enteroviruses between apes and humansJ. Gen. Virol.201495Pt 240340710.1099/vir.0.059048-0
- MomboIMFirst detection of an enterovirus C99 in a captive chimpanzee with acute flaccid paralysis, from the Tchimpounga Chimpanzee Rehabilitation Center, Republic of CongoPLoS ONE201510e013670010.1371/journal.pone.0136700
- MomboIMAfrican non-human primates host diverse enterovirusesPLoS ONE201712e016906710.1371/journal.pone.0169067
- ObersteMSEnteroviruses 76, 89, 90 and 91 represent a novel group within the species Human enterovirus AJ. Gen. Virol.20058644545110.1099/vir.0.80475-0
- ObersteMSJiangXMaherKNixWAJiangBThe complete genome sequences for three simian enteroviruses isolated from captive primatesArch. Virol.20081532117212210.1007/s00705-008-0225-4
- NielsenSCProbable transmission of coxsackie B3 virus from human to chimpanzee, DenmarkEmerg. Infect. Dis.2012181163116510.3201/eid1807.111689
- ObersteMSNaturally acquired picornavirus infections in primates at the Dhaka zooJ. Virol.20138757258010.1128/JVI.00838-12
- ObersteMSCharacterizing the picornavirus landscape among synanthropic nonhuman primates in Bangladesh, 2007 to 2008J. Virol.20138755857110.1128/JVI.00837-12
- SmuraTEvolution of newly described enterovirusesFuture Virol.2011610913110.2217/fvl.10.62
- ZhangGHaydonDTKnowlesNJMcCauleyJWMolecular evolution of swine vesicular disease virusJ. Gen. Virol.19998063965110.1099/0022-1317-80-3-639
- BruhnCAViral meningitis epidemics and a single, recent, recombinant and anthroponotic origin of swine vesicular disease virusEvol. Med. Public Health2015128930310.1093/emph/eov026
- LomakinaNFYu ShustovaEStrizhakovaOMFelix DrexlerJLukashevANEpizootic of vesicular disease in pigs caused by coxsackievirus B4 in the Soviet Union in 1975J. Gen. Virol.201697495210.1099/jgv.0.000318
- HardingJDProgress in genetics and genomics of nonhuman primatesILAR J.201354778110.1093/ilar/ilt051
- PrüferKThe bonobo genome compared with the chimpanzee and human genomesNature201248652753110.1038/nature11128
- NaratVAlcayna-StevensLRuppSGiles-VernickTRethinking human–nonhuman primate contact and pathogenic disease spilloverEcohealth20171484085010.1007/s10393-017-1283-4
- WolfeNDDaszakPKilpatrickAMBurkeDSBushmeat hunting, deforestation, and prediction of zoonotic diseaseEmerg. Infect. Dis.2005111822182710.3201/eid1112.040789
- BetsemERuaRTortevoyePFromentAGessainAFrequent and recent human acquisition of simian foamy viruses through apes’ bites in Central AfricaPLoS Pathog.20117e100230610.1371/journal.ppat.1002306
- FuentesACharacterizing human–macaque interactions in SingaporeAm. J. Primatol.20087087988310.1002/ajp.20575
- WolfeNDDunavanCPDiamondJOrigins of major human infectious diseasesNature200744727910.1038/nature05775
- HahnBHShawGMDeKMCockSharpPMAIDS as a zoonosis: scientific and public health implicationsScience200028760710.1126/science.287.5453.607
- VasilakisNCardosaJHanleyKAHolmesECWeaverSCFever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public healthNat. Rev. Microbiol.2011953210.1038/nrmicro2595
- de Carvalho Dominguez SouzaBFDrexlerJFde LimaRSde Oliveira Hughes Veiga do RosárioMNettoEMTheories about evolutionary origins of human hepatitis B virus in primates and humansBraz. J. Infect. Dis.20141853554310.1016/j.bjid.2013.12.006
- FaustCDobsonAPPrimate malarias: diversity, distribution and insights for zoonotic plasmodiumOne Health20151667510.1016/j.onehlt.2015.10.001
- McClenahanSDDiscovery of a bovine enterovirus in AlpacaPLoS ONE20138e6877710.1371/journal.pone.0068777
- BorosAacutePankovicsPKnowlesNJReuterGNatural interspecies recombinant bovine/porcine enterovirus in sheepJ. Gen. Virol.2012931941195110.1099/vir.0.041335-0
- Enterovirus (The Pirbright Institute, 2018), http://www.picornaviridae.com/enterovirus/enterovirus.htm.
- Virus Taxonomy: 2017 Release (International Committee on Taxonomy of Viruses, 2017), https://talk.ictvonline.org/taxonomy/.
